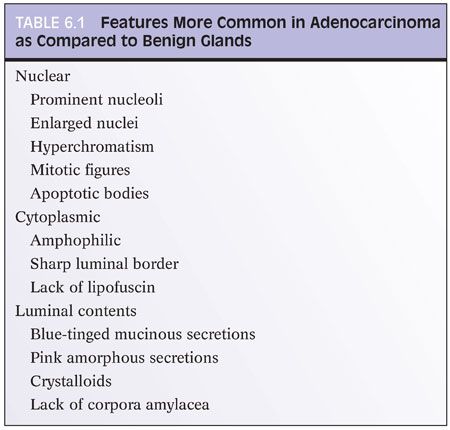
Evaluating an atypical focus in a needle biopsy of the prostate should be a methodical process. When reviewing needle biopsies, one should develop a mental balance sheet where on one side of the column are features favoring the diagnosis of carcinoma and on the other side of the column are features against the diagnosis of cancer (Table 6.1). At the end of evaluating a case, hopefully all of the criteria are listed on one side of the column or the other such that a definitive diagnosis can be made. It is always helpful to first identify glands that you are confident are benign, and then compare these benign glands to the atypical glands that you are considering to diagnose as adenocarcinoma of the prostate. The greater the number of differences between the recognizable benign glands and the atypical glands, the more confidently a malignant diagnosis can be established. It will be stressed throughout this chapter that the diagnosis of cancer should be based on a constellation of features rather than relying on any one criterion by itself.
ARCHITECTURAL FEATURES IN THE DIAGNOSIS OF CANCER
It is important when examining needle biopsy specimens to gain an appreciation of what the overall architecture of the nonneoplastic prostate looks like. In order to identify limited amounts of cancer on needle biopsy material, one first has to identify the normal nonneoplastic prostate and then look for glands that do not fit in. Although most prostates are relatively similar in their histologic appearance, some contain numerous small foci of crowded glands similar to adenosis. In such a case, the diagnosis of cancer based on a small focus of crowded glands with minimal cytologic atypia should be performed with caution. Other men’s prostate glands are characterized by widespread atrophy; one should in these cases hesitate to diagnose cancer if the atypical glands have scant cytoplasm.
In general, scanning of prostate needle biopsies should be performed with either a 4× or 10× objective. Reviewing needle biopsies at lower magnifications runs the risk of overlooking limited foci of carcinoma. Evaluation of prostate needle biopsies at higher magnification is also nonproductive as glands with slight nuclear atypia taken out of context of their architectural pattern will often be erroneously confused with adenocarcinoma.
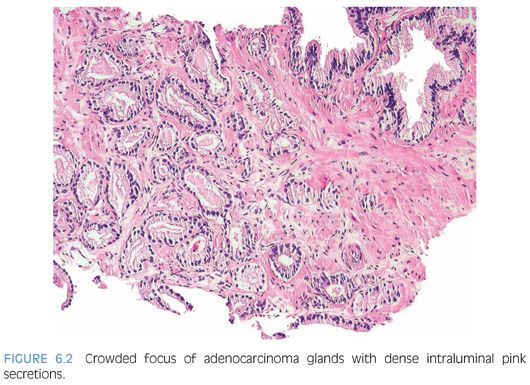
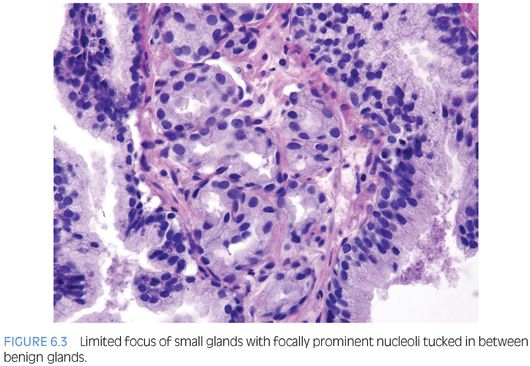
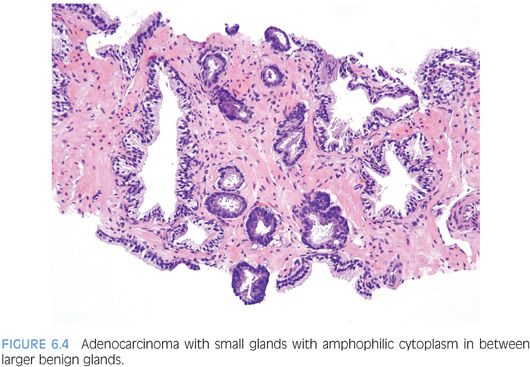
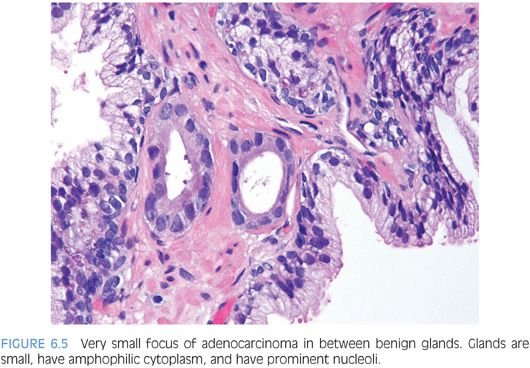
One pattern seen at low magnification that should raise a suspicion of carcinoma is the presence of a focus of crowded glands (Figs. 6.1 and 6.2, eFigs. 6.1 to 6.9). The second architectural pattern that is suspicious for adenocarcinoma of the prostate is the presence of small glands situated next to larger benign glands (Figs. 6.3 and 6.4, eFigs. 6.10 to 6.13). In most adenocarcinomas, the neoplastic glands are smaller than adjacent benign glands. Benign glands are recognized by their larger size, papillary infolding, and branching. Even if there are only a few small atypical glands, if they are crowded tightly in between benign glands, then they cannot be a tangential section off of high-grade prostatic intraepithelial neoplasia (PIN) (Fig. 6.5).
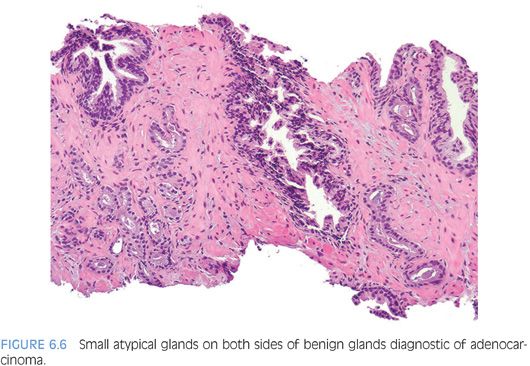
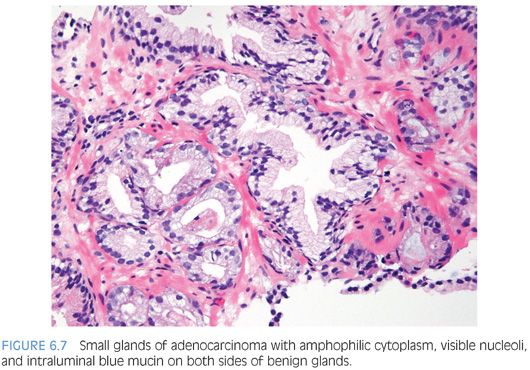
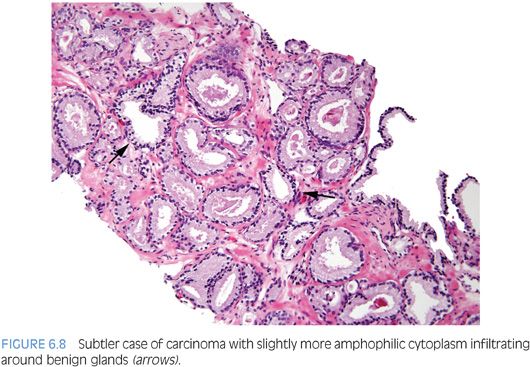
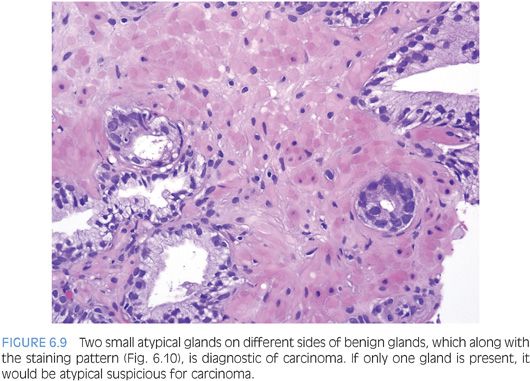
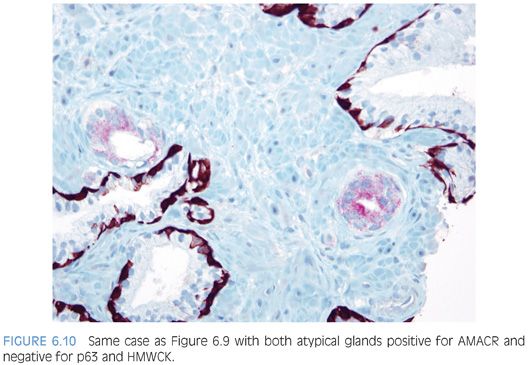
An infiltrative pattern, characterized by the presence of small atypical glands on both sides of a benign gland, is even more diagnostic of malignancy (Figs. 6.6 to 6.10, eFigs. 6.14 to 6.28). In contrast, mimickers of cancer will appear infiltrative as a collection of glands in between benign glands, but do not intercalate as isolated glands in between and around benign glands.
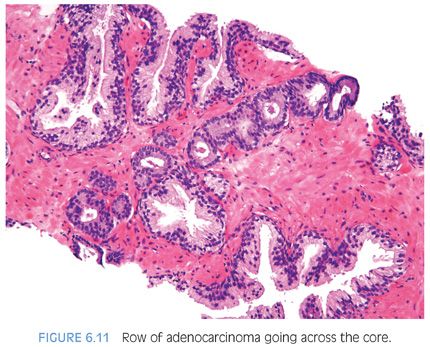
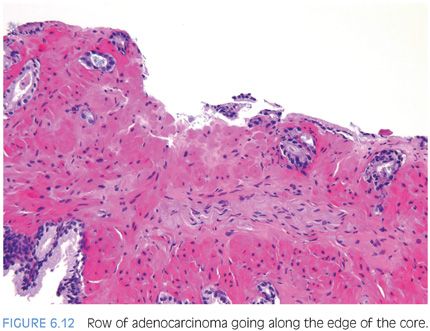
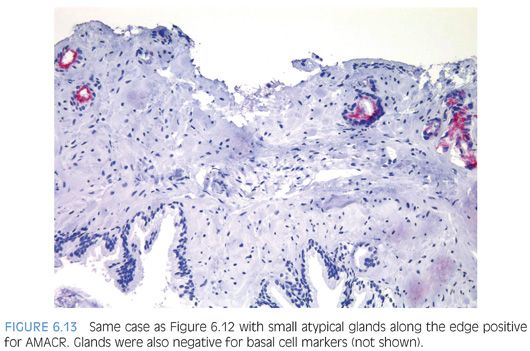
Another characteristic pattern in prostate cancer is the finding of a linear row of atypical glands going either across the width of the core or along the edge of the core (Figs. 6.11 to 6.13). Linear growth is not a feature of mimickers of prostate adenocarcinoma.
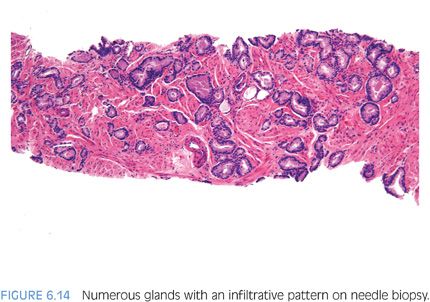
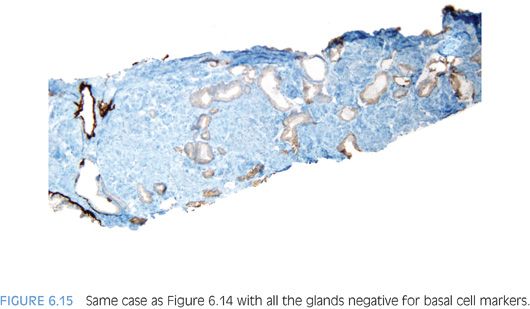
In the evaluation of an atypical focus, the presence of several of the features can help establish a diagnosis of cancer even when limited tumor is present. It is uncommon for the diagnosis of limited tumor to be solely based on the architectural pattern.2 In these cases, when none of the features listed in Table 6.1 are present and the diagnosis is made on the architectural pattern, one should be extremely cautious and only diagnose cancer when the pattern is overtly malignant (Figs. 6.14 and 6.15).
NUCLEAR FEATURES IN THE DIAGNOSIS OF CANCER
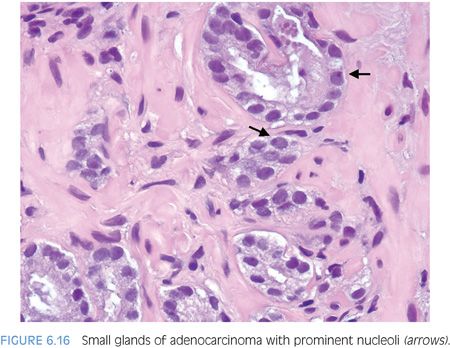
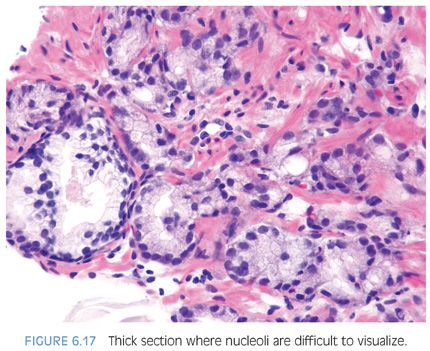
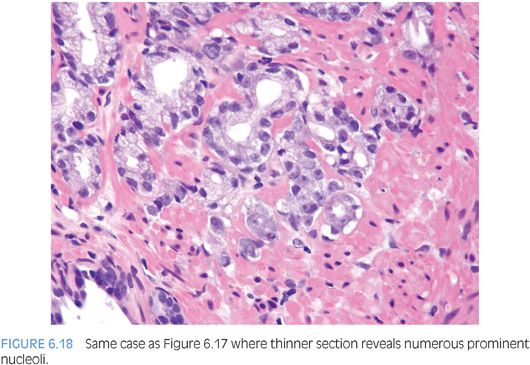
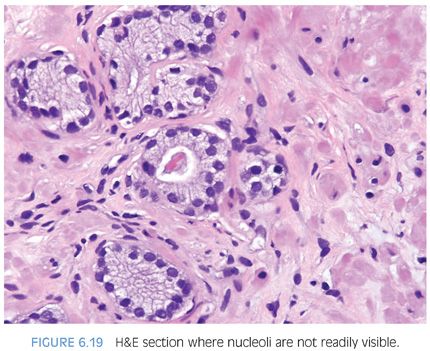
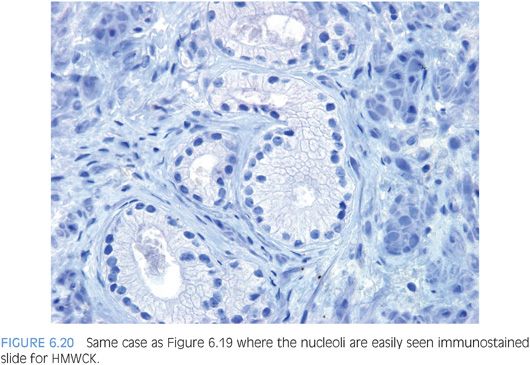
Prominent nucleoli, although important in the diagnosis of cancer on needle biopsy, should not be the sole criterion used to establish the diagnosis (Fig. 6.16, eFigs. 6.17 to 6.28). Reliance on prominent nucleoli for the diagnosis of prostate cancer will potentially lead both to an overdiagnosis as well as to an underdiagnosis of prostate cancer. Overdiagnosis of cancer can arise from prominent nucleoli being seen in various mimickers of cancer (see Chapter 7). Underdiagnosis of cancer relates to prominent nucleoli being seen in only 76% of cancers in consultation-based needle biopsy material, although more frequently present in routine biopsy material.2,3 In addition, a significant minority of cancers may reveal only rare prominent nucleoli. In many of these cases, referring pathologists specifically noted that the lack of prominent nucleoli prevented them from definitively establishing a malignant diagnosis. The lack of prominent nucleoli in many of these cases probably reflected a sampling problem where areas of the tumor with prominent nucleoli were not biopsied. In other cases, overstained or thick sections obscured nuclear detail (Figs. 6.17 and 6.18). In some cases, greater nuclear detail can be seen on the immunostained sections, which tend to be cut thinner than the routine hematoxylin and eosin (H&E) sections (Figs. 6.19 and 6.20). If prominent nucleoli are relied upon to establish a diagnosis of limited prostate cancer on needle biopsy, a significant number of carcinomas will go underdiagnosed.
It has been stated that multiple nucleoli, especially those eccentrically located in the nucleus, are diagnostic of cancer.3,4 In contrast to prior studies, we have analyzed not only cancer and benign tissue but a wide range of mimickers of cancer.5 In our study, we found that multiple nucleoli was a rare (1%) finding in normal glands. It was most common in high-grade PIN (54%) and cancer (38%). Although the overall frequency of noneccentrically located nucleoli was higher than centrally positioned nucleoli in cancer and very uncommon in normal glands, various benign mimickers of prostate cancer had a sufficiently high frequency of multiple nucleoli and peripherally located nucleoli to render these features not useful in diagnostic practice. For example, in postatrophic hyperplasia, 37% of cases had more than one nucleolus per nucleus and 52% had some peripherally located nucleoli.
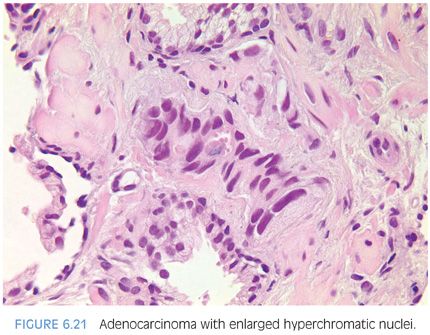
Often, nuclear enlargement may be present when prominent nucleoli are not and is an important diagnostic feature.2 Nuclear hyperchromasia is another cytologic feature that may help to distinguish cancerous from benign glands (Fig. 6.21, eFigs. 6.29 to 6.36).
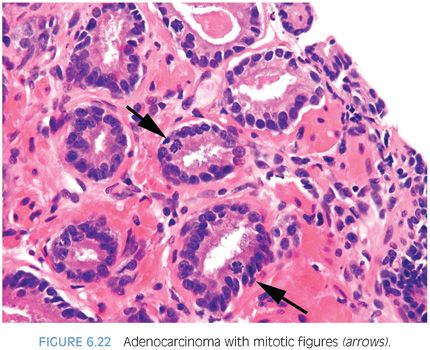
Fewer works have examined the frequency of mitotic figures in prostate cancer (Fig. 6.22, eFigs. 6.37 and 6.38). We noted in a series of limited adenocarcinoma on needle biopsy that 11% contained mitotic figures.2 A comparable frequency of 10% was noted in a study of minimal volume cancers on biopsy by Iczkowski and Bostwick.6 Vesalainen et al.7 demonstrated that although mitotic figures were not uncommon in Gleason score 8–10 cancers, the mean number of mitotic figures per 10 high power fields was only 4.3 for Gleason score 5–7 tumors. Mitotic figures were also shown by Aihara et al.8 to correlate with Gleason grade. In accordance with these studies, we found mitotic figures more commonly in high-grade PIN (12%) and cancer (13%) as compared to its infrequency (≤3%) in benign glandular mimickers of cancer with the exception of 6% of cases of benign glands with inflammation having mitoses.5 Mitotic figures are, therefore, a helpful diagnostic feature that favors the diagnosis of prostate cancer, although its infrequency in focal Gleason score 6 cancer on needle biopsy limits its utility.
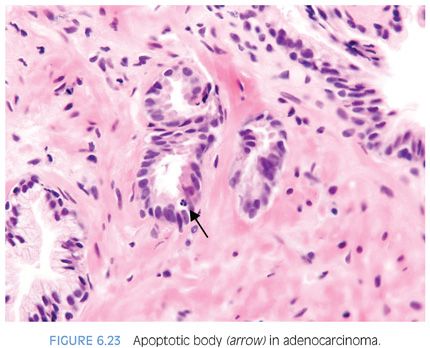
Most studies have found between 1% and 2% of benign prostate cells have apoptotic bodies using specialized techniques.9,10 Identifying apoptotic bodies on routine H&E-stained sections, Montironi et al.11 found that the frequency of apoptotic bodies increased from benign prostatic hyperplasia (BPH) (0.3%) to high-grade PIN (0.75%) to “small acinar carcinoma” (1.0%) to “cribriform cancer” (1.3%) to “solid carcinoma” (2.1%). Aihara et al.8 also investigated the frequency of apoptotic bodies on H&E-stained sections and showed that the frequency increased across Gleason grade pattern 1 (0.04%), pattern 2 (0.15%), pattern 3 (0.3%), pattern 4 (0.5%), and pattern 5 (0.7%). Apoptotic bodies were rare in normal glands (0.07%).8 We reported apoptotic bodies as being present or absent per small focus of cancer on needle biopsy and found that apoptotic bodies were fairly common in cancer, seen in 34% of small foci of cancer sent in for consultation. Apoptotic bodies were next most prevalent in high-grade PIN (13%). In contrast, apoptotic bodies were uncommon (≤3%) in normal glands and benign mimickers of cancer, such that the presence of apoptotic bodies is helpful in the diagnosis of challenging cases of prostate cancer on needle biopsy (Fig. 6.23, eFig. 6.39).5
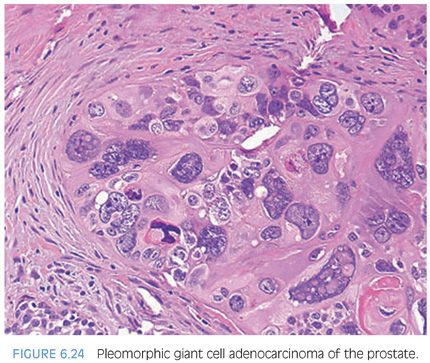
Conventional prostate cancer, even when very high grade, typically consists of cells with relatively uniform nuclei. We have described rare cases on needle biopsy of prostate carcinoma with pleomorphic bizarre nuclei (Fig. 6.24, eFig. 6.40).12 The presence of a more conventional prostatic adenocarcinoma component may clue one into the correct diagnosis once one realizes that prostate cancer can rarely display such prominent nuclear atypia. In cases where the conventional component is very poorly differentiated or absent, a battery of immunostains including melanoma, lymphoid, and epithelial markers, and antibodies against thrombomodulin, GATA3, and prostate-specific antigen (PSA), P501S, and NKX3.1 (see Chapter 15) should be performed. A further pitfall that must be recognized with evaluation of the immunohistochemical stains is that PSA is typically negative in the giant cell component and often only focal in the conventional adenocarcinoma component. Based on the limited number of cases in this study and other published studies, pleomorphic giant cell prostatic adenocarcinoma heralds a particularly aggressive clinical outcome.12,13
CYTOPLASMIC FEATURES IN THE DIAGNOSIS OF CANCER
Although in the past there has been much less consideration paid to cytoplasmic features as compared to nuclear qualities, the nature of the cytoplasm may be critical in the diagnosis of some carcinomas. In some adenocarcinomas of the prostate, the cytoplasm of the malignant glands is more amphophilic than the surrounding benign glands that have pale to clear cytoplasm (Figs. 6.25 and 6.26, eFigs. 6.41 to 6.50). In order for this criterion to be helpful, the benign prostate glands must be appropriately stained such that they have a pale to clear appearance. In a study of consult cases, we found that in 32% of the cases, this criterion was not applicable since the benign glands also exhibited amphophilic cytoplasm.2 Because this feature is helpful in a large number of cases, one’s H&E stains should be adjusted so that the cytoplasm of the benign glands appears pale to clear.
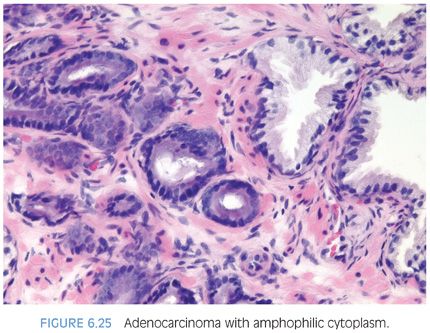
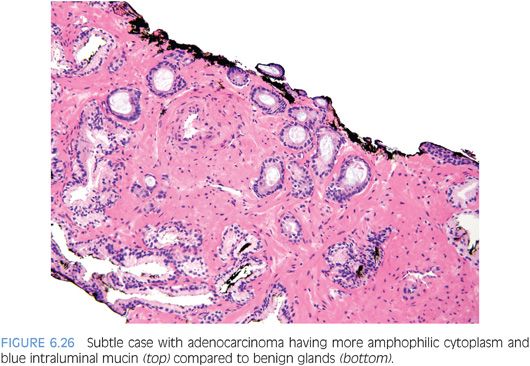
The lack of lipofuscin in atypical prostatic glands suspicious for cancer, if there is prominent pigment in the surrounding benign glands, may help to establish a definitive diagnosis of cancer. Lipofuscin is uncommon in high-grade PIN and rare in cancer (eFigs. 6.51 to 6.55).14
Abundant cytoplasm with straight luminal borders in larger glands is also a feature of cancer, which is also described under the entity of “pseudohyperplastic cancer” (eFigs. 6.56 to 6.58). The only time benign prostate glands typically have straight luminal borders is when either the glands are small or large but with markedly atrophic cytoplasm.
INTRALUMINAL CONTENTS IN THE DIAGNOSIS OF CANCER
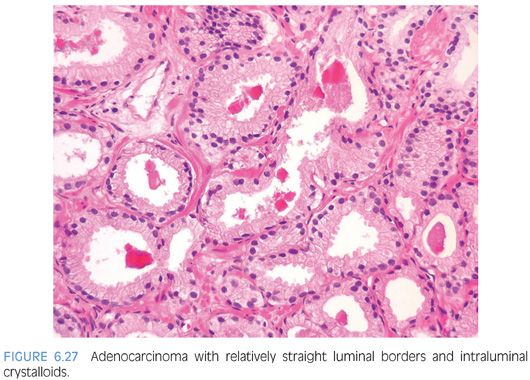
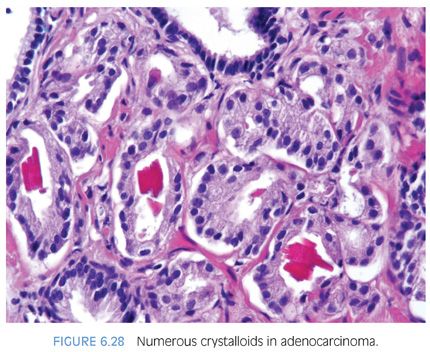
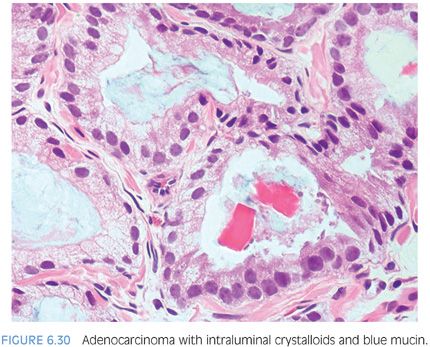
Prostatic crystalloids are dense eosinophilic crystal-like structures that appear in various geometric shapes such as rectangular, hexagonal, triangular, and rodlike structures (Figs. 6.27 and 6.28, eFigs. 6.59 to 6.62). Prostatic crystalloids have been reported in 25% of cancers seen on biopsy material, yet may also be seen in benign prostate acini.2,15,16 The likelihood of finding crystalloids is dependent on the number of malignant glands present and the grade; crystalloids are inversely correlated with the Gleason grade. Crystalloids, although not diagnostic of carcinoma, are more frequently found in cancer than in benign glands. The one condition that mimics cancer where crystalloids are frequently seen is adenosis, which consists of a lobule of pale-staining glands (see Chapter 7). Consequently, if crystalloids are seen in small glands with an infiltrative appearance in between benign glands, where adenosis is not in the differential, they may help to establish a diagnosis of cancer. The finding of prostatic crystalloids in benign glands does not indicate an increased risk of cancer on subsequent biopsy.17
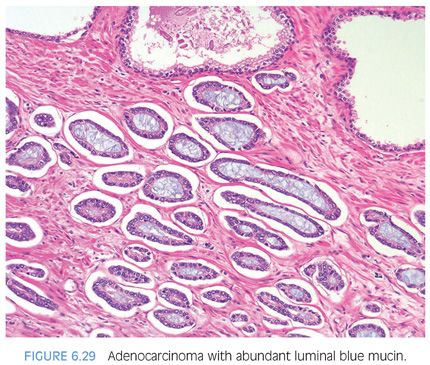
Another diagnostic criterion relates to the nature of intraluminal secretions. Blue-tinged mucinous secretions seen on H&E-stained sections are mostly observed in carcinomas and only rarely identified in benign glands (Figs. 6.29 and 6.30)2 (eFigs. 6.63 to 6.74). The prevalence of these blue-tinged secretions is in part influenced by the nature of the H&E stain. In some institutions’ referral material, this feature appears to be fairly prevalent, whereas in other institutions, it is uncommonly seen or faint and wispy. Some laboratory’s H&E stains are too basophilic, where even benign glands contain blue-tinged mucinous secretions. When normal colonic glands that are present on many prostate biopsies show an intense blue appearance, pathologists have to be cautious in placing too much weight on blue-tinged mucin in prostate glands as a diagnostic criterion for cancer. Although initial reports suggested that acid mucin stains could distinguish malignant from benign glands, subsequent articles demonstrated that acid mucin is variably present in mimickers of carcinoma, such as adenosis and atrophic glands.18,19
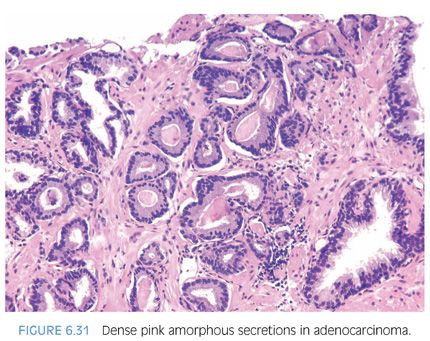
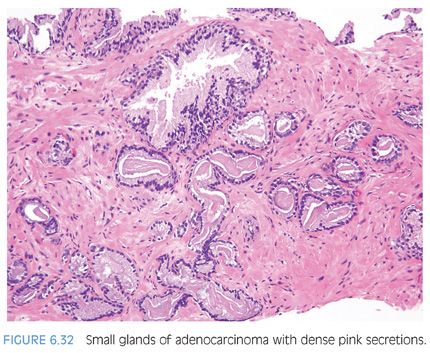
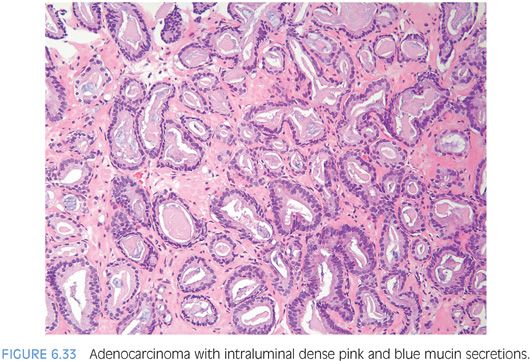
Another type of intraluminal secretion that may aid in the diagnosis of limited cancer is pink dense amorphous acellular secretions identified in approximately half of cancers on needle biopsy and only occasionally seen in benign glands (Figs. 6.31 and 6.32, eFigs. 6.75 to 6.81).2 These amorphous secretions should be distinguished from corpora amylacea, which are well-circumscribed round to oval structures with concentric lamellar rings that are prominent in benign glands and uncommonly seen in cancer (eFig. 6.82).2,20,21 Both pink and blue secretions often coexist in the same glands (Fig. 6.33, eFigs. 6.83 to 6.91). As with all of the criteria mentioned to this point, this feature is not specific for carcinoma. Rather, the presence of intraluminal secretions should be taken in context of the architectural pattern and the nuclear and cytoplasmic features.
HISTOLOGIC FEATURES SPECIFIC FOR PROSTATE CANCER
There are three features that have not to date been identified in benign glands, and which are in and of themselves diagnostic of cancer (Table 6.2). These are mucinous fibroplasia (collagenous micronodules), glomerulations, and perineural invasion.

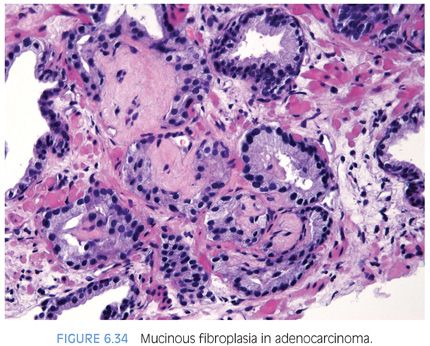
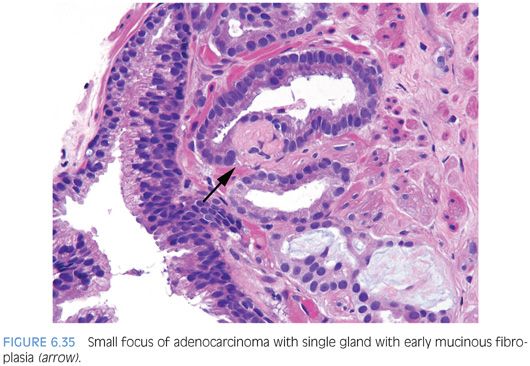
Occasionally, intraluminal mucinous secretions are so extensive that they become focally organized.22,23 This lesion, known as either mucinous fibroplasia or collagenous micronodules, is typified by very delicate loose fibrous tissue with an ingrowth of fibroblasts (Figs. 6.34 and 6.35, eFigs. 6.92 to 6.111). Mucinous secretions can displace the epithelium, resulting in atrophic cytoplasm and small pyknotic nuclei, whereby these foci can be difficult to recognize as cancer (see Chapter 9 for grading).
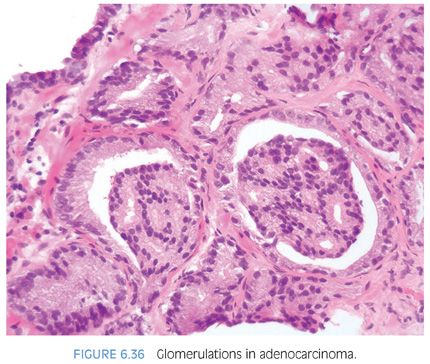
Glomerulations consists of glands with a cribriform proliferation that is not transluminal (Fig. 6.36, eFigs. 6.112 to 6.119). Rather, these cribriform formations are attached to only one edge of the gland resulting in a structure superficially resembling a glomerulus (see Chapter 9 for grading).
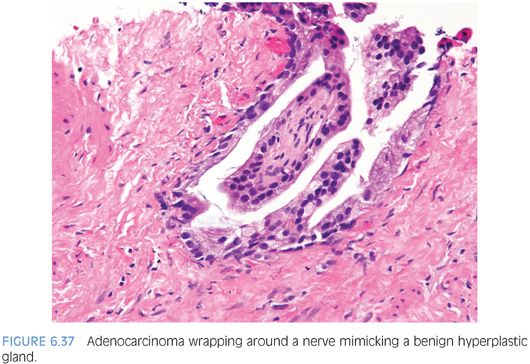
Perineural invasion is seen in approximately 20% of needle biopsies of the prostate showing adenocarcinoma.24 In a difficult case where the diagnosis of carcinoma hinges on perineural invasion, the glands in question should circumferentially surround the nerve (eFigs. 6.120 to 6.125). Uncommonly, the only atypical glands in a case are those wrapping around a nerve.22 Occasionally, cancer glands with perineural invasion have a peculiar proclivity to resemble a benign hyperplastic gland (Fig. 6.37). In other cases where there are some atypical features to the glands, perineural invasion can be diagnosed when the nerve is only partly encircled by the gland (Fig. 6.38). Perineural invasion must be distinguished from perineural indentation by benign prostate glands25,26 (eFigs. 6.126 to 6.133). The most common pattern of this phenomenon is perineural indentation by benign glands. We have called attention to other patterns of neural involvement by benign glands that are not as widely known, including intraneural and incomplete perineural involvement (Figs. 6.39 and 6.40).25 Complete circumferential wrapping was not observed in our cases as is often noted by cancerous acini, although one case did show up to 95% wrapping. Our study demonstrates that if one is going to use perineural involvement as the key diagnostic feature to establish malignancy in a given case, complete circumferential growth around the nerve is required especially if the glands have cytologic and architectural features more typically associated with benign glands. If the diagnosis of cancer is established based on other criteria, then the diagnosis of perineural invasion for prognostic purposes (see Chapter 8) can be made with less stringent criteria, including perineural tracking, intraneural involvement, and subtotal circumferential growth.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


