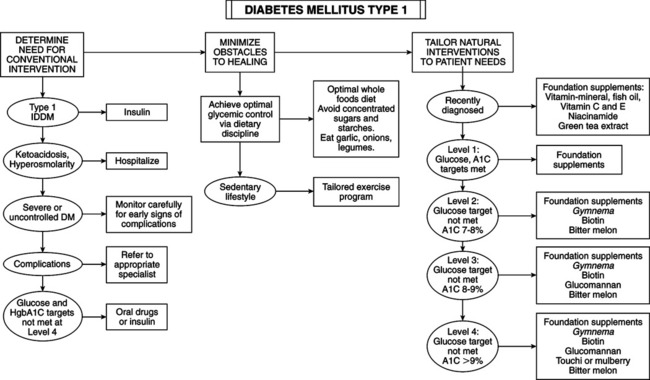
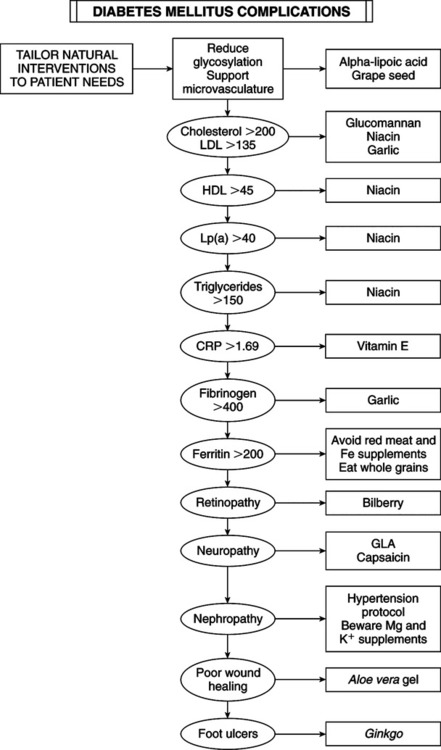
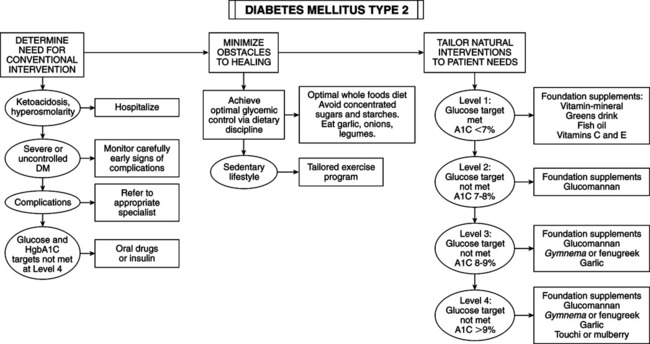
• Elevated blood glucose determination — Fasting (overnight): venous plasma glucose ≥126 mg/dL on at least two separate occasions — After ingestion of 75 g glucose: venous plasma glucose ≥200 mg/dL at 2 hours postingestion and at least one other sample during 2-hour test — Random blood glucose of 200 mg/dL or more plus presence of suggestive symptoms • Classic symptoms: polyuria, polydipsia, polyphagia • Presenting symptoms: fatigue, blurred vision, poor wound healing, periodontal disease, frequent infections • Type 1 (insulin-dependent diabetes mellitus [IDDM]): most often in children and adolescents (juvenile-onset diabetes). • Type 2 (non-insulin-dependent diabetes mellitus [NIDDM]): onset after age 40 years (adult-onset diabetes). Incidence of this type is rising in sedentary, obese children. Fifteen percent of adults diagnosed with type 2 actually have type 1. • IDDM (type 1): complete destruction of pancreas beta-cells, which manufacture insulin. Patients require lifelong insulin supplementation and must manage blood sugar daily by modifying insulin types and dosage schedules according to results of regular blood sugar testing. Ten percent of diabetics are type 1. Current causal theory focuses on injury to beta-cells plus a defect in tissue regeneration. Autoimmune component is revealed by antibodies to beta-cells present in 75% of cases compared with 0.5%-2.0% of normals. Antibodies to beta-cells may develop in response to cell destruction from other mechanisms (chemical, free radical, viral, food allergy). • NIDDM (type 2): affects 90% of diabetics. Insulin is elevated because of a loss of sensitivity to insulin by cells of body. Obesity is the major contributor; 90% of type 2 diabetics are obese. Achieving ideal body weight is linked to restoring normal blood sugar in most cases. Diet is of primary importance, implemented diligently before drug use. Most type 2 diabetics can be controlled by diet alone. — Secondary diabetes: a result of conditions or syndromes (pancreatic disease, hormone disturbances, drugs, malnutrition) — Gestational diabetes: glucose intolerance during pregnancy — Impaired glucose tolerance: includes prediabetic, chemical, latent, borderline, subclinical, and asymptomatic diabetes • Fasting blood glucose: taken after fasting at least 10 hours but not more than 16 hours. Normal fasting blood glucose is 70-99 mg/dL. Fasting blood glucose >126 mg/dL (7 mmol/L) on two separate occasions is diagnostic of diabetes. Fasting glucose ≥110 and <126 mg/dL indicates impaired glucose tolerance. Levels <50 mg/dL indicate fasting hypoglycemia. • Postprandial glucose: 2 hours after meal. Level >200 mg/dL (11 mmol/L) indicates DM. • Random glucose: anytime regardless of last meal. Level >200 mg/dL (11 mmol/L) indicates DM. • Glucose tolerance test (GTT): gold standard because of its longstanding use but less reliable than fasting blood glucose if combined with hemoglobin A1c (HgbA1c). • Normal: no level >160 mg/dL (9 mmol/L) and <150 mg/dL (8.3 mmol/L) at end of first hour; <120 mg/dL (6.6 mmol/L) at end of second hour. • Flat: no variation >±20 mg/dL (1.1 mmol/L) from fasting value. • Prediabetic: 140 mg/dL (7.8 mmol/L) to 180 mg/dL (10 mmol/L) at end of second hour. • Diabetic: >180 mg/dL (10 mmol/L) during first hour; 200 mg/dL (11.1 mmol/L) or higher at end of first hour; 150 mg/dL (8.3 mmol/L) or higher at end of second hour. • Glycosylated hemoglobin: proteins with attached glucose molecules (glycosylated peptides) are elevated severalfold in DM. Normal levels are 5%-7% of Hgb. Mild glucose elevations cause A1c increase to 8%-10%; high glucose can increase A1c to 20%. A1c assay is a time-averaged indicator for preceding 2-4 months. It is simple and useful for assessing treatment efficacy and patient compliance. A1c plus fasting glucose can diagnose DM but not as only criteria. A third of diabetics diagnosed with GTT have normal A1c. Couple A1c with fasting glucose and 2-hour postprandial glucose for a more accurate diagnosis. A1c assay can determine relative glucose load and monitor therapy. • Environmental and dietary risk factors: abnormalities of gut immune system: poor protein digestion induces development of antibodies by the gastrointestinal (GI) immune system that attack beta-cells. Diet can modify the development of autoimmune DM. Absorbed whole proteins can trigger formation of antibodies. Antibodies cross-react with beta-cell antigens. Incriminated food proteins are in milk (bovine serum albumin and bovine insulin) and wheat (gluten). Antibodes may attack human insulin and activate T-killer cells to attack beta-cells. Breastfeeding helps optimize intestinal immunity, reducing type 1 DM risk, food allergies, and intestinal infection risk. Ingestion of cow’s milk at any age may increase risk of type 1 DM. Gluten sensitivity produces celiac disease, another autoimmune disorder. Breastfeeding may be preventive; early introduction of cow’s milk may be causative. Risk of type 1 DM is higher in children with celiac disease. Celiac patients have the highest level of antibodies to cow’s milk proteins. • Factors that set the stage for autoimmunity in type 1 diabetes: immune system usually develops tolerance to bovine insulin. Factors affecting autoimmunity response: gut microflora, breastfeeding, GI infections, nutritional status. • Enteroviruses and type 1 diabetes: type 1 DM can arise from GI viral infection. Enteroviruses (polioviruses, coxsackieviruses, echoviruses) and rotavirus are common in children, can activate immune cells, and can infect beta-cells, triggering a white blood cell attack. GI virus infections may increase intestinal permeability. Small intestinal leaky gut during and after rotavirus infections exposes gut immune cells to intact protein. • Vitamin D deficiency: cod liver oil vitamin D may be protective. Supplementation during early childhood can prevent type 1 DM. Protective dosage is 2000 IU daily. Children taking vitamin D from cod liver oil have an 80% reduced risk of type 1 DM; vitamin D–deficient children have a 300% increased risk. Vitamin D from cod liver oil during pregnancy reduces type 1 DM frequency. Lack of sun exposure during childhood may explain higher type 1 DM rates in Northern countries. Vitamin D helps normalize immune system development and inhibit autoimmune reactions against beta-cells. • Omega-3 fatty acid deficiency: omega-3 fatty acids in cod liver and other fish oils are also protective. Compounds that generate free radicals (e.g., nitrosamines, alloxan) destroy beta-cells. Fish oil can prevent chemically induced type 1 DM by improving cell membrane function, enhancing antioxidant status, and suppressing formation of inflammatory cytokines. • Nitrates: the increase in nitrates in diet and water is linked to an increased rate for type 1 DM. Sources of nitrates include agricultural fertilizer runoff and cured and smoked meats. Nitrates are converted in the body into nitrosamines, which can cause DM. Infants and young children are particularly vulnerable. Nitrates in water may be a key factor in the 3% annual growth in type 1 DM. High-quality water purifiers are recommended. Early intervention resolving autoimmunity or oxidation may slow or reverse the disease process. • Niacinamide (nicotinamide): may help prevent immune-mediated destruction of beta-cells and reverse disorder. It must be given soon enough at onset of DM to restore beta-cells or retard destruction. A dosage of 3 g niacinamide daily may prolong non-insulin-requiring remission, lower insulin requirements, improve metabolic control, and increase beta-cell function as determined by C-peptide secretion. Time-released niacinamide may not provide sufficient peak concentrations to block autoimmunity (e.g., cytokine production). Complete reversal in some patients makes its use worth the effort because no reasonable alternative is available. Dosage is based on body weight: 25-50 mg niacinamide per kilo (2.2 lb) of body weight to maximal dosage of 3 g q.d. in divided doses. No side effects were reported in type 1 DM clinical trials. It can harm the liver; monitor liver enzymes every 3 months. • Epicatechin: bark from the Malabar kino tree (Pterocarpus marsupium) is used in India for DM. Epicatechin extract prevents beta-cell damage in rats. Epicatechin and crude extract of Pterocarpus marsupium can regenerate beta-cell function in diabetic animals. Green tea (Camellia sinensis) extract has a higher epicatechin content and broader range of beneficial effects. Green tea polyphenols have antiviral activity against rotavirus and enterovirus, suspected of causing type 1 DM. Dosage for children younger than 6 years is 50-150 mg; children 6-12 years, 100-200 mg; children >12 years and adults, 150-300 mg. Decaffeinated green tea extracts with a polyphenol content of 80% are preferred. • Obesity (excess body fat): 80% to 90% of type 2 diabetics are obese (body mass index >30). Abdominal adipocytes, engorged with fat, secrete resistin, leptin, tumor necrosis factor, and free fatty acids that dampen the effect of insulin, impair glucose use in skeletal muscle, promote liver gluconeogenesis, and impair beta-cell insulin release by pancreatic beta-cells. The increasing size of adipocytes reduces secretion of compounds (adiponectin) that improve insulin sensitivity, reduce inflammation, lower triglycerides, and inhibit atherosclerosis. Adipose is part of the endocrine system. Blood levels of adiponectin/adipohormone may predict type 2 DM. Increasing insulin resistance leads to compensatory increased insulin secretion to the point of beta-cell exhaustion or burn out. • Family history: parent or sibling with type 2 DM • Increasing age beginning at age 45 years • Race/ethnicity (African American, Hispanic, Native American/First Nation, Native Australian or New Zealander, Asian American, Pacific Islander) • Impaired fasting glucose (IFG) or impaired glucose toler-ance (IGT) • History of gestational diabetes or delivery of infant larger than 9 lb • Hypertension (blood pressure >140/90 mm Hg) • Low adiponectin, elevated fasting insulin • Polycystic ovary syndrome (adult women: overweight, acne, infertile) • Genetics of type 2 diabetes and obesity: even with the strongest genetic predisposition, DM can be avoided in most cases. When DM-prone ethnic groups follow the traditional diet and lifestyle of their original culture, DM incidence is extremely low. These groups are simply sensitive to the Western lifestyle. • Diet, exercise, lifestyle, and diabetes risk: type 2 DM is a disease of diet and lifestyle: lack of exercise, failure to eat whole plant foods, high intake of harmful fat, and obesity. Lifestyle changes alone are associated with a 58% reduced risk of developing DM in people with impaired glucose tolerance. • Glycemic index and glycemic load (GL): glycemic index is the numeric value expressing a rise of blood glucose after eating a particular food. The standard of 100 applies to the ingestion of glucose itself. The insulin response matches blood sugar elevation. Glycemic index reflects the quality of carbohydrates, not the quantity of sugar to be metabolized. Glycemic index is not related to portion size. GL is calculated by amount of carbohydrate in a serving multiplied by the food’s glycemic index (compared with glucose), then divided by 100. The higher the GL, the greater the stress on insulin. GL has a stronger link in predicting DM than glycemic index. A high-GL diet increases risk for heart disease because of lower high-density lipoprotein (HDL) and higher triglyceride levels. Increased DM and heart risk start at daily GL of 161. GL should not exceed 150 daily. • Fiber and glycemic index/GL: inadequate dietary fiber increases DM risk. Fibers most beneficial to glycemic control are water-soluble forms (hemicelluloses, mucilages, gums, and pectin, as in legumes, oat bran, nuts, seeds, psyllium seed husks, pears, apples, most vegetables). They slow down digestion and absorption, preventing rapid glycemic rises, increasing tissue insulin sensitivity, and improving uptake of glucose by muscles and liver. Recommend 35+ g fiber daily from a variety of food sources, especially vegetables. Fiber supplements can also be used. • The wrong type of fats: saturated fat and trans fats (hydrogenated vegetable oils, as in margarine, vegetable shortening) with relative insufficiency of monounsaturated and omega-3 fats. Cell membrane fluidity, affected by fatty acid composition, affects insulin binding to receptors. Monounsaturated fats (olive oil, nuts, nut oils) and omega-3 oils improve insulin action and protect against onset of type 2 DM. Eating nuts reduces risk of type 2 DM. Raw or lightly roasted and unsalted fresh nuts and seeds are preferred over commercially roasted and salted. • Low intake of antioxidants: Cumulative free radical damage ages cells and contributes to type 2 DM. Fruits and vegetables (even dark green salads) improve glycemic control and reduce risk. Vitamins C and E and carotenes lower risk. Antioxidant deficits and lipid peroxides increase risk. • Free radicals: type 2 diabetics have higher levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which exhaust antioxidant mechanisms while damaging DNA, proteins, and membrane fatty acids. ROS, RNS, high blood glucose, and saturated fat activate inflammatory compounds (nuclear factor kappa B), increase insulin resistance and impair insulin secretion. • Lifestyle vs. drugs to prevent type 2 DM: the benefits of drugs pale compared with the efficacy of diet and lifestyle changes. • Urine glucose monitoring: kidneys conserve glucose until blood glucose reaches 180 mg/dL (10 mmol/L). A negative urine glucose means blood glucose since previous voiding has been <180 mg/dL. Urine testing is crude; it gives no indication of blood glucose below 180 mg/dL. It is worthless for severe hypoglycemia or hyperglycemia. • Urine ketone testing: when fat is the primary source of energy, ketones are produced. High ketone level spills over into urine. In the diabetic, ketonuria occurs with severe deficits in insulin availability or activity. Examples: a patient with IDDM accidentally or purposefully fails to take insulin; or a diabetic is ill, injured, or is given high doses of cortisone-related drugs. Loss of insulin effect impairs glucose uptake and use of glucose. The result is hyperglycemia, fat burning for energy, and toxemia from acidic ketones, with severe dehydration from water loss caused by glycosuria. This state is the deadly medical emergency termed “diabetic ketoacidosis,” necessitating intravenous (IV) insulin, IV fluids, and intensive care unit monitoring. Urine ketone testing is critical, especially in IDDM. Urine ketones (with hyperglycemia) may indicate impending or established ketoacidosis, requiring immediate intervention. Advise patients to test urine for ketones during acute illness; during severe stress, when blood glucose is consistently elevated (>300 mg/dL [16.7 mmol/L]), regularly during pregnancy; or in the presence of symptoms of ketoacidosis (nausea, vomiting, abdominal pain). • Self-monitoring of blood glucose: glycemic control is the most important factor in the long-term risk of serious complications in type 1 and type 2 DM. Self-monitoring of blood glucose allows the patient to modify treatment to maintain glycemic control, detect hypoglycemia, adjust care to daily circumstances (food intake, exercise, stress, illness), detect and treat severe hyperglycemia, increase therapeutic compliance, and improve motivation from immediate feedback. • Optimal schedule for self-monitoring of blood glucose — Awakening and just before each meal. Ideal before meals is <120 mg/dL (6.7 mmol/L). Optimal Range for Self-Monitored Blood Glucose* *Slightly higher values may be acceptable in elderly or young children because of the higher risk of dangerous hypoglycemia. — Two hours after each meal. Ideal 2 hours after meals is <140 mg/dL (7.7 mmol/L). • Type 1 diabetes and self-monitoring of blood glucose: all type 1 diabetics must frequently monitor blood glucose to make ongoing adjustments to insulin injections, diet, and exercise. Until recently, type 1 diabetics often were prescribed combinations of short- and medium-action or long-action insulin one to two times per day. Many diabetics monitored glucose infrequently, some less than once daily. Diabetics are now trained to maintain ideal levels 24/7 by intensive insulin therapy (IIT) plus frequent glycemic monitoring. Using rapid-acting and short-duration insulins, IIT involves either frequent injections of insulins (Humalog [Insulin Lispro] or Novolog [Insulin Aspart]) or an insulin pump (continuous electronic injection of very-short-acting insulins with extra boluses before meals). Rapid-acting, short-duration insulin allows timing and size of doses to be adjusted to daily events. Despite multiple injections (before meals and at bedtime) and glycemic testing six or more times daily, IIT allows greater freedom, higher quality of life, and superior glycemic control. • Type 2 diabetes and self-monitoring of blood glucose: every type 2 diabetic must own a monitor and be intimately familiar with its use. Even if glucose is well controlled by diet, lifestyle, and supplements, they should monitor regularly. Type 2 NIDDM patients with good glucose control according to regular lab testing, including A1c, should designate a day every 1-3 weeks as intensive glycemic measuring day. Check before breakfast (fasting), just before meals, 2 hours after meals, and before bed. Record measurements, diet, exercise, and supplements in a journal to increase awareness of the effects of factors on blood glucose. Type 2 diabetics with poor control should monitor intensively each day and seek professional help to regain control. Diabetics in advanced stages, with diminished insulin secretion, may need IIT. Blood test for C-peptide estimates insulin output. IIT requires self-monitoring of blood glucose as frequently as type 1 diabetics (before and 2 hours after each meal). Advanced type 2 diabetics with diminished insulin production have less than normal C-peptide. Common intervention trend: one injection of new, very-long-acting insulin (insulin glargine [Lantus]) for smooth, continual 24-hour release plus diet and medication. With this regimen, self-monitoring should be done before and 2 hours after each meal. • C-Peptide determination: assessing insulin secretion influences treatment, especially in a diabetic hoping to avoid or stop using insulin, and the type of pharmaceuticals and nutriceuticals likely to be effective. Pancreas manufactures proinsulin first. Enzymes snip off a piece of this proinsulin (C-peptide); both C-peptide and insulin are secreted. For type 2 diabetics with poor glycemic control, high or normal C-peptide confirms their pancreatic output and directs attention to insulin resistance and sensitivity. A patient with low C-peptide may need insulin replacement.
Diabetes Mellitus
DIAGNOSTIC SUMMARY
GENERAL CONSIDERATIONS
Classification
Features
Type 1
Type 2
Age at onset
Usually <40 years
Usually >40 years
Percentage of all diabetics
<10%
>90%
Seasonal trend
Fall and winter
None
Family history
Uncommon
Common
Appearance of symptoms
Rapid
Slow
Obesity at onset
Uncommon
Common
Insulin levels
Decreased
Variable
Insulin resistance
Occasional
Often
Treatment with insulin
Always
Not required
Beta-cells
Decreased
Variable
Ketoacidosis
Frequent
Rare
Complications
Frequent
Frequent
DIAGNOSIS
Glucose Tolerance Test Response Criteria
CAUSES OF DIABETES
Risk Factors for Type 1 Diabetes Mellitus
Early Treatment and Possible Reversal of Type 1 Diabetes Mellitus
Risk Factors for Type 2 Diabetes Mellitus
MONITORING THE DIABETIC PATIENT
Fasting or before meals
80-110 mg/dL (4.4-6.7 mmol/L)
Two hours after eating (postprandial)
<140 mg/dL (7.8 mmol/L)
At bedtime
100-140 mg/dL (5.6-7.8 mmol/L)
C-Peptide Results
Interpretation
Normal
The insulin production is at normal level
Less than normal
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
