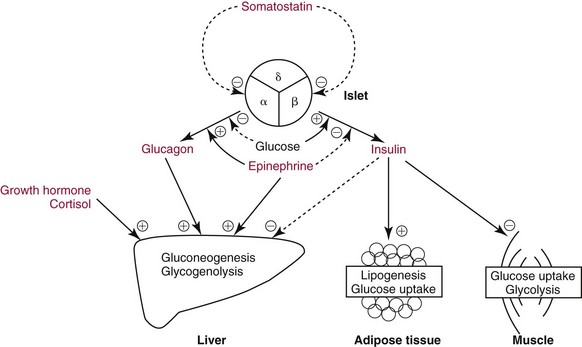
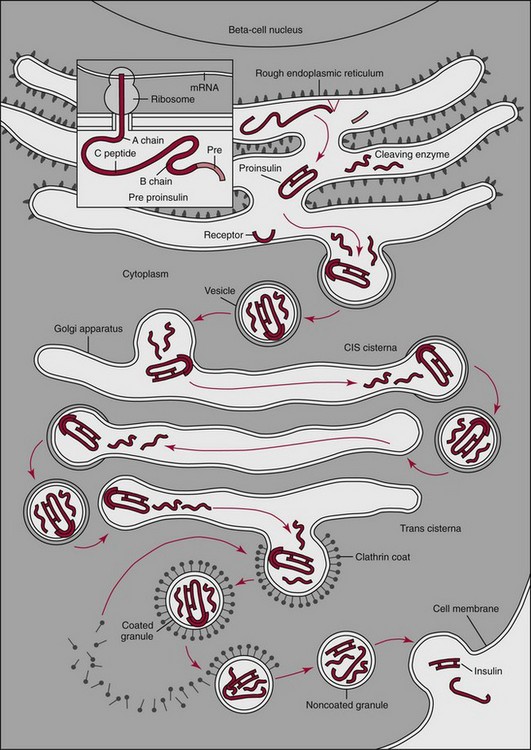
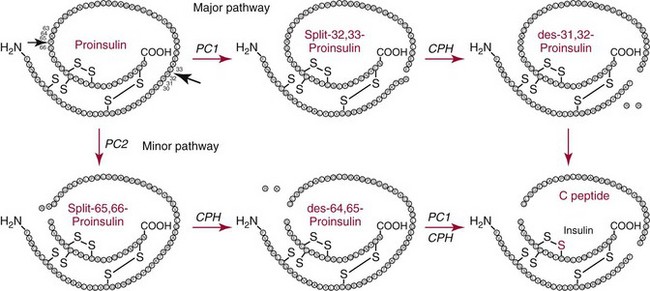
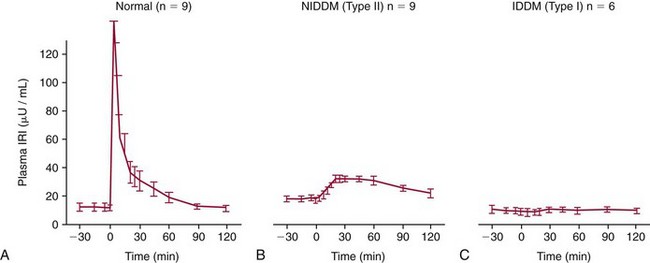
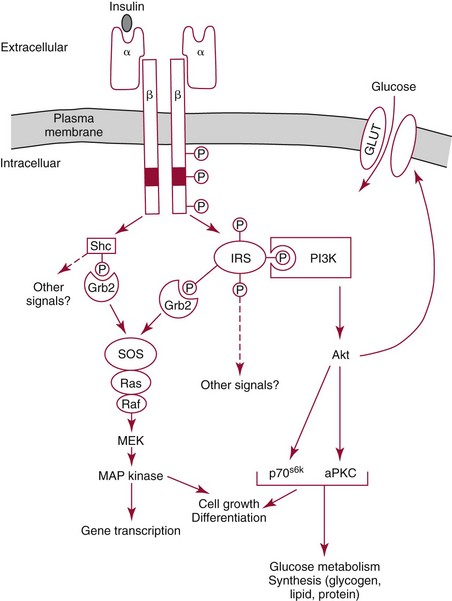
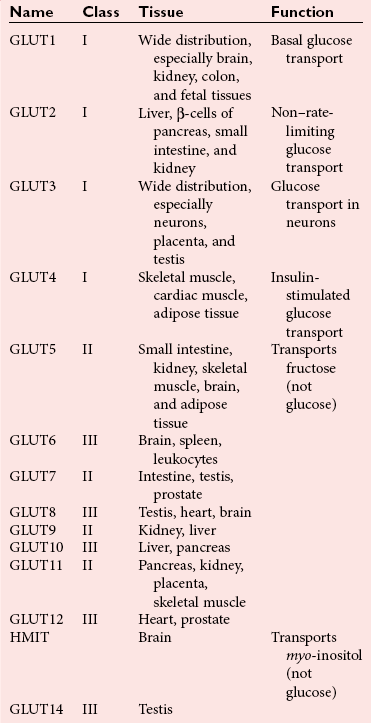
Chapter 46 Diabetes mellitus is a group of metabolic disorders of carbohydrate metabolism in which glucose is underused, producing hyperglycemia. Some patients may experience acute life-threatening hyperglycemic episodes, such as ketoacidosis or hyperosmolar coma. As the disease progresses, patients are at increased risk for the development of specific complications, including retinopathy leading to blindness, nephropathy leading to renal failure, and neuropathy (nerve damage), collectively known as microvascular complications, as well as atherosclerosis, which is considered a macrovascular complication.169,218 The last may result in stroke, gangrene, or coronary artery disease. Diabetes is a common disease, although the exact prevalence is unknown. It is estimated that ≈250 million people currently have diabetes, and by 2025 this number will reach 280 million, 80% of whom will live in developing countries. In the United States, the number of people with diabetes has increased dramatically. The prevalence in 1999-2002 was 9.3%, 30% of whom were undiagnosed.57 Analysis of the 2005-2006 National Health and Nutritional Examination Survey (NHANES) using both fasting glucose and oral glucose tolerance testing (OGTT) shows a prevalence of diabetes in the United States in persons 20 years of age and older of 12.9% (equivalent to ≈40 million people).58 Of these, ≈40% are undiagnosed. Similarly, the prevalence of diabetes in Asian populations has increased rapidly in recent decades, reaching more than 110 million in 2007.50 These statistics led to a description of diabetes as “one of the main threats to human health in the twenty-first century.”267 The prevalence of diabetes mellitus increases with age, and approximately half of all cases occur in people older than 55 years. In the United States, more than 20% of the population older than 65 years have diabetes.99 A racial predilection has been noted, and by the age of 65, 33%, 25%, and 17% of Hispanics, blacks, and whites, respectively, in the United States have diabetes. In 2007, diabetes mellitus was estimated to be responsible for $174 billion in healthcare expenditures in the United States.17 The direct costs were $116 billion, with 56% of that total incurred by those 65 years and older. An estimated 3.8 million people worldwide died from diabetes-related causes in 2007.115 Diabetes is the fourth most common cause of death in the developed world. Most populations have plasma glucose values that exhibit a unimodal, log-normal distribution (a distribution curve that is skewed to the high end but becomes bell shaped on a logarithmic axis). Ethnic groups with a high prevalence of diabetes, such as the Pima Indians and Nauruans, exhibit bimodal blood glucose distributions.80 Optimal distinction between normal and diabetic individuals in these groups occurs at a fasting glucose around 140 mg/dL and glucose concentrations greater than 200 mg/dL 2 hours after an oral glucose load. Furthermore, the specific microvascular complications of diabetes were believed to be rare in patients with fasting or 2 hour postprandial glucose concentrations less than 140 or 200 mg/dL, respectively. These observations formed the basis for the criteria proposed in 1979 by a workgroup of the National Diabetes Data Group174 and later endorsed by the World Health Organization (WHO) Committee on Diabetes. The 1979 classification scheme recognized two major forms of diabetes: type I (insulin-dependent) diabetes mellitus (IDDM) and type II (non–insulin-dependent) diabetes mellitus (NIDDM).174 The terms juvenile-onset and adult-onset diabetes were abolished. To base the classification on cause rather than on treatment, the American Diabetes Association (ADA) established a workgroup in 1995 to re-examine the classification and diagnosis of diabetes mellitus. The revised classification, published in 1997,9 eliminates the terms insulin-dependent diabetes mellitus and non–insulin-dependent diabetes mellitus, which now are termed type 1 and type 2 diabetes, respectively (Box 46-1). Furthermore, the categories of previous abnormality of glucose tolerance and potential abnormality of glucose tolerance have been eliminated. This group accounts for approximately 90% of all cases of diabetes. Patients have minimal symptoms, are not prone to ketosis, and are not dependent on insulin to prevent ketonuria. Insulin concentrations may be normal, decreased, or increased, and most people with this form of diabetes have impaired insulin action. Obesity is commonly associated, and weight loss alone usually improves hyperglycemia in these persons. However, many individuals with type 2 diabetes may require dietary manipulation, oral hypoglycemic agents, or insulin to control hyperglycemia. Most patients acquire the disease after age 40, but it may occur in younger people. Type 2 diabetes in children and adolescents is an emerging, significant problem.12,59,267 Among children in Japan, type 2 diabetes is now more common than type 1.267 This subclass includes uncommon patients in whom hyperglycemia is due to a specific underlying disorder, such as genetic defects of β-cell function; genetic defects in insulin action; disease of the exocrine pancreas; endocrinopathies (e.g., Cushing’s syndrome, acromegaly, glucagonoma); administration of hormones or drugs known to induce β-cell dysfunction (e.g., dilantin, pentamidine) or to impair insulin action (e.g., glucocorticoids, thiazides, β-adrenergics); infection; uncommon forms of immune-mediated diabetes; or other genetic conditions (e.g., Down syndrome, Klinefelter syndrome, porphyria; see Reference 18 for a detailed list). This was formerly termed secondary diabetes. This is defined as any degree of glucose intolerance with onset or first recognition during pregnancy157 (i.e., diabetic women who become pregnant are not included in this category). Estimates of the frequency of abnormal glucose tolerance during pregnancy range from 1 to 14%, depending on the population studied and the diagnostic tests employed.131 In the United States, gestational diabetes mellitus (GDM) occurs in 6 to 8% of pregnancies (≈200,000 cases annually). Women with GDM are at significantly increased risk for the subsequent development of type 2 diabetes mellitus, which occurs in 6 to 62%.130 The risk is particularly high in women who have marked hyperglycemia during or soon after pregnancy, women who are obese, and those whose GDM was diagnosed before 24 weeks’ gestation.135 At 6 to 12 weeks postpartum, all patients who had GDM should be evaluated for diabetes using nonpregnant OGTT criteria. If diabetes is not present, patients should be re-evaluated for diabetes at least every 3 years.19 Impaired glucose tolerance (IGT) is diagnosed in people who have fasting blood glucose concentrations less than those required for a diagnosis of diabetes mellitus, but have a plasma glucose response during the OGTT between normal and diabetic states. The 2 hour postload plasma glucose following an OGTT is 140 to 199 mg/dL for this classification. An OGTT is required to assign a patient to this class. Development of overt diabetes occurs at a rate of 1 to 5% per year, but a large proportion of cases spontaneously revert to normal glucose tolerance. Microvascular disease is rare in this group, and patients usually do not experience the renal or retinal complications of diabetes. Patients have an increased prevalence of atherosclerosis and mortality from cardiovascular disease.65 During a brief fast, a precipitous decline in the concentration of blood glucose is prevented by breakdown of glycogen stored in the liver and synthesis of glucose in the liver. Some glucose is derived from gluconeogenesis in the kidneys.84 These organs contain glucose-6-phosphatase, which is necessary to convert glucose-6-phosphate (derived from gluconeogenesis or glycogenolysis) to glucose. Skeletal muscle lacks this enzyme; muscle glycogen therefore cannot contribute directly to blood glucose. With more prolonged fasting (>42 hours), gluconeogenesis accounts for essentially all glucose production. In contrast, after a meal, the absorbed glucose is converted to glycogen (for storage in the liver and skeletal muscle) or fat (for storage in adipose tissue). Despite large fluctuations in the supply and demand of carbohydrates, the concentration of glucose in the blood is normally maintained within a fairly narrow range by hormones that modulate the movement of glucose into and out of the circulation. These include insulin, which decreases blood glucose, and the counter-regulatory hormones (glucagon, epinephrine, cortisol, and growth hormone), which increase blood glucose concentrations (Figure 46-1).84 Normal glucose disposal depends on (1) the ability of the pancreas to secrete insulin, (2) the ability of insulin to promote uptake of glucose into peripheral tissue, and (3) the ability of insulin to suppress hepatic glucose production. The major insulin target organs are liver, skeletal muscle, and adipose tissue. These organs exhibit some differences in their responses to insulin. For example, the hormone stimulates glucose uptake through a specific glucose transporter—GLUT4—into muscle and fat cells, but not into liver cells. Preproinsulin, a protein of about 100 amino acids (MW 12,000 Da), is formed by ribosomes in the rough endoplasmic reticulum of the pancreatic β-cells (Figure 46-2). Preproinsulin is not detectable in the circulation under normal conditions because it is rapidly converted by cleaving enzymes to proinsulin (MW 9000 Da), an 86 amino acid polypeptide. This is stored in secretory granules in the Golgi complex of the β-cells, where proteolytic cleavage to insulin and connecting peptide (C-peptide) occurs.179 Cleavage of proinsulin is catalyzed by two Ca2+-regulated endopeptidases: prohormone convertases 1 and 2 (PC1 and PC2).195 PC1 (sometimes designated PC3) hydrolyzes the molecule on the C-terminal end of Arg-31 and Arg-32 (at the BC junction) to yield split-32, 33-proinsulin (Figure 46-3). PC2 cleaves proinsulin on the C-terminal side of dibasic residues Lys-64 and Arg-65 (at the AC junction) to generate split-65,66-proinsulin. Each enzymatic hydrolysis is rapidly followed by the removal of two newly exposed C-terminal basic amino acids by carboxypeptidase-H to produce insulin and C-peptide. The split proinsulin intermediates are rarely detected in patient samples because of the relatively high quantity of carboxypeptidase-H. This enzyme produces the more commonly observed proinsulin intermediates, des-31,32-proinsulin and des-64,65-proinsulin (see Figure 46-3). Most proinsulin processing is sequential. Intact proinsulin is initially hydrolyzed by PC1 or carboxypeptidase-H. The resultant des-31,32-proinsulin is converted by PC2 and carboxypeptidase-H to insulin and C-peptide. Less than 10% of proinsulin is metabolized via des-(64-65)-proinsulin, which is present in negligible amounts in humans. Des-31,32-proinsulin is the major proinsulin conversion intermediate.223 Glucose regulates biosynthesis of both proinsulin and PC1, but has no effect on PC2 or carboxypeptidase-H. At the cell membrane, insulin and C-peptide are released into the portal circulation in equimolar amounts. In addition, small amounts of proinsulin and intermediate cleavage forms enter the circulation. Glucose, amino acids, pancreatic and gastrointestinal hormones (e.g., glucagon, gastrin, secretin, pancreozymin, gastrointestinal polypeptide), and some medications (e.g., sulfonylureas, β-adrenergic agonists) stimulate insulin secretion. Insulin release is inhibited by hypoglycemia, somatostatin (produced in the pancreatic δ-cells), and various drugs (e.g., α-adrenergic agonists, β-adrenergic blockers, diazoxide, phenytoin, phenothiazines, nicotinic acid).184 In healthy individuals, insulin is secreted in a pulsatile fashion, with glucose and insulin the main signals in the feedback loop. Glucose elicits the release of insulin from the pancreas in two phases. The first phase begins 1 to 2 minutes after intravenous injection of glucose and ends within 10 minutes. This phase, illustrated by the sharp spike in Figure 46-4, A, represents the rapid release of stored insulin. The second phase, beginning at the point where the first phase ends, depends on continuing insulin synthesis and release and lasts until normoglycemia has been restored, usually within 60 to 120 minutes. With progressive failure of β-cell function, the first-phase insulin response to glucose is lost, but other stimuli such as glucagon or amino acids may be able to elicit this response. Although the second-phase insulin response is preserved in most patients with type 2 diabetes mellitus, both the first-phase response (Figure 46-4, B) and normal pulsatile insulin secretion174 are lost. In contrast, patients with type 1 diabetes mellitus exhibit minimal or no insulin response (Figure 46-4, C). Proinsulin, which has relatively low biological activity (approximately 10% of insulin potency), is the major storage form of insulin.198 Normally, only small amounts (about 3% of the amount of insulin, on a molar basis) of proinsulin enter the circulation. However, the hepatic clearance rate for proinsulin is only 25% of that for insulin, and the half-life of proinsulin is ≈30 minutes. Therefore in the fasting state, circulating proinsulin concentrations are approximately 10 to 15% of insulin concentrations. Proinsulin is cleaved to a 31 amino acid connecting (C) peptide (MW 3600 Da) and insulin (see Figure 46-3). C-peptide is devoid of biological activity but appears necessary to ensure the correct structure of insulin.104 Although insulin and C-peptide are secreted into the portal circulation in equimolar amounts, fasting C-peptide concentrations are fivefold to 10-fold higher than those of insulin owing to the longer half-life of C-peptide (≈35 minutes). The liver does not extract C-peptide, which is removed from the circulation by the kidneys and degraded, with a fraction excreted unchanged in the urine. Antibodies to insulin develop in almost all patients who are treated with exogenous insulin.197 These antibodies are usually present at low titer and produce no adverse effects. On rare occasions (usually in patients with type 2 diabetes), high titers of insulin antibodies may cause insulin resistance. Improvement in the purity of animal insulins and the widespread use of human insulin have reduced, but not totally eliminated, antibody production. Recent advances in insulin delivery systems, namely, continuous subcutaneous insulin infusion and inhaled insulin, have significantly increased concentrations of insulin antibodies.189 Antibodies to insulin rarely develop in patients who have not received exogenous insulin. Although rare, patients with antibodies to the insulin receptor have been described.79 On binding the receptor, these antibodies act as antagonists, producing hyperglycemia (e.g., in patients with acanthosis nigricans), or agonists, resulting in hypoglycemia. Although the metabolic effects produced by insulin are well known, the molecular mechanism of insulin action remains incompletely understood.40,231 It is generally accepted that the initial event is the binding of insulin to specific receptors in the plasma membrane (Figure 46-5). The human insulin receptor, which is well characterized, is a heterotetramer, comprising two α- and two β-subunits. The α-subunit (MW 135,000 Da) is located on the outer surface of the plasma membrane and contains the site where insulin binds. The β-subunit (MW 95,000 Da) extends intracellularly through the plasma membrane and contains an intrinsic tyrosine kinase. Binding of insulin to the α-subunits induces a conformational change in the receptor, resulting in activation of tyrosine kinase, which catalyzes the phosphorylation of tyrosine residues on several proteins. One of the major substrates for this tyrosine kinase is the receptor itself. In addition to phosphorylating itself, the insulin receptor catalyzes the tyrosine phosphorylation of various specific intracellular proteins (see Figure 46-5). These include the four members of the family of insulin-receptor substrate (IRS) proteins (termed IRS-1, IRS-2, IRS-3, and IRS-4), Shc, and Gab-1. The phosphorylated tyrosines on these target proteins act as docking sites for selected intracellular signal transducer proteins.231 Most of these transducer proteins contain one or more Src homology 2 (SH2) domains. The SH2 domain is a sequence of approximately 100 amino acids that recognizes phosphotyrosine.183 Sequence differences in the SH2 domain dictate the specificity of binding. SH2-containing proteins depicted in Figure 46-5 include those labeled phosphatidylinositol 3-kinase (PI3K) and growth factor receptor–bound protein 2 (Grb2), both of which mediate downstream signal transduction events. Similar to other growth factors, insulin stimulates the mitogen-activated protein (MAP) kinase cascade via Ras. In addition, phosphatidylinositol 3′-kinase activates atypical protein kinase C (aPKC) via Akt. The latter enzymes regulate glucose transport by modulating translocation of GLUT4 (the insulin-sensitive glucose transporter) to the plasma membrane. Akt also phosphorylates and inactivates GSK-3, thereby enhancing glycogen synthesis. Some of these events are listed in Figure 46-5. The pathways are elaborate, and although several components have been identified, there remain considerable gaps in our knowledge and understanding. Recent studies have clarified a fundamental concept—that insulin-mediated signaling events are highly redundant. For example, when two key insulin-signaling molecules, IRS-1 and GLUT4, were knocked out in transgenic mouse experiments, the resulting animals had minor metabolic defects rather than overt diabetes.196 Similarly, mice with knockout of insulin receptors from skeletal muscle or liver do not develop diabetes. The transport of glucose into cells is modulated by two families of proteins.263 The sodium-dependent glucose transporters (SGLTs) use the electrochemical sodium gradient to transport glucose against its concentration gradient. SGLTs promote the uptake of glucose and galactose from the lumen of the small bowel and their reabsorption from urine in the kidney. Members of the second family of glucose carriers are called facilitative glucose transporters (GLUT) (Table 46-1). These transporters are designated GLUT1 to GLUT14, based on the order in which they were identified.209 Eleven have been shown to catalyze sugar transport. They can be divided into three classes, based on sequence similarities and characteristics. The best characterized are class I. Less is known about those in classes II and III. GLUT1 is widely expressed and provides many cells with their basal glucose requirement. GLUT1 in the blood-brain barrier and GLUT3 in neuronal cells provide the constant high concentrations of glucose required by the brain. GLUT2 is expressed in hepatocytes, β-cells of the pancreas, and basolateral membranes of intestinal and renal epithelial cells. It is a low-affinity, high-capacity transport system that allows non–rate-limiting movement of glucose into and out of these cells. GLUT4 catalyzes the rate-limiting step for glucose uptake and metabolism in skeletal muscle, the major organ of glucose consumption. GLUT4 is also present in adipose tissue. Insulin-like growth factors 1 and 2 (IGF-1 and IGF-2) are polypeptides structurally related to insulin.190 These hormones (previously referred to as nonsuppressible insulin-like activity or somatomedin) exhibit metabolic and growth-promoting effects similar to those of insulin. Accumulating evidence implicates the IGF axis in the development of several common cancers.207 IGF-1 (previously known as somatomedin C) is an important mediator of growth hormone action and is one of the major regulators of cell growth and differentiation. The physiologic role of IGF-2 is not known. Synthesis of IGF-1 depends on growth hormone and occurs predominantly in the liver. In addition, many other cells produce IGF-1 that does not enter the circulation but acts locally. Circulating IGF concentrations are approximately 1000-fold higher than insulin concentrations, and the hormone is kept inactive by binding to a family of at least six specific binding proteins.113 These proteins regulate IGF by protecting the ligands in the circulation and delivering them to their target tissue. In contrast to insulin, which is unbound in the circulation, less than 10% of total serum IGF-1 is free. The biological actions of IGF are exerted through specific IGF receptors or the insulin receptor. The IGF-1 receptor is closely related to the insulin receptor in structure and biochemical properties. In contrast, the IGF-2 receptor is quite different; it lacks tyrosine kinase activity, and its physiologic relevance is not understood. The IGF-1 receptor has a high affinity for both IGF-1 and IGF-2, but a low affinity for insulin. The IGF-2 receptor has high, low, and no affinity for IGF-2, IGF-1, and insulin, respectively. The insulin receptor binds insulin with high affinity and IGF-1 and IGF-2 with low affinity. The significance of IGFs in normal carbohydrate metabolism is not known. Exogenous administration produces hypoglycemia, whereas a deficiency of IGF-1 results in dwarfism (pygmies and Laron dwarfs). IGFs, particularly IGF-2, may be produced in excess by extrapancreatic neoplasms, and patients may have fasting hypoglycemia.60 The high concentrations of both IGF-2 protein in the blood and IGF-2 messenger RNA (mRNA) in tumor extracts have led to the proposal that IGF-2 is the humoral mediator of non–islet cell tumor–induced hypoglycemia.217 Measurement of plasma IGF-1 concentration may be useful in evaluating growth hormone deficiency and excess (acromegaly), and in monitoring response to nutritional support. Several hormones have actions opposite to those of insulin. These counter-regulatory hormones are catabolic and increase hepatic glucose production initially by enhancing the breakdown of glycogen to glucose (glycogenolysis), and later by stimulating the synthesis of glucose (gluconeogenesis).83,84 The initial response (within minutes) to low blood glucose is an increase in glucose production, stimulated by glucagon and epinephrine. Over time (3 to 4 hours), growth hormone and cortisol increase glucose mobilization and decrease glucose use (see Figure 46-1). Evidence also suggests that glucose production by the liver is an inverse function of ambient glucose concentration, independent of hormonal factors (glucose autoregulation). The role of other hormones or neurotransmitters is not clear but appears relatively unimportant. Multiple counter-regulatory hormones exhibit both redundancy and hierarchy. Glucagon is the most important, and epinephrine becomes critical when glucagon is deficient. The other factors have lesser roles. These hormones, briefly described here, are discussed further in Chapters 30, 51, 53, and 54. Glucagon is a 29 amino acid polypeptide secreted by α-cells of the pancreas. The major target organ for glucagon is the liver, where it binds to specific receptors and increases both intracellular adenosine-5′-monophosphate and calcium. Glucagon stimulates the production of glucose in the liver by glycogenolysis and gluconeogenesis.144 In addition, glucagon enhances ketogenesis in the liver. A minor target organ for glucagon is adipose tissue, where the hormone increases lipolysis. Glucagon secretion is regulated primarily by plasma glucose concentrations, with low and high plasma glucose being stimulatory and inhibitory, respectively. Long-standing diabetes mellitus impairs the glucagon response to hypoglycemia, resulting in an increased incidence of hypoglycemic episodes. Stress, exercise, and amino acids induce glucagon release. Insulin inhibits glucagon release from the pancreas and decreases glucagon gene expression, thereby attenuating its biosynthesis. Increased glucagon concentrations, secondary to insulin deficiency, are believed to contribute to the hyperglycemia and ketosis of diabetes. Proglucagon is also produced in the distal gut by L-cells, which process it into glucagon, glucagon-like peptide-1 (GLP-1), and GLP-2. Food ingestion stimulates release of GLP-1, which acts on β-cells of the pancreas to stimulate insulin gene transcription and potentiate glucose-induced insulin secretion. GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) are incretin hormones that are responsible for 70% of postprandial insulin secretion.55 GLP-1 reduces hyperglycemia by regulating insulin and glucagon secretion, thus providing gastric emptying and satiety. For these reasons, GLP-1 analogs are generating interest in the treatment of type 2 diabetes,55 and two (exenatide and liraglutide) have received Food and Drug Administration (FDA) approval. Epinephrine, a catecholamine secreted by the adrenal medulla, stimulates glucose production (glycogenolysis) and decreases glucose use, thereby increasing blood glucose concentrations. It also stimulates glucagon secretion and inhibits insulin secretion by the pancreas (see Figure 46-1). Epinephrine appears to have a key role in glucose counter-regulation when glucagon secretion is impaired (e.g., in type 1 diabetes mellitus). Physical or emotional stress increases epinephrine production, releasing glucose for energy. Tumors of the adrenal medulla, known as pheochromocytomas, secrete excess epinephrine or norepinephrine and produce moderate hyperglycemia as long as glycogen stores are available in the liver. Box 46-2 lists the clinical conditions in which hormones that regulate glucose, namely, insulin, proinsulin, C-peptide, and glucagon, have been measured. Although there is interest in the possible clinical value of measurement of the concentrations of insulin and its precursors, the assays are useful primarily for research purposes. There is no role for routine testing for insulin, proinsulin, or C-peptide in most patients with diabetes mellitus.204 Measurement of C-peptide is sometimes necessary in the United States for patients to obtain insurance coverage for continuous subcutaneous insulin infusion pumps. It must be emphasized that the diagnostic criteria for diabetes mellitus do not include measurements of hormones, which remain predominantly research tools. The primary clinical application of insulin measurement is in the evaluation of patients with fasting hypoglycemia (discussed in more detail in Chapter 26). Measurement of circulating insulin could be helpful in evaluating insulin resistance and insulin secretion. Insulin determination has also been proposed to be of value in selecting the optimal initial therapy for patients with type 2 diabetes mellitus. In theory, the lower the pretreatment insulin concentration, the more appropriate might be insulin or an insulin secretagogue as the treatment of choice. Although intellectually appealing, no evidence suggests that knowledge of the insulin concentration leads to more efficacious treatment. Evidence indicates that increased concentrations of insulin in nondiabetic individuals predict the development of coronary artery disease.96 Nevertheless, it is not clear whether the increased insulin is responsible for the risk of coronary disease, and the clinical value is questionable.204 In the past, measurement of insulin was advocated in the evaluation and management of patients with polycystic ovary syndrome.204 Women with this condition have insulin resistance and abnormal carbohydrate metabolism that may respond to oral hypoglycemic agents. However, it is not clear whether assessing insulin resistance by measuring insulin concentrations affords any advantage over clinical signs of insulin resistance (body mass index, acanthosis nigricans), and the American College of Obstetrics and Gynecology does not recommend routine measurements of insulin.5 Although a few investigators have recommended measuring insulin along with glucose during an OGTT as an aid to the early diagnosis of diabetes mellitus, this approach is not recommended.204 High proinsulin concentrations are usually noted in patients with benign or malignant β-cell tumors of the pancreas. Most patients with β-cell tumors have increased insulin, C-peptide, and proinsulin concentrations, but occasionally only proinsulin is increased82 because the tumors have defective conversion of proinsulin to insulin. Despite its low biological activity, proinsulin production may be adequate to produce hypoglycemia. In addition, a rare form of familial hyperproinsulinemia, produced by impaired conversion to insulin, has been described. Measurement of proinsulin can be useful to determine the amount of proinsulin-like material that cross-reacts in an insulin assay. Patients with type 2 diabetes have increased proportions of proinsulin and proinsulin conversion intermediates,143 high concentrations of which are associated with cardiovascular risk factors.167 Even relatively mild hyperglycemia produces hyperproinsulinemia, with values greater than 40% of insulin concentration in type 2 diabetes.143 Similarly, women with GDM have higher concentrations of proinsulin and split-32,33-proinsulin than pregnant normoglycemic control subjects. An increased ratio of proinsulin-like molecules to insulin-like molecules at screening may be a better predictor of GDM than age, obesity, or hyperglycemia.226 Increased proinsulin concentrations may also be detected in patients with chronic renal failure, cirrhosis, or hyperthyroidism. Accurate measurement of proinsulin has been difficult for several reasons: the blood concentrations are low; antibody production is difficult; most antisera cross-react with insulin and C-peptide, which are present in much higher concentrations; the assays measure intermediate cleavage forms of proinsulin; and reference preparations of pure proinsulin are not readily available. However, a more sensitive nonequilibrium RIA method for measuring proinsulin was developed by adsorbing the initial antiserum with biosynthetic human C-peptide coupled with agarose to eliminate cross-reactivity with C-peptide.42,101 An enzyme-linked immunosorbent assay (ELISA) has been described that employs an antibody to C-peptide as the coating antibody and anti-insulin antibody for detection.85 The detection limit is 0.25 pmol/L.134 Measurement of C-peptide has a number of advantages over insulin measurement. Because hepatic metabolism is negligible, C-peptide concentrations are better indicators of β-cell function than is peripheral insulin concentration.186 Furthermore, C-peptide assays do not measure exogenous insulin and do not cross-react with insulin antibodies, which interfere with the insulin immunoassay. The primary indication for measuring C-peptide is for the evaluation of fasting hypoglycemia. Some patients with insulin-producing β-cell tumors, particularly if hyperinsulinism is intermittent, may exhibit increased C-peptide concentrations with normal insulin concentrations. When hypoglycemia is due to surreptitious insulin injection, insulin concentrations will be high but C-peptide values will be low108; this occurs because C-peptide is not found in commercial insulin preparations and exogenous insulin suppresses β-cell function. Basal or stimulated (by glucagon or glucose) C-peptide concentrations provides estimates of a patient’s insulin secretory capacity and rate. For example, diabetic patients with C-peptide concentrations greater than 1.8 µg/L (1.8 ng/mL) after stimulation with glucagon behave clinically like patients with type 2 diabetes, and those with low peak C-peptide values (<0.5 µg/L) behave like patients with type 1 diabetes.105 In rare cases, this strategy may be helpful before discontinuation of insulin treatment (e.g., in an obese adolescent). Urinary and fasting serum C-peptide concentrations appear to be of some value in differentiating patients with type 1 diabetes from those with type 2 diabetes.124 In addition, patients who have type 1 diabetes but who have no C-peptide response are usually more labile than those with some residual β-cell function. Despite these observations, C-peptide measurement has a negligible role in the routine management of patients with diabetes. A relatively new indication for C-peptide analysis is the recent requirement that Medicare patients in the United States must have low C-peptide concentrations to be eligible for coverage of insulin pumps.204 Measurement of C-peptide is used to monitor patients’ response to pancreatic surgery. C-peptide should be undetectable after a radical pancreatectomy and should increase after a successful pancreas or islet cell transplant. In addition, a stable C-peptide concentration is used as an endpoint in immunomodulatory trials for the prevention of type 1 diabetes.1 Measurements of urine C-peptide are useful when continuous assessment of β-cell function is desired, or when frequent blood sampling is not practical. The 24-hour urine C-peptide content (in the absence of renal failure, which produces increased concentrations) correlates well with fasting serum C-peptide concentration or with the sum of C-peptide concentrations in sequential specimens after a glucose load. However, the fraction of secreted C-peptide that is excreted in the urine exhibits high intersubject and intrasubject variability, limiting the value of urine C-peptide as a measure of insulin secretion.237 Very high concentrations of glucagon are seen in patients with α-cell tumors of the pancreas called glucagonomas. Patients with this tumor frequently have weight loss, necrolytic migratory erythema, diabetes mellitus, stomatitis, and diarrhea.252 Skin lesions often occur first and are frequently overlooked. Most tumors have metastasized when finally diagnosed. Low glucagon concentrations are associated with chronic pancreatitis and long-term sulfonylurea therapy. Although insulin has been assayed for over 50 years, no highly accurate, precise, and reliable procedure is available to measure the amount of insulin in a patient sample. Many insulin assays are commercially available.52,150,160 The techniques most widely used are immunometric.150,160 Bioassays, although of greater physiologic relevance because they measure biological activity, are labor intensive and are not widely used. A stable isotope dilution mass spectrometry assay yields lower values than an immunoassay.132 General comments on the measurement of insulin include the following: 1. The term immunoreactive insulin is used in reference to assays that may recognize, in addition to insulin, substrates that share antigenic epitopes with insulin. Examples include proinsulin, proinsulin conversion intermediates, and insulin derivatives, produced by glycation or dimerization. 2. Various insulin preparations, including human insulin, are used as insulin calibrators. For ease of comparison of results among laboratories, the insulin calibrator is expressed in terms of international units (IU). One international unit of insulin is equal to approximately 43 µg of the World Health Organization (WHO) first International Reference Preparation (1st IRP) Code 66/304 (National Institute of Biological Standards and Control, South Mimms, Potters Bar, Hertfordshire, United Kingdom), which is 100% human insulin. 3. Antisera raised against insulin show some cross-reactivity with proinsulin but not with C-peptide. Specificity is not a problem in healthy individuals because the low proinsulin concentrations do not appreciably affect the absolute values of insulin. In certain situations (e.g., islet cell tumors and diabetic individuals), proinsulin is present at higher concentrations, and direct assay of plasma may falsely overestimate the true insulin concentration. Because proinsulin has very low activity, incorrect conclusions regarding the availability of biologically active insulin may be reached in patients with diabetes. The magnitude of the error depends on the concentration of proinsulin and the extent of cross-reactivity of the antiserum with proinsulin. Monoclonal antibody-based assays that are specific for insulin and do not measure proinsulin,234 although theoretically advantageous, are not superior to nonspecific assays.197 4. A stable isotope dilution mass spectrometry (IDMS) assay has been developed to measure insulin, proinsulin, and C-peptide.132 The difference in mass among the three analytes allows specific measurement of each protein. Comparison of patient samples revealed that most, but not all, results were higher by immunoassay than by mass spectrometry.132 Thus immunoassays may overestimate insulin, particularly at low concentrations. The high protein concentration in the serum requires extraction of proteins (e.g., by immunoaffinity) and purification by high-performance liquid chromatography (HPLC) before quantification by mass spectrometry. This method is not suitable for routine laboratory analysis, but is the best higher-order measurement procedure available and can be used as a candidate reference measurement procedure. 5. The American Diabetes Association (ADA) appointed a task force to standardize the insulin assay.197 Evaluation of unknown samples by 17 different laboratories revealed a wide range in insulin values, with interlaboratory variation up to threefold.197 Large differences were observed even among laboratories using the same assays. Use of a common calibrator did not improve agreement among laboratories. Assay coefficients of variation (CVs) ranged from less than 2% to greater than 30%, with ELISAs exhibiting the lowest imprecision. Certain characteristics of some assays, including commercial kits, were unacceptable. The task force judged available proficiency and certification programs for insulin to be inadequate, and recommended the establishment of a central laboratory to provide certification for insulin assays. Complete interlaboratory standardization was deemed to be neither practical nor universally acceptable. ADA recommendations for analysis of insulin197 are as follows: a. Each laboratory should carefully evaluate its insulin assay to ensure acceptable assay performance. b. Each laboratory should compare the performance of its assay with others using common calibrators and unknown samples. c. Because assay performance may change with time or with new reagents or equipment, performance characteristics must be remeasured periodically. 6. In 2004 the ADA convened an international workgroup to establish guidelines for acceptability of insulin assays and to develop a standardization program that can be used to achieve uniform accuracy-based values.152 Evaluation of 10 commercial insulin methods from 9 manufacturers revealed within-assay CVs ranging from 3.7 to 39%, and 7 assays had a CV ≤10.6%.152 Among-assay CVs ranged from 12 to 66%. Results from six assays agreed within a total error of 32%.152 Cross-reactivity with proinsulin and split-32,33-proinsulin was <2% for nine methods and <3% for eight methods, respectively. A common insulin reference preparation failed to improve harmonization of results. The workgroup concluded that not all commercial insulin assays have acceptable performance characteristics. A study in the United Kingdom published at the same time150 compared 11 commercially available insulin assays and made analogous observations. Insulin values among the different assays varied up to twofold. The ADA workgroup subsequently compared results of 10 commercial insulin assays against IDMS. Four methods were within 32% of the IDMS concentration.160 Most methods had bias greater than 15.5%. Bias was reduced by calibration with serum pools, but remained high for many methods at low insulin concentrations (<60 pmol/L) (10 µIU/mL). 7. Based on biological variability, desirable measurement bias of ±15%, imprecision of 10.6% CV, and total analytical error of 32.0% have been proposed for a single insulin measurement.152 8. Patient samples with high values should be diluted with the zero calibrator. 9. The presence of antibodies to insulin produces spuriously increased or decreased (depending on the method used) insulin values. Reference intervals vary among assays, and each laboratory should establish its own reference intervals. After an overnight fast, insulin concentrations in healthy, normal, nonobese people vary from 12 to 150 pmol/L (2 to 25 µIU/mL).* More specific assays that have minimal cross-reactivity with proinsulin reveal a fasting plasma insulin concentration of less than 60 pmol/L (10 µIU/mL). Concentrations up to 1200 pmol/L (200 µIU/mL) can be reached during a glucose tolerance test. Representative values for insulin concentrations after glucose are shown in Figure 46-4. Fasting insulin values are higher in obese, nondiabetic people and lower in trained athletes. Assays for insulin antibodies fall into three categories: (1) quantitative radioimmunoelectrophoresis, which measures the binding of immunoglobulin (Ig)G antibody to radiolabeled insulin by rocket immunoelectrophoresis into anti–IgG-containing agarose; (2) RIAs with separation of bound and free insulin by precipitation with PEG or a second antibody; and (3) solid-phase immobilization of insulin to test tubes or Sepharose. These are discussed in greater detail in Reeves.194 Accurate measurement of proinsulin has been difficult for several reasons: the blood concentrations are low; antibody production is difficult; most antisera cross-react with insulin and C-peptide, which are present in much higher concentrations; the assays measure intermediate cleavage forms of proinsulin; and reference preparations of pure proinsulin were not readily available.247 Therefore few accurate data are available in the literature on plasma proinsulin. These problems have, to a large extent, been overcome by the availability of biosynthetic proinsulin, which has allowed the production of monoclonal antibodies to proinsulin67,223 and has provided reliable proinsulin calibrators and reference preparations. An International Reference Preparation for human proinsulin (code 84/611) is available from the National Institute of Biological Standards and Controls (Potters Bar, United Kingdom). Earlier assays may have overestimated proinsulin concentrations.180 C-peptide undergoes minimal liver metabolism, and, in contrast to proinsulin assays, assays are not affected by anti-insulin antibodies. However, several methodologic problems produce large between-method variation. These difficulties include variable specificity among different antisera, variable cross-reactivity with proinsulin, and various types of C-peptide preparation used as a calibrator. A 2008 comparison of 40 serum samples using nine commercial C-peptide assay methods showed within- and between-run CVs varying from less than 2% to greater than 10%, and from less than 2% to greater than 18%, respectively.146 Some methods had high imprecision, with between-run CVs exceeding 15%. Two isotope-dilution liquid chromatography–mass spectrometry methods for measuring C-peptide have been developed.48,199 Calibrating C-peptide measurements to a reference method using mass spectrometry increased comparability among laboratories.146 Type 1 diabetes mellitus results from cellular-mediated autoimmune destruction of the insulin-secreting cells of pancreatic β-cells.22,100 In the vast majority of patients, destruction is mediated by T cells. This is termed type 1A or immune-mediated diabetes (see Box 46-1). The α-, δ-, and other islet cells are preserved. The islet cells have a chronic mononuclear cell infiltrate, called insulitis. The autoimmune process leading to type 1 diabetes begins months or years before the clinical presentation, and an 80 to 90% reduction in the volume of β-cells is required to induce symptomatic type 1 diabetes. The rate of islet cell destruction is variable and is usually more rapid in children than in adults. The most practical markers of β-cell autoimmunity are circulating antibodies, which have been detected in the serum years before the onset of hyperglycemia. The best characterized antibodies are as follows22,100,204,251: 1. Islet cell cytoplasmic antibodies (ICAs) react with a sialoglycoconjugate antigen present in the cytoplasm of all endocrine cells of the pancreatic islets. These antibodies are detected in the serum of 0.5% of normal subjects and 75 to 85% of patients with newly diagnosed type 1 diabetes. The antibodies are detected by immunofluorescence microscopy on frozen sections of human pancreatic tails. Results are compared with standard serum of the Immunology of Diabetes Workgroup162 and are expressed in Juvenile Diabetes Foundation (JDF) units. Although not universal, many laboratories use 10 JDF units on two separate occasions or a single result of greater than or equal to 20 JDF units as a significant titer. The ICA assay is labor intensive and difficult to standardize. Few clinical laboratories are likely to implement this assay, which has marked interlaboratory variability in sensitivity and specificity.204 2. Insulin autoantibodies (IAAs) are present in more than 90% of children who develop type 1 diabetes before age 5, but in less than 40% of individuals who develop diabetes after age 12. Their frequency in healthy people is similar to that of ICA. A radioisotopic method that calculates the displaceable insulin radio ligand binding after the addition of excess nonradiolabeled insulin is recommended for IAA. Results are positive when concentrations exceed the 99th percentile or the mean + 2 (or 3) standard deviations (SDs) in healthy controls. Proficiency evaluation revealed poor concordance for IAA among laboratories.35 An important caveat is that insulin antibodies develop after insulin therapy, even in those persons who use human insulin. 3. Antibodies to the 65 kDa isoform of glutamic acid decarboxylase (GAD65)24 have been found up to 10 years before the onset of clinical type 1 diabetes and are present in ≈60% of patients with newly diagnosed diabetes. GAD65 antibodies may be used to identify patients with apparent type 2 diabetes who will subsequently progress to type 1 diabetes. Several different assay formats have been used for the measurement of anti-GAD65 antibodies, including enzymatic immunoprecipitation assay, radiobinding assay, ELISA, immunofluorescence, and Western blotting.214 Considerable variability among laboratories has been significantly reduced by the Second International GADAb Workshop.214 A monoclonal antibody, MICA 3, was suggested as a reference standard. A dual micromethod and RIA performed with 3H-labeled human recombinant GAD65 in a rabbit reticulocyte expression system is used by many laboratories. Methods for measurement of GAD65 are now commercially available. 4. Insulinoma-associated antigens (IA-2A and IA-2βA), directed against two tyrosine phosphatases, have been detected in more than 50% of newly diagnosed type 1 diabetes patients. A widely used method to measure IA-2A uses 35S-labeled recombinant IA-2 in a dual micromethod and RIA. Concurrent analysis of IA-2 and GAD65 in a single assay has been reported.205 5. Zinc transporter ZnT8 was identified recently as a major autoantigen in type 1 diabetes.251 Initial analysis identified ZnT8 in 60 to 80% of patients with new-onset type 1 diabetes compared with less than 2% of controls and less than 3% of individuals with type 2 diabetes.
Diabetes Mellitus
Classification
Type 2 Diabetes Mellitus
Other Specific Types of Diabetes Mellitus
Gestational Diabetes Mellitus
Impaired Glucose Tolerance
Hormones that Regulate Blood Glucose Concentration
Insulin
Synthesis
Release
Degradation
Proinsulin
C-Peptide
Antibodies to Insulin
The Mechanism of Insulin Action
Glucose Transport
Insulin-like Growth Factors
Counter-Regulatory Hormones
Glucagon
Epinephrine
Clinical Utility of Measuring Insulin, Proinsulin, C-Peptide, and Glucagon
Insulin
Proinsulin
C-Peptide
Fasting Hypoglycemia
Insulin Secretion
Monitoring Therapy
Glucagon
Methods for the Measurement of Specific Hormones
Comments
Reference Intervals
Insulin Antibodies
Proinsulin
C-Peptide
Pathogenesis of Type 1 Diabetes Mellitus
Antibodies
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Diabetes Mellitus