Fig. 14.1
Cytotoxicity test methods. Agar diffusion method is one of the methods of “test by indirect contact”
Most metallic biomaterials are alloys which consist of several kinds of metallic elements. Although their cytotoxicity has been investigated by using the materials themselves, it was difficult to clarify from the obtained results which metallic element had cytotoxicity on cells and responsible to adverse reactions to natural tissues. To solve these problems, metal salts or solutions of metal cations have been used for the in vitro cytotoxicity tests [4]. Such studies have revealed that the cytotoxicity and cell reactions depend on the concentration of metal ions.
The biomedical use of inorganic nanoparticles has enjoyed an increasing interest over the past decade. The nanoparticles enable noninvasive and long-term imaging of the whole body, potential treatment of cancer as currently being studied in clinical trials for magnetite and fold particles, or shedding of light on the complex cellular environment [5]. Rivera Gil et al. mentioned that several properties of nanoparticles have been demonstrated to change the in vitro (and partly also in vivo) toxicity of nanoparticles as compared to the bulk state, and the properties are (1) a higher surface-to-volume ratio and thus an enhanced contact area with their surroundings than bulk materials of the same mass do, (2) their retention in many cells and organs to a larger extent than larger particles, and (3) their crucial role in determining response based on the nanoparticle shape [6]. Thus, the state of metallic materials used for cytotoxicity tests, such as bulk, ion, and nanoparticles, plays an important role in their cytotoxicity and cell reactions.
Naturally, the choice of cytotoxicity test model, such as cell and tissue types, is critical to a more thorough understanding of the safety of metallic implants. This must depend on application fields in which the metallic implants are used, such as orthopedic or dental field. Various types of cells (e.g., osteoblasts, fibroblasts) have been used for in vitro tests. In addition, several types of assay have been applied to the tests: cells are cultured on metallic implant surface directly or in extracts of the implants, in media containing metal ions, etc. Most types of adherent cells are influenced by surface properties of the substrates used, such as roughness, hydrophilicity, chemical component, etc., in their reactions. We need to pay attention to not only the type of metallic materials used but also the culture conditions to understand cytotoxicity of the materials by in vitro tests.
The assessment of cytotoxicity of metallic implants has been complicated due to a great variety in sample shapes (including ions), physicochemical parameters of samples, type of cells used, type of assay used, etc. It would be difficult to compare results based on varying methods and sample types. In addition, in vitro tests focus on specific interactions between metallic samples (including ions) and cells. Extrapolation to in vivo is not straightforward. Comprehensive investigation must be required to thoroughly understand cytotoxicity of metallic implants.
Many cases, in which human beings or experimental animals have adverse reactions to metallic implants, are reported. However, positive reactions by metallic implants have enjoyed an increasing interest recently. Effects of metallic materials on specific cellular functions have been focused to identify the material biocompatible to bone or tooth rather than to evaluate general cytotoxicity. Characteristic reactions of several metal ions with cells have been found via in vitro tests, for example, upregulation of proliferation and mineralization of osteogenic cells by several metal ions was reported.
This chapter provides a comprehensive update of the cytotoxicity of metallic materials (implants themselves, ions, and particles), differences in cell reactions to metallic materials depending on cell type, and positive cell reactions to metallic materials.
14.2 Metallic Samples for Cytotoxicity Test
14.2.1 Plates and Disks
Direct method of cytotoxicity tests using plates, disks, and commercialized devices of metallic materials has been applied in order to assess the cell response to such material surfaces (Table 14.1). Generally, cells are seeded on sample surfaces or cultured with samples in the same well and then examined in their adhesion, viability, and proliferation. Cell functions are influenced by not only the type of metallic component but also several surface properties, such as hydrophilicity, roughness, etc. Therefore, to take account of such properties is important when we compare the cytotoxicity of several materials.
Table 14.1
Reports on cytotoxicity of metal bulks
Metallic material | Specimen | Cell type | Assay | Ref. |
|---|---|---|---|---|
Ti | – | Mouse fibroblast (NIH/3T3) | MTT | [7] |
Ti–Cr–Mo–Fe | ||||
Stainless steel | ||||
Ag–Sn–Co–Hg | ||||
Au alloy | Cast disk | Mouse fibroblast (NCTC clone 929) | Agar overlay | [8] |
Unalloyed Cu | Millipore filter | |||
Unalloyed Ag | ||||
Cr–Co alloy | MTT | |||
Au–Pt–Pd–Ag | Block | Human fibroblast | BrdU immunocytochemistry | [9] |
Au–Pt–Pd–Ag–Cu–In–Ir | ||||
Au–Pt–Pd–Ag–In–Ru–Zn | ||||
Au–Pt–Pd–Ag–Sn–In–Ga | ||||
Au–Pt–Pd–Ag–Cu–In | ||||
Au–Pt–Pd–Ag–Cu |
Retamoso et al. reported the cell culture test for ten kinds of orthodontic devices including metallic, polycarbonate, monocrystalline ceramic, and polycrystalline ceramic samples using mouse fibroblasts (NIH/3T3) [7]. The cells were cultured in a well containing specimen. After 24 h of culturing, the cytotoxicity was evaluated by MTT assay, which is based on the ability of the mitochondrial enzyme succinate dehydrogenase to convert the yellow water-soluble tetrazolium salt into formazan crystals in metabolically active cells. Three types of commercialized nickel-free samples, including two types of 100 % of Ti and one alloy (Ti–Cr–Mo–Fe), were found to have better biocompatibility than the others. The alloy sample showed some toxic effects compared with the titanium one. This might be due to effects of molybdenum released from the sample, referring the past literatures. The release of ions from the tested samples, however, was not evaluated in this report. Nickel-free and steels with reduced nickel content have been tried in orthodontic use. There are biocompatibility concerns from the use of nickel alloys in the human oral cavity for extended periods of time, while nickel has been incorporated into the alloys to decrease corrosion. In this report, austenitic stainless steel (17–20 mass % Cr, 8–10.5 mass % Ni, 65–69 mass % Fe) was used as a negative control (showing a low toxicity). The titanium and its alloy had significant lower cell viability than the negative control and cellular control (no sample).
The viability of mouse fibroblast contacting several types of dental metallic materials (Au alloys, unalloyed Cu or Ag, Cr–Co alloy, etc.) intended for fixed and removable prostheses was reported by Sjögren et al. [8]. The materials were placed on an agar surface where the cells were seeded and then incubated for 24 h. After the culturing, unstained area in cell monolayer by neutral red was evaluated, as damaged or dead cells appear decolorized in comparison to healthy control cells (Fig. 14.2). In their report, Millipore filter and MTT tests (in direct method) were also applied for the cytotoxicity test of the samples. From the results of all tests, the authors concluded that the cytotoxicity was related to the alloy composition and treatment. The alloys releasing Cu and Zn had lower cell compatibility in comparison with the others. Especially, the adverse effects of released Cu depended on its amount. Surface treatment for the materials influenced the cytotoxicity; sandblasting and polishing reduced both cytotoxicity and release of metal ions from the test specimens.

Fig. 14.2
Cytotoxicity of unalloyed Ag assessed by agar overlay test and presented as unstained area in cell monolayer (Reprinted from Ref. [8], Copyright 2000, with permission from Elsevier)
The influence of six types of dental alloys (Au–Pt–Pd–Ag, Au–Pt–Pd–Ag–Cu–In–Ir, Au–Pt–Pd–Ag–In–Ru–Zn, Au–Pt–Pd–Ag–Sn–In–Ga, Au–Pt–Pd–Ag–Cu–In, and Au–Pt–Pd–Ag–Cu) on the proliferation and fibronectin arrangement in human fibroblasts was reported by Grill et al. [9]. The cells were cultured in a dish in which the alloy block was placed. The cytotoxicity was evaluated by calculating the percentage of cells in the S-phase and observing fibronectin organization. The authors concluded that the alloy with the highest Au content had the most biocompatibility among the samples tested, and this agreed with the results reported by other literatures. The reason why Au content in alloy was effective for high cell compatibility was unclear. The authors also mentioned that a correlation exists between fibronectin organization and cell proliferation, and the observation of the fibronectin arrangement could be a farther useful tool in evaluating the biocompatibility of biomaterials. Some reports suggested such correlation [10]; each type of fibronectin organization is strictly related to cell proliferation; fibronectin organized in focal adhesions enhances cell entry into the S-phase and therefore stimulates the growth cycle; different morphological and functional features of fibroblasts can influence cell proliferation.
14.2.2 Particles
Several types of nanoparticles and microparticles have been investigated in their cell compatibility by in vitro tests (Table 14.2). Nanoparticles have been investigated for the application in the invasive and long-term imaging of the whole body, cancer treatment, etc. Therefore, cells are exposed to a wide array of nanoparticles. Understanding the interaction between nanoparticles and cell must be necessary.
Table 14.2
Reports on cytotoxicity of metal particles
Metallic material | Specimen | Cell type | Assay | Ref. |
|---|---|---|---|---|
Iron oxide | Nanoparticle (around 13 nm) | Primary human fibroblast (h-TERT BJ1) | MTT | [11] |
Iron oxide | Nanoparticle (40–45 nm) | Primary human fibroblast (h-TERT BJ1) | MTT | [12] |
Iron oxide | Nanoparticle (60 nm) | Professional phagocyte | XTT | |
Cu | Microparticle (1–147 μm) | Human osteoblast-like cell (MG-63) | Neutral red | [17] |
Al | ||||
Ti | ||||
Zr | ||||
V | ||||
Nb | ||||
Ta | ||||
Cr | ||||
Mo | ||||
W | ||||
Mn | ||||
Fe | ||||
Co | ||||
Ni | ||||
Cd | Microparticle (0.1–150 μm) | Human erythrocyte | Hemolysis measurement | [18] |
Cr | ||||
Co | ||||
Fe | ||||
Mo | ||||
Ni | ||||
Ta | ||||
Ti | ||||
Zn | ||||
Co–Cr alloy | ||||
Au–Cu–Ag–Pd–others | Sphere (9.1 mm) | Mouse fibroblast (L929) | Neutral red | [19] |
Ag–Pd–Cu–others | ||||
Ti–others | ||||
Co–Cr–Mo–others | ||||
Ni–Cr–others |
Iron oxide and gold nanoparticles are one of the frequently used inorganic nanoparticles. As iron oxide is a ferromagnetic material, its nanoparticles are useful for many kinds of biomedical applications, such as magnetic resonance imaging (MRI), mediators in magnetic cancer hyperthermia, and magnetically enhanced and targeted drug or gene delivery. Therefore, small particles are preferably employed.
The diameter of the iron oxide core is smaller than the superparamagnetic limit. Although iron oxide nanoparticles have been regarded to be degraded and released, resulting in the normal iron metabolism, there are concerns that its small size pose an additional hazard. Gold nanomaterials have enjoyed an increasing interest in biomedical field such as drug or gene delivery, cancer treatment, and biological imaging, as they have unique optical features, such as localized surface plasmon resonance. Although bulk gold has been expected not to have toxicity, its nanoparticles have been suggested to induce toxicity by penetrating the nuclear compartment and binding to DNA.
“Nanofactor” itself appears to cause several adverse effects on cells [5]. Nanoparticles reach places where larger particles cannot enter, such as the nucleus; nanoparticles internalized into cells [11, 12] and passed across a cellular barrier (Fig. 14.3) [13], and ultrafine particles (<100 nm) were transferred to the brain [14]. Their high surface area is available for interaction with cellular components. They suggested several factors which are expected to relate the cytotoxicity of nanoparticles: (1) the generation of reactive oxygen species, (2) the cell morphology and cytoskeleton defects, (3) the intracellular signaling pathways and genotoxicity, (4) the intracellular nanoparticle degradability, (5) the interaction with biological molecules, etc. Thus, the cytotoxicity of metallic nanoparticles is influenced by not only the type of metal component but also these nanofactors of the materials.


Fig. 14.3
TEM image of a nanoparticle-treated BeWo cell (human trophoblast choriocarcinoma-derived cell line) barrier showing aggregates of CoCr nanoparticles (NP) internalized in the upper layers of the barrier (Reprinted by permission from Macmillan Publishers Ltd: Ref. [13], copyright 2009)
Reactive oxygen species have been expected to be of major importance in the toxicological prolife of nanoparticles. They are generated by cultured cells upon exposure to nanoparticles. Their generation has been controlled by coating nanoparticles with various materials, such as dextran, citrate, etc. The intercellular volume of nanoparticles influences cellular morphology or the structure of the cellular cytoskeleton network. In the case of iron oxide nanoparticles, their intracellular localization is related to the disruption of the cell cytoskeleton network [11]. The coating is effective for controlling the adverse effects, because the effects have been found to depend on the concentration of generated reactive oxygen species and the particles penetrating into the cells (Fig. 14.4) [11].
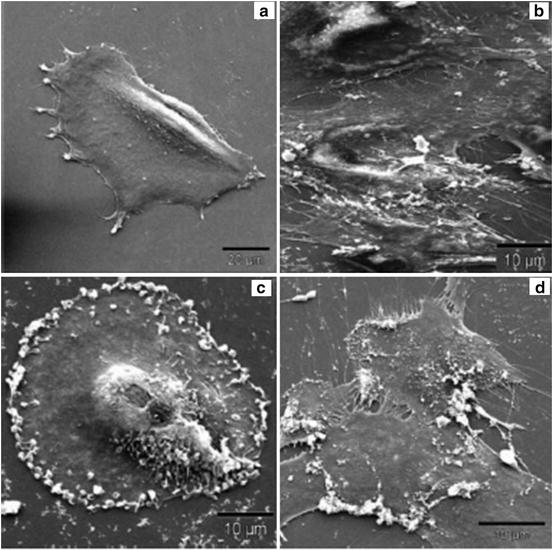

Fig. 14.4
SEM pictures of human fibroblasts incubated with magnetic nanoparticles: (a) control cells, (b) plain-uncoated particles, (c) lactoferrin-coated, and (d) ceruloplasmin-coated nanoparticles. The picture shows that lactoferrin- and ceruloplasmin-coated nanoparticles adhere to the cell surface, whereas plain-uncoated particles were found to be phagocytosed by the cells (Reprinted from Ref. [11], Copyright 2004, with permission from Elsevier)
Nanoparticles influence intracellular signaling pathways [13]. This is suggested to be carried out via the high levels of reactive oxygen species, change in the level of protein or gene expression, altered activation status of proteins, and influenced gene expression [5]. The nanoparticle degradation inside of the cells has been reported to play a significant role for the cell reactions [15, 16]. The local pH at the nanoparticle surface increases or decreases compared with the physiological condition through the degradation of the particles. Subsequently, free metal ions are released from the particles. The nanoparticle degradation inside of the cells has been reported to induce adverse effects on cell functions, such as apoptosis and inhibition of differentiation. On the other hand, different cell reactions are observed when gold nanoparticles are induced into cells. Gold nanoparticles possess high stability; therefore, the degradation hardly happens inside of the cells. Most of the cytotoxicity of gold nanoparticles has been regarded to be due to the particle size and the coating. Thus, the adverse effects on cells by the nanoparticle degradation depend on the kinds of metal component of the nanoparticles.
Microparticles are produced by the wear on metallic implant surfaces implanted in the body and have been recognized as one of the major factors responsible for loosening implants. Two materials placed together under load develop electrorepulsive and atomic binding interactions in the area of contact. These reactions are disrupted, when the surfaces slide across each other. Thus, wear debris (particles of the implant material) are produced and may attach to the counter face, remain between the two surfaces, or disperse into the system of the host.
The cytotoxicity of metal particles (Cu, Al, Si, Ti, Zr, V, Nb, Ta, Cr, Mo, W, Mn, Fe, Co, and Ni) with several micrometers in size was reported by Sakai et al. [17]. They cultured human osteoblast-like cell (MG-63) with the particles and also in the culture medium containing extract of each particle. The cytotoxicity was assessed by evaluating the viability with neutral red assay after 3 and 6 days of culturing. Nb and Zr showed no inhibition of the cell viability in any conditions where the amount of particles in the medium changed, while other metal materials inhibited the viability depending on the dose of particles. Mn particles showed the most severe inhibition. The extracts of Al, Ti, Zr, Nb, Ta, Cr, and Fe had no adverse effects on the cell viability, while the viability was decreased by the metal particles in the case of direct culturing. This result suggested that there was a direct interaction between the particle surface and cellular membranes, which influenced the cell viability.
Rae et al. revealed effects of the direct interaction on the hemolytic properties [18]. Erythrocytes were incubated in a buffer solution containing metal particles (Cd, Cr, Co, Fe, Mo, Ni, Ta, Ti, Zn, and Co–Cr alloy), and the hemolysis was measured. Cd, Ta, Ti, and Zn possessed a mild hemolysis, while Co, Ni, and Co–Cr alloy had a severe hemolysis. The others appeared to cause some hemolysis which was intermediate to the above groups. The authors also did similar tests for soluble nickel and cobalt chlorides, respectively, to clarify if soluble metallic product of the Co, Ni, and Co–Cr alloy related to the hemolysis. Results demonstrated no measurable hemolysis by these metal ions. Therefore, the direct interaction between particle surfaces and erythrocyte surfaces was suggested to be responsible for the observed hemolysis. Thus, cell activities are affected by such direct interaction rather than metal ions released, when metal samples have low cytotoxicity. We, however, need to pay attention to the size of particles tested. As determined in this section, nanoparticles internalized into cells, whereas microparticles did not. A route to adverse effects on cell viability is different between the two types of particles. The particle size must relate to the development of the direct interaction.
Takeda et al. reported that wear debris of some alloys, rather than the extract from them, were responsible for adverse effects on fibroblasts [19]. They did culture tests using L929 mouse fibroblast cells for three different culture media conditioned by metal particles (Au–Cu–Ag–Pd–others, Ag–Pd–Cu–others, Ti–others, Co–Cr–Mo–others, and Ni–Cr–others), (1) medium containing extracts of metal particles prepared with static condition, (2) medium after mixing with metal particles (dynamic system), and (3) medium after the treatment in the dynamic system followed by filtration with 0.22 μm membrane filter. After 3 days of culturing the cells in the three different types of medium, the cytotoxicity was determined by evaluating the cell viability with neutral red assay. No adverse effects of the metal particles tested on the cell viability were observed in the results of culture tests using medium 1. However, the cell viability in media 2 and 3 varied with the metal type. Figure 14.5 shows possible interactions with the cell response. In the case of Au-based alloy, the viability in the medium 2 was lower than that in the medium 3. Wear debris of the alloy were generated by the action of mechanical forces in the medium 2 and influenced the viability via a direct interaction between debris surfaces and cells. The authors suggested based on these results that even if metallic biomaterials are not toxic due to excellent corrosion resistance, cytotoxic effects can occur when wear debris is produced. Co-based alloy possessed a severe deterioration of the cell viability in both cases of media 2 and 3. Although the alloy tested had good corrosion resistance, Co or Cr ions dissolved from the alloy affected the cell viability. The ion dissolution was suggested to increase due to a break of the protective surface layer (passivation layer) of the alloy through the mixing. Ni-based alloy also had a severe deterioration of the cell viability in both cases of media 2 and 3. This was due to Ni dissolution.
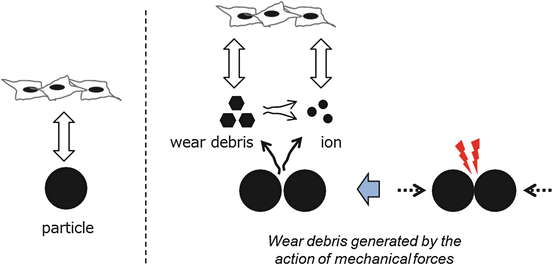

Fig. 14.5
Schematic image of possible interactions between metal specimen and cells
14.2.3 Ions
Extracts of metallic biomaterials (a solution containing ions released from the materials) have been applied for cytotoxicity tests (Table 14.3). This is because metal ions have been reported to be released from some biomaterials in vivo and may be associated with local inflammation or other adverse reactions. The international standards complied as ISO 10993 recommend in vitro cellular toxicity testing on established cell lines and using the extracts. In the case of alloys, several ions are simultaneously released from them.
Table 14.3
Reports on cytotoxicity of metal extracts
Metallic material | Specimen | Cell type | Assay | Ref. |
|---|---|---|---|---|
Stainless steel | Extract | Mouse fibroblast (L929) | MTT | [20] |
Au-plated steel | ||||
Ti | ||||
Ni–Ti alloy | ||||
Ti–Mo alloy | ||||
Ag-based soldering alloy | ||||
Au–Pt alloy | Extract | Human gingival fibroblast | MTT | [21] |
Co–Cr alloy | ||||
Ni–Cr alloy | ||||
4 types of Ti alloys | Extract | Human gingival fibroblast and mouse osteoblast (MC3T3-E1) | Impedance-based real-time cell analyzer | [22] |
316 stainless steel containing alloy |
To investigate in the effects of single metal ion on cell functions, culture media containing single ion which are prepared using metal salts, such as metal chlorides, are also applied for the cytotoxicity tests (Table 14.4).
Table 14.4
Reports on cytotoxicity of metal ions
Metallic material | Specimen | Cell type | Assay | Ref. |
|---|---|---|---|---|
Ag | Ag2SO4 | Mouse fibroblast (L929) | Trypan blue | [23] |
Al | AlCl3 | |||
Be | BeCl2
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|