The study of congenital anomalies continues to be hampered by misunderstandings at a number of levels. In many circles, for example, the statement that “the baby was born with a genetic deformity” is often heard. In fact, this is often not the case, for many congenital anomalies are neither genetic in origin nor do they constitute a physical deformation per se. Another common, but erroneous, opinion is that hundreds or even thousands of substances in the environment cause birth defects. In fact, only 30 to 40 exogenous substances (i.e., teratogens) have been proven to have this potential. But if these issues continue to hamper our dealings professionally, they also tug at the souls of grieving parents who ask “What caused my baby’s problem?” or “How did this happen?” or “Will it happen again?” These questions take on added complexity when multiple anomalies are encountered in a single patient. It can fall to the pathologist, as well as clinical specialists, to help explain these findings. The purpose of this chapter, then, is to provide a broad context for understanding the basic patterns of anomalies. As such, descriptions will emphasize gross features over microscopic appearances or discussions of intricate pathologic processes. For help with these latter matters, the reader is referred to chapters that cover specific organ systems.
ETIOLOGY AND PATHOGENESIS
The question of causation—etiology—is not at all simple, for etiology may be heterogeneous, that is, multiple factors may bring about a given defect. Holoprosencephaly is a powerful example, for it arises sporadically or is associated with several gene mutations, aneuploidies (e.g., trisomy 13), diseases such as maternal diabetes mellitus, and teratogens (e.g., ethyl alcohol). Robin sequence (micrognathia, cleft palate, glossoptosis) is another example, in which causes may be chromosomal, teratogenic, monogenic, disruptive (i.e., amniotic bands), or unknown.
The mechanism—or pathogenesis—responsible for a defect may be varied as well. In Robin sequence, for example, deformations may occur secondary to intrauterine constraint produced by oligohydramnios. However, reduced amniotic fluid volume may occur from premature rupture of membranes (particularly chronic leakage), renal anomalies, or placental or maternal factors.
CONCEPTS AND TERMS OF MORPHOGENESIS
In 1982, a set of standardized terms for describing human developmental abnormalities was established (
1). The definitions are essential for pediatric pathologists, pediatricians, medical geneticists, and others dealing with congenital anomalies. Several additional discussions of the terminologic, historic, diagnostic, nosologic, and morphologic aspects of congenital anomalies in humans are available (
2,
3).
Hypoplasia refers to underdevelopment and hyperplasia to overdevelopment of an organism, organ, or tissue and result from a change in cell number. Hypotrophy and hypertrophy refer to a decrease and increase, respectively, in the size of an organ, tissue, or cells. Agenesis is the absence of a part of the body caused by a presumed absence of the anlage, or primordium. Aplasia refers to absence of a rudimentary structure caused by failure of the anlage to develop completely. Aplasia can be regarded as an extreme degree of hypoplasia. Atrophy describes the shrinkage of a previously normally developed tissue mass or organ because of a decrease in cell size or cell number.
A
developmental field is the portion of the embryo that reacts as a coordinated unit to inductive effects with differentiation and growth (
4). Developmental fields represent, then, the major branches on the morphogenetic tree. It has been suggested that the embryo itself constitutes the
primary developmental field (
5) and that other more constricted ones are operational at later stages of development. A
monotopic field defect represents a defect in organogenesis and includes contiguous anomalies (e.g., cyclopia and holoprosencephaly; cleft lip and cleft palate). Such alterations are more likely to
arise late in gestation and produce more confined defects (
6). By contrast, a
polytopic field defect is thought to result from an earlier defect during blastogenesis—the first 4 weeks of development—and occurs if abnormal inductive processes produce more distantly located and diverse defects (
6,
7).
The midline also acts as a developmental field (
8). It represents the normal plane of cleavage in monozygotic twinning and the plane around which symmetry of visceral position is determined. It is an especially vulnerable site in terms of developmental anomalies. Morphogenetic events involving the midline include fusions, segmentation, programmed cell death with morphogenetic “necroses” or resorptions, rotations, and other developmental movements. In some anomalies involving the midline, the incidence of monozygotic twinning may be increased (e.g., sirenomelia, cloacal anomalies). Other examples of midline anomalies include the holoprosencephaly complex, agenesis of the corpus callosum, cleft lip, cleft palate, midface cleft complex, spina bifida, omphalocele, congenital heart defects, hypospadias, and imperforate anus.
A
malformation is “a morphologic defect of an organ, part of an organ, or larger region of the body resulting from an intrinsically abnormal developmental process” (
1).
A
disruption, or secondary malformation, is “a morphologic defect of an organ, part of an organ, or a larger region of the body, resulting from the extrinsic breakdown of, or interference with, an originally normal developmental process” (
1). Disruptions are causally heterogeneous and may bear close resemblance to malformations anatomically. In a given case, the distinction between a disruption and a malformation may be made on the basis of the associated malformations or the history of gestational exposure to a teratogenic agent or event. The general prevalence of birth defects is given as 3% to 5%.
A
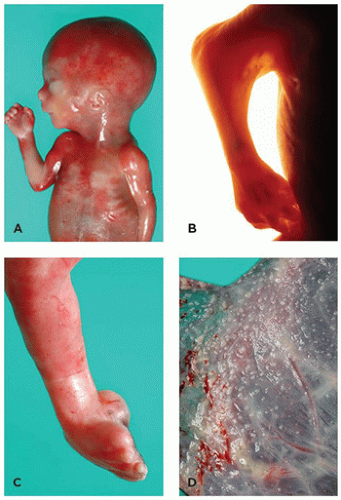
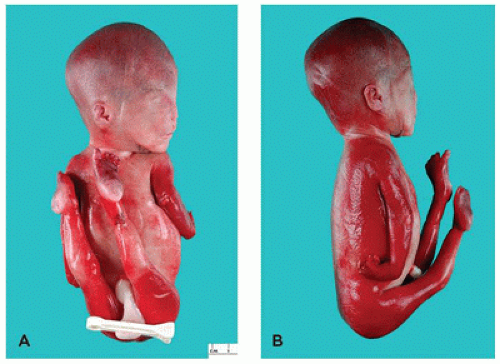
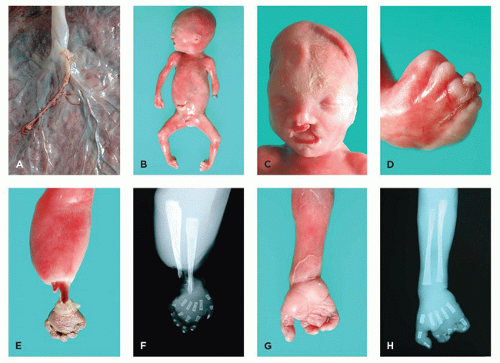
deformation is “an abnormal form, shape, or position of a part of the body caused by mechanical forces.” It may be extrinsic, due to intrauterine constraint (e.g., lack of amniotic fluid), or intrinsic, due to a defect of the nervous system that causes hypomobility (
1). Examples of deformities are talipes equinovarus and arthrogryposis. About 1% to 2% of newborn infants have deformations of some sort.
Dysplasia represents “an abnormal organization of cells into tissue(s) and its morphologic result(s)” (
1). Dysplasia is therefore a process and the consequence of
dyshistogenesis, an abnormal differentiation of tissue structure. This is in contrast to a malformation, which is a defect in morphogenesis of the organ structure. Dysplasias may or may not be metabolically induced, may involve one or several germ layers, and may be generalized or localized; they often demonstrate a sporadic pattern of occurrence (
1).
Mild dysplasias, common in the normal population, include freckling, capillary hemangioma over the glabella and metopic suture area of the forehead, café au lait spots, moles, and nevi. If they are mendelian traits, they usually represent autosomal dominant mutations. Dysplasias are components of every aneuploidy syndrome and probably are one reason for the increased incidence of associated cancers. Dysplasias can be induced environmentally by radiation, viruses, and carcinogens.
Anomalies sometimes occur as groups of defects, which require additional classification. The terms described below help in categorizing anomalies but are only aids. Placing a name on a cluster of anomalies helps in organizing thoughts about a given condition but does not identify cause or mechanism or suggest recurrence risk (
9).
That being said, a
syndrome is “a pattern of multiple anomalies thought to be pathogenetically related and not known to represent a single sequence or a polytopic field defect” (
1). No structural anomaly of any malformation syndrome is obligatory, and no one component is pathognomonic of any syndrome.
A
sequence is a “pattern of multiple anomalies derived from a single known or presumed prior anomaly or mechanical factor” (
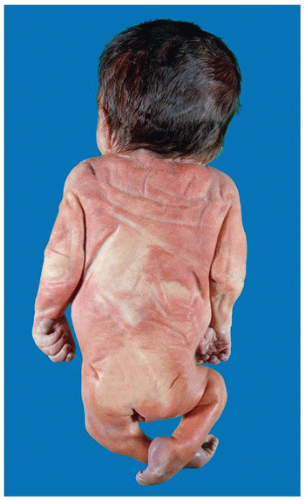
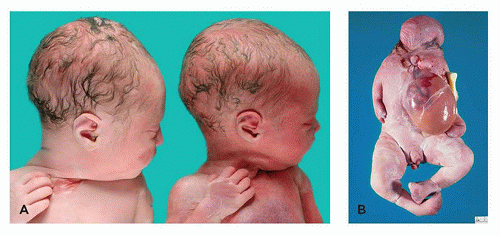
1). In the Potter sequence, the pathogenetic event is oligohydramnios arising from a genetic or nongenetic cause; the causal event represents a malformation (e.g., renal agenesis or dysplasia, as in polycystic kidney) or a mechanical factor (e.g., amniotic fluid leakage). Lack of amniotic fluid restricts fetal movement and causes fetal compression, producing the typical changes of Potter sequence (
Figure 4-1).
A malformation complex consists of “those groups of heterogeneous disorders with overlapping characteristics that are difficult to separate into specific conditions,” for example, facio-auriculo-vertebral spectrum and hypoglossia-hypodactylia.
An
association consists of “a nonrandom occurrence in two or more individuals of multiple anomalies not known to be a polytopic field defect, sequence, or syndrome” (
1). Associations have also been defined as the results of “disruptive events acting on developmental fields” (
10). However, the diversity of findings in associations highlights how much remains unknown about possible developmental fields. The term “association” is a temporary category that should change as conditions become better understood. An example is CHARGE syndrome, which for many years was classified as an association, until mutations in the
CHD7 gene were identified in numerous patients (
11).
Jones has offered a valuable policy for naming patterns of malformations (
12):
When the etiology is known and easily remembered, the appropriate term should be used to designate the disorder.
Time-honored designations should be continued unless there is good reason to change.
In the absence of a reasonably descriptive designation, eponyms, some of them multiple, may be used until the basic defect for the disorder is recognized. However, use of an eponym should thereafter be limited to one proper name.
The use of the possessive form of an eponym should be discontinued, because the author neither had nor owned the disorder.
Designation of a disorder by one or more of its manifestations does not necessarily imply that they are either specific or consistent components of that disorder.
Names that may have an unpleasant connotation for the affected individual or family should be avoided.
The syndrome should not be designated by the initials of the originally described patients.
Names that are too general for a specific syndrome should be avoided.
Unless acronyms are extremely pertinent or appropriate, they should be avoided.