system. However, innate immune mechanisms are also relatively impaired in neonates as compared to older children and adults, thereby increasing neonatal susceptibility to infections (9). Further, the neonate is unable to produce antibody to thymus-independent antigens such as bacterial polysaccharides, although transplacentally acquired maternal antibodies confer some protection for the first few months of life. Neonatal B-cells have an immature phenotype, and the neonatal spleen has a different cellular composition; unlike adults, neonatal accessory cells, that is, macrophages and dendritic cells, appear to produce less stimulatory cytokines and an overabundance of inhibitory cytokines (10).
TABLE 6-1 INFECTIONS ASSOCIATED WITH PRIMARY IMMUNODEFICIENCIES | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
nosocomial environments as intensive care units, neonatal nurseries, day care centers, schools, and summer camps play a role either by serving as reservoirs for pathogenic microbes or by facilitating their spread in a susceptible population. Biofilm formation may also be important in bacterial pathogenesis (15). Hospitalized or chronically ill children are exposed additionally to equipment and pharmaceutical agents that may be the source of iatrogenic infections. Lack of personal hygiene and exposure to pets, soil, and natural environments are additional factors to consider in children.
TABLE 6-2 CONGENITAL IMMUNODEFICIENCIES WITH AN INCREASED RISK OF SEPSISa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 6-3 PRIMARILY NONIMMUNE DISORDERS INFLUENCING INCIDENCE AND SEVERITY OF INFECTION | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 6-4 INFLUENCE OF ENVIRONMENTAL FACTORS ON INFECTIOUS DISEASES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
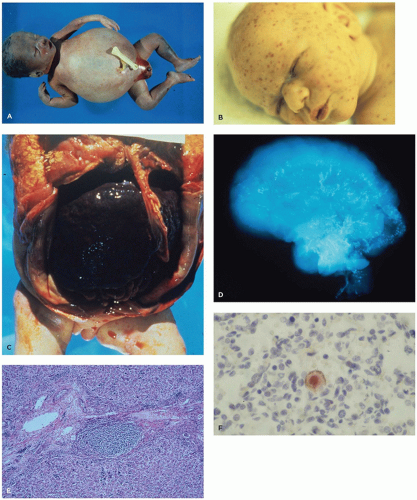
advanced developmental state of the infant. Nonetheless, they produce acute and possibly fatal disease (e.g., neonatal herpes virus infection), persistent tissue or organ dysfunction (e.g., postnatal CMV infection), or late complications (e.g., obstructive hydrocephalus resulting from neonatal meningitis).
TABLE 6-5 VIRAL INFECTIONS ASSOCIATED WITH ADVERSE FETAL OUTCOMES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
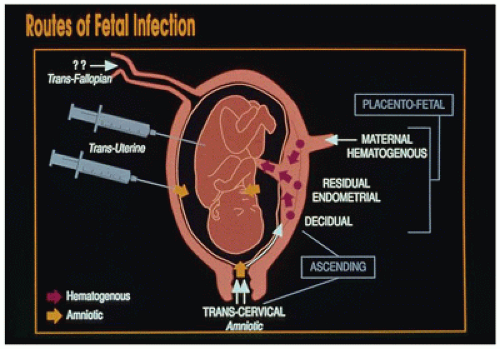
bacteria, and Candida. Hematogenous spread of maternal blood-borne organisms across the placenta is the pathway used by most viruses and protozoa such as plasmodia and toxoplasma.
TABLE 6-6 PREDOMINANT PATHWAYS OF THE MAJOR FETAL AND NEONATAL INFECTION | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||
otitis media). Breast milk enhances nonpathogenic flora, decreases colonization with enteropathogens, aids development of the respiratory and intestinal mucosal barriers, provides secretory IgA and functioning immune cells (neutrophils, macrophages, T and B lymphocytes), and prevents gut inflammation and immunomodulation (20). Breast milk-associated infections include HIV-1, human T-lymphotropic virus-1 (HTLV-1), CMV, measles virus, and streptococci (21). Bacterial infections are rarely, if ever, transmitted to infants through breast milk. Although infections associated with breast milk are more commonly transmitted through other mechanisms, at least temporary cessation of breast-feeding has been recommended in certain maternal bacterial infections associated with Neisseria gonorrhoeae, Haemophilus influenzae, group B streptococci (GBS; Streptococcus agalactiae), staphylococci, Borrelia burgdorferi, Treponema pallidum, and Mycobacterium tuberculosis.
transmitted. Virus is present in urine; oropharyngeal, cervical, and vaginal secretions; breast milk; semen; and tears and can be shed intermittently for years.
TABLE 6-7 VIRAL INFECTION IN THE FETUS AND NEONATE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 6-8 COMMON SYSTEMIC VIRAL INFECTIONS IN INFANTS AND CHILDREN | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CMV-infected babies. During infancy, sequelae such as sensorineural deafness, psychomotor retardation, and cerebral palsy may develop even in infants lacking symptoms at birth. About 20% of all infected neonates suffer sequelae of a congenital CMV infection (30).
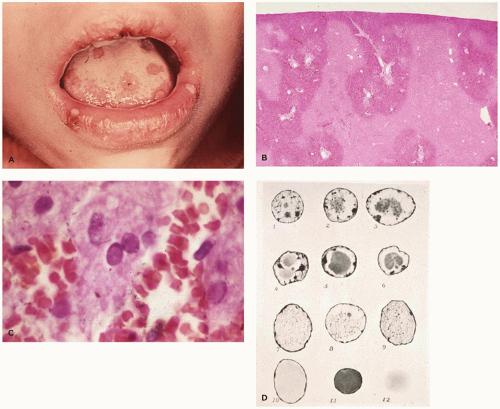
push the chromatin to the nuclear membrane. The later inclusions (Cowdry type A) are smaller, deeply eosinophilic, round, or polygonal and separated from the nuclear membrane by a clear halo. Multinucleated cells are more likely to contain Cowdry type B inclusions. Type A inclusions reflect excess viral capsid material following extrusion of encapsidated viral DNA. Seventy-five to eighty percent of cases of disseminated HSV involve liver (Figure 6-3B, C) and adrenal glands. Lesions may also be seen in the lung, brain, spleen, bone marrow, and GIT. Care must be exercised in the evaluation of necrotizing and ulcerated skin or mucous membrane lesions; HSV inclusions can usually be found at the periphery of such lesions, as secondary bacterial or yeast infection may obscure the underlying viral lesion.
phenotypic and genotypic methods. Phenotypic methods are cumbersome but allow clear interpretation of results, whereas genotypic methods are faster but may be diagnostically less conclusive (36).
eliminated from the United States through 2011, but international importation continues (44).
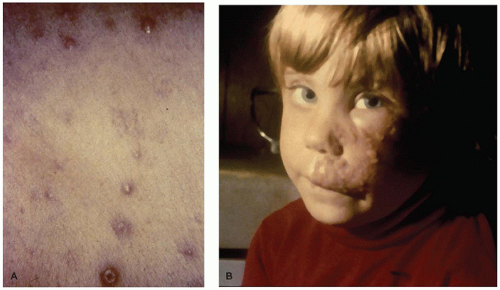
syndrome, necrotizing pneumonia, encephalitis, hepatitis, and/or Reye syndrome (51).
due to immunologic factors. Immune factors may be responsible for the adverse effects even in infants who escape actual infection (62).
TABLE 6-9A 2008 SURVEILLANCE CASE DEFINITION FOR HIV INFECTION AMONG CHILDREN AGED <18 MONTHSa | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 6-9B 2008 SURVEILLANCE CASE DEFINITION FOR HIV INFECTION AMONG CHILDREN AGED 18 MONTHS TO <13 YEARSa | ||||
|---|---|---|---|---|
|
TABLE 6-10 AIDS-DEFINING ILLNESSES IN THE PEDIATRIC AGE GROUP | |||
|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree