Columnar Cell Change with or without Flat Epithelial Atypia
Key Facts
Terminology
Columnar cell change (CCC)
Flat epithelial atypia (FEA)
Encountered with increasing frequency in breast biopsies performed for mammographic microcalcifications
Microscopic Pathology
CCC
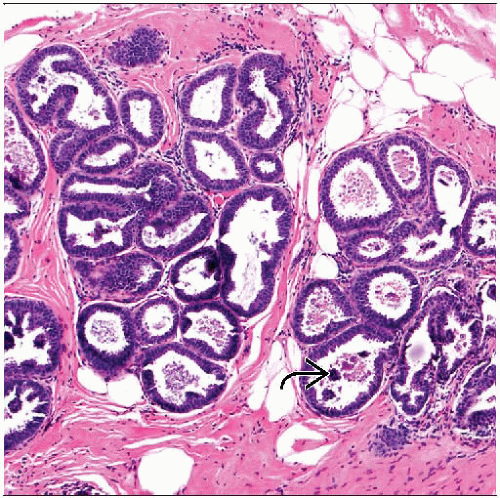
TDLUs with variably dilated acini lined by 1 or 2 layers of columnar epithelial cells
Cells are uniform with ovoid to elongated nuclei oriented perpendicular to basement membrane
Cystic spaces contain luminal secretions, flocculent material, and frequent microcalcifications
FEA: Similar architectural features to CCC and columnar cell hyperplasia (CCH)
Epithelial cells demonstrate low-grade, monomorphic-type cytologic atypia
Cells show nuclear enlargement, stratification, and variable nucleoli
May coexist with areas that fulfill diagnostic criteria for ADH or low-grade DCIS
Diagnostic Checklist
FEA may show genetic changes similar to those seen in low-grade DCIS and invasive carcinomas
Risk of FEA progressing to invasive cancer appears to be low when present as isolated finding
Presence of FEA should alert pathologist to search for other frequently associated lesions
ADH, DCIS, lobular neoplasia, and invasive carcinoma
TERMINOLOGY
Abbreviations
Columnar cell change (CCC)
Flat epithelial atypia (FEA)
Synonyms
Columnar cell hyperplasia
Columnar alteration with prominent apical snouts and secretions
Atypical cystic lobules
Unfolding breast lobules
Term CCC is preferred
Encompasses spectrum of lesions with characteristic cytoarchitectural features
Definitions
CCC characterized by presence of columnar epithelial cells
Cells line dilated terminal ductal lobular units (TDLUs)
Cystic spaces frequently contain luminal secretions and flocculent material
Frequently associated with microcalcifications
Encountered with increasing frequency in breast needle core biopsies
Most frequently seen in biopsies performed for mammographic microcalcifications
ETIOLOGY/PATHOGENESIS
Genetic Changes
Molecular studies show genetic changes similar to those found in low-grade DCIS and invasive cancer
May represent nonobligate precursor lesion
Likely early lesion in low-grade breast neoplasia development
CLINICAL ISSUES
Site
Often a multifocal process that may be bilateral
Rarely will produce palpable abnormality
Reported association and coexistence with other more serious low-grade neoplastic processes
Atypical ductal hyperplasia, atypical lobular hyperplasia
Low-grade ductal carcinoma in situ, lobular carcinoma in situ
Invasive low-grade ductal carcinomas (tubular carcinoma), invasive lobular carcinoma
Presentation
Microcalcifications on screening mammography
Finding will prompt needle core biopsy
Treatment
Surgical approaches
FEA found on needle core biopsy
Surgical excision is recommended
Diagnosis is upgraded to more serious lesion in 20-30% of cases
CCC found on needle core biopsy (without atypia)
Most likely incidental finding as result of microcalcifications
Can be followed as long as there are no other worrisome clinical or mammographic findings
Prognosis
Follow-up studies suggest low risk of progression to invasive cancer
Need to exclude association with more serious lesion, such as low-grade DCIS or tubular carcinoma
IMAGE FINDINGS
Mammographic Findings
Presence of microcalcifications frequent finding
MACROSCOPIC FEATURES
General Features
Typically no gross findings in absence of other associated lesions
MICROSCOPIC PATHOLOGY
Histologic Features
CCC
TDLUs with variably dilated acini lined by 1 or 2 layers of columnar epithelial cells
Enlargement and cystic dilatation of TDLUs
Flat growth pattern by lining epithelial cells, may show some stratification
Cells are uniform with ovoid to elongated nuclei
Nuclei show polarity, oriented in regular fashion
Typically perpendicular to basement membrane
Evenly dispersed chromatin without conspicuous nucleoli or atypia
Mitotic figures rarely encountered
Apical cytoplasmic blebs or snouts often present at luminal surface of epithelial cells
Flocculent secretions typically present in lumina of involved acini
Luminal calcifications frequently present and may be prominent
Columnar cell hyperplasia (CCH)
Features similar to CCC
Epithelial lining cells show varying cellular stratification, > 2 cell layers
Nuclei ovoid to elongated and, for the most part, oriented perpendicular to basement membrane
Lack conspicuous nucleoli or atypia
Proliferating columnar cells form small mounds, tufts, or abortive micropapillations
Cellular tufts and mounds are broader at base than at tips
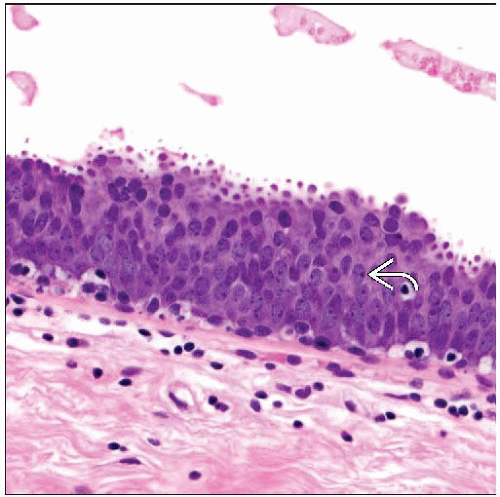
FEA
Similar architectural features as seen in CCC and CCH
Morphologic spectrum based on presence and degree of epithelial atypia
FEA represents columnar cell lesion with varying degrees of cytologic atypia
Epithelial cells demonstrate low-grade, monomorphic-type cytologic atypia
Typically show relatively round or ovoid enlarged nuclei
Increased nuclear to cytoplasmic ratio
Nuclei can show stratification
Loss of polarity
Loss of perpendicular orientation to basement membrane
Nuclear chromatin may be evenly dispersed or slightly marginated
Nucleoli may be present and variably prominent
Cytologic features similar to those seen in low-grade DCIS, lesions lack architectural changes
FEA may coexist with areas that fulfill diagnostic criteria for ADH or low-grade DCIS
Finding of FEA should prompt diligent search for such areas
May require careful examination of deeper levels &/or additional sections
ANCILLARY TESTS
Immunohistochemistry
Cells are strongly ER(+) throughout lesion
Luminal low molecular weight keratin 8/18 positive
High molecular weight keratin 5/6 negative
Array CGH
FEA is clonal proliferation
Genetic changes have been described, including losses of 16q
Changes similar to those seen in low-grade DCIS and invasive carcinoma
DIFFERENTIAL DIAGNOSIS
Apocrine Metaplasia
May show cystically dilated spaces and calcifications similar to CCC
Apocrine metaplasia shows low nuclear to cytoplasmic ratio, eosinophilic granular cytoplasm, and round nuclei with nucleoli
Flat growth pattern or micropapillary growth pattern
Atypical Ductal Hyperplasia/Low-Grade Ductal Carcinoma In Situ
Complex architectural patterns
Well-developed micropapillations
Rigid cellular bridges, bars, and arcades
“Punched-out” fenestrations
Complex architectural patterns should be considered either ADH or DCIS
FEA may coexist with ADH and DCIS
High-Grade Ductal Carcinoma In Situ, Flat or “Clinging” Pattern
High-grade cytologic atypia with marked nuclear pleomorphism is not a feature of FEA
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree