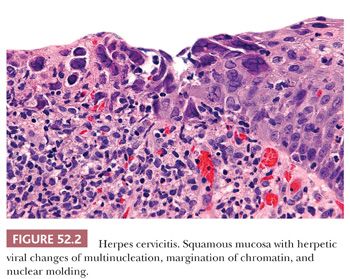
Infections. Herpes simplex virus (HSV) and Chlamydia trachomatis cause characteristic histologic findings, whereas Trichomonas vaginalis and Neisseria gonorrhoeae do not (2). HSV infections of the anogenital region are more commonly type 2 and although HSV more commonly involves the vulva or anal area, it can also affect the ectocervix and vagina where the majority present as superficial ulcerations that may appear necrotic (3). In rare cases, herpes infection can lead to the formation of a fungating, necrotic mass that clinically mimics malignancy. On histologic sections, mucosal cells infected with HSV can be identified at the edge of the ulcer and exhibit the characteristic nuclear changes of multinucleation, margination of chromatin, and nuclear molding (Fig. 52.2). The bed of the ulcer is notable for necrosis and an underlying chronic inflammatory infiltrate. In cases where characteristic nuclear inclusions are not identified, immunohistochemical stain for HSV can be helpful.
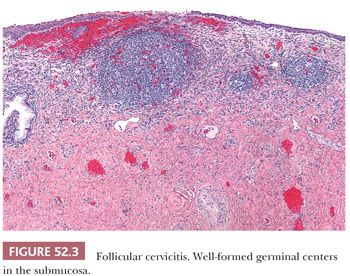
In contrast to HSV, infections with chlamydia, trichomonas, and gonorrhea are rarely associated with ulceration. Chlamydia infections are characterized by follicular cervicitis with well-formed germinal centers and a marked plasmacytic infiltrate (Fig. 52.3). However, because chlamydia and gonorrhea infections are often asymptomatic but can lead to pelvic inflammatory disease and subsequent infertility, testing for these infections has become a routine adjunct to cervical cytology screening.
Ligneous Cervicitis. Ligneous cervicitis is a very rare noninfectious form of pseudomembranous inflammation that is associated with plasminogen deficiency (4–6). This condition, which is more commonly associated with conjunctival pathology, is characterized by fibrin plaques with associated inflammation deposited in the submucosa. The overlying surface mucosa is often denuded or disrupted. The fibrin plaques may appear hyalinized and resemble amyloid. Since amyloid can also occur in the cervix as a localized process or in association with squamous cell carcinoma (7–9), trichrome and Congo red stains can be helpful in the distinction.
SQUAMOUS INTRAEPITHELIAL LESIONS
Human papillomavirus (HPV) DNA has been detected in virtually all cases of cervical dysplasia and carcinoma, and persistent infection with high-risk HPV is considered to be a necessary but not sufficient cause for the development of high-grade squamous dysplasia and the vast majority of invasive cervical carcinomas (10–13).
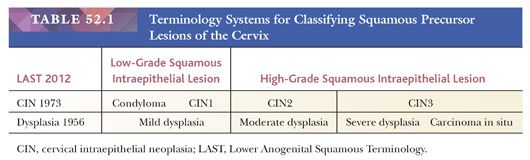
Historically, there have been different classification systems for classifying preneoplastic lesions of the cervix, which have shifted towards fewer, more clinically relevant categories (14–16) (Table 52.1). In 2012, the College of American Pathologists (CAP) and the American Society for Colposcopy and Cervical Pathology (ASCCP) sponsored a consensus conference to propose uniform terminology for HPV-related squamous intraepithelial lesions (SILs) and early invasive carcinoma (16). The Lower Anogenital Squamous Terminology (LAST) Project recommended a two-tiered nomenclature system—low-grade squamous intraepithelial lesion (LSIL) and high-grade squamous intraepithelial lesion (HSIL)—which can be further qualified by the grade of cervical intraepithelial neoplasia (CIN). The SIL terminology parallels the Bethesda System for Reporting Cervical Cytology, which has been in use since 1988 and was developed for providing uniform diagnostic terminology for cervical cytology specimens. The LSIL category encompasses condyloma and CIN grade 1, whereas the HSIL category encompasses CIN2 and CIN3. CIN1 is equivalent to mild dysplasia, CIN2 to moderate dysplasia, and CIN3 to severe dysplasia and carcinoma in situ. The LAST Project recommendation for standardized terminology applies across the anogenital tract for HPV-related preneoplastic squamous lesion.
Gross Pathology
Precursor lesions can only be visualized by colposcopic examination after application of 3% to 5% acetic acid to the cervix. LSIL lesions often appear white leading to the designation of an acetowhite lesion, whereas HSIL lesions tend to demonstrate changes such as punctation, mosaicism, and atypical vessels.
Low-Grade Squamous Intraepithelial Lesion (Condyloma, Cervical Intraepithelial Neoplasia Grade 1)
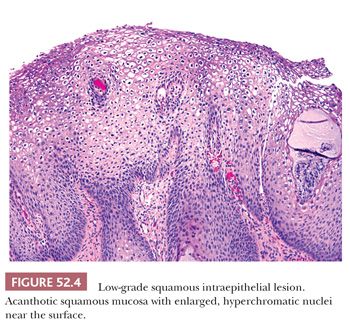
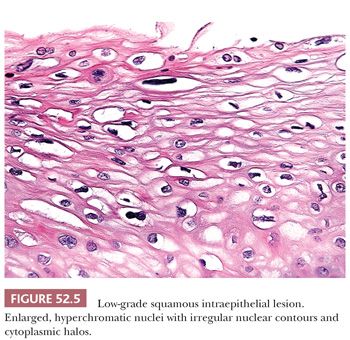
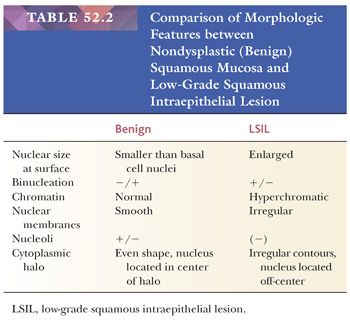
Histology. It is best to initially evaluate cervical biopsies on low scanning power (4× or 10×). On high power, everything looks atypical and it becomes easy to overdiagnose benign, reactive lesions as LSIL. LSIL should be visible from low power as an area with acanthosis and low nuclear-to-cytoplasmic (N:C) ratio cells with enlarged, hyperchromatic nuclei in the superficial mucosa (Fig. 52.4). There is usually adjacent nondysplastic mucosa that is helpful as a comparison for determining whether the mucosa is thickened and whether the nuclei are hyperchromatic. The nuclei of dysplastic cells will be darker than those of the uninvolved squamous mucosa. Normal squamous cell nuclei also typically become smaller as they mature and move towards the surface. In contrast, dysplastic nuclei are enlarged and are similar in size or larger than the basal cell nuclei. Binucleated cells are present in 90% of LSIL and when surrounded by a cytoplasmic halo are termed koilocytes. Koilocytic halos are sharply punched out and irregularly shaped with a variable distance from the edge of the halo to the edge of the nucleus (Fig. 52.5). Parakeratosis may also be present (Table 52.2).
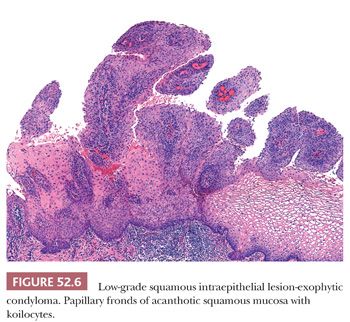
LSIL in the cervix is typically flat and occurs in the transformation zone; it is often associated with intermediate and high-risk HPV types. Exophytic condylomas, or condyloma acuminatum, are less frequent in the cervix and are more commonly found in the vulva; they are often associated with low-risk HPV types 6 and 11 (Fig. 52.6).
Diagnostic Reproducibility and Prognosis. The ASCUS–LSIL Triage Study (ALTS) on interobserver variability found poor reproducibility for the histologic diagnosis of LSIL with kappa statistics of 0.46 to 0.49 (17). A substantial proportion of LSIL cases are positive for high-risk HPV: 76.6% according to a meta-analysis drawing data based on Digene Hybrid Capture 2 (HC2) HPV testing from 62 studies (18). However, HPV-16 is less common in LSIL (CIN1) than HSIL (CIN3) (19). LSIL represents a heterogeneous group of lesions with a high rate of regression (70% to >90%) and uncommon progression to HSIL within the first 24 months (20).
Differential Diagnosis
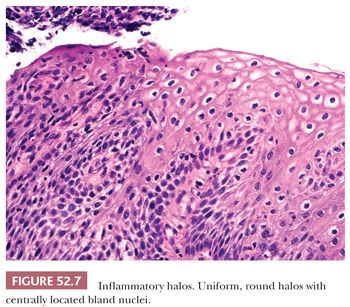
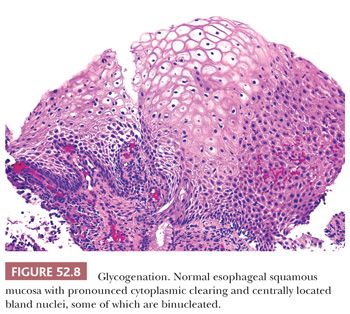
REACTIVE CHANGES AND GLYCOGENATION. Reactive, inflammatory changes and glycogenation can be associated with binucleation and cytoplasmic halos (Table 52.2). With reactive change, the halo is round, uniform and less distinct, and the nuclei are minimally enlarged and normochromic (Fig. 52.7). Similarly, glycogenation appears as uniform cytoplasmic clearing surrounding nuclei that lack atypia (Fig. 52.8). With both reactive change and glycogenation, the nuclei are more centrally located within the halo.
FIBROEPITHELIAL POLYP. Fibroepithelial polyps (also known as squamous papilloma or stromal polyp) rarely occur in the cervix and although uncommon are more typically found along the vaginal wall (21,22). The exophytic architecture can mimic LSIL (condyloma) but the absence of koilocytes and the central fibrovascular core are distinguishing features.
Management. According to the ASCCP consensus guidelines for management of women with a histologic diagnosis of LSIL (CIN1), management varies depending on the prior cytologic diagnosis (23). A large population-based study by Kaiser Permanente Northern California in collaboration with the National Cancer Institute reported a 3.8% 5-year risk of CIN3+ for women with LSIL on biopsy who had a prior Pap test showing HPV-positive atypical squamous cells of undetermined significance (ASCUS), LSIL, or atypical glandular cells (AGC). The 5-year risk increased to 7.8% for women with a prior Pap test diagnosis of atypical squamous cells cannot exclude HSIL (ASC-H) and to 15% for HSIL+ (24).
Cotesting with cytology and HPV is recommended at 1 year for women with a prior cytologic diagnosis of ASCUS or LSIL, or who are known to harbor HPV-16, HPV-18, or persistent untyped oncogenic HPV. Repeat endocervical sampling is also recommended at 12 months for women with LSIL (CIN1) previously detected on endocervical sampling. For LSIL (CIN1) that persists for at least 2 years, continued follow-up or treatment with excision or ablation is acceptable if colposcopy is adequate. Hysterectomy is unacceptable as the primary treatment for a histologic diagnosis of LSIL (CIN1).
Women with a prior cytologic diagnosis of ASC-H or HSIL and histologic LSIL can be managed conservatively with cotesting at 12 months and 24 months if colposcopy is adequate and endocervical sampling is negative, or with diagnostic excision. In these cases, it is helpful to review the prior Pap test and correlate with the histologic findings. If the cytologic diagnosis is revised based on cytologic–histologic correlation, the patient can be managed based on the revised interpretation. Additional level sections of the biopsy can also be helpful in detecting small foci of HSIL.
For women aged 21 to 24 years, histologic LSIL (CIN1) should be managed conservatively with repeat cytology only at 12 months after an ASCUS or LSIL cytology, or with colposcopy and cytology at 6-month intervals for 24 months after an ASC-H or HSIL cytology. Follow-up with HPV testing is not acceptable. Pregnant women should also be managed conservatively with follow-up and no treatment.
High-Grade Squamous Intraepithelial Lesion (Cervical Intraepithelial Neoplasia Grades 2 and 3)
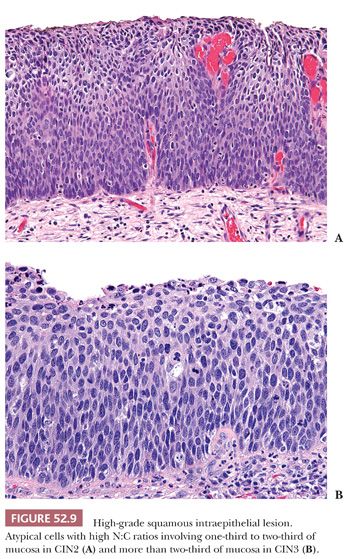
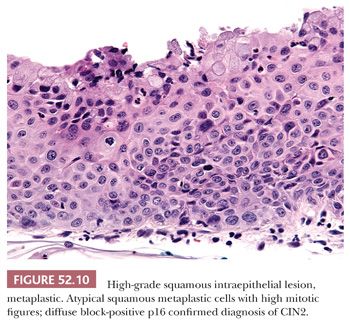
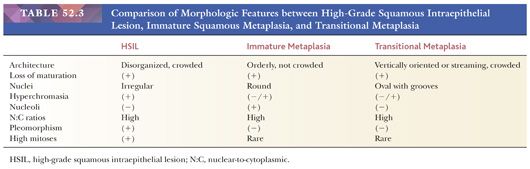
Histology. Classic HSIL is characterized by atypical cells with high N:C ratios (at least 1:1), irregular nuclear contours, and coarse chromatin. These abnormalities should involve one-thirds to two-thirds of the mucosal thickness in CIN2 (Fig. 52.9A) and more than two-thirds in CIN3 (Fig. 52.9B). The involved mucosa is notable for disorderly arrangement of cells with crowding and loss of polarity that is best seen along the basal layer. Mitotic figures are commonly found in the upper half of the mucosa. Nucleoli are typically absent but may be seen in rare cases of HSIL. HSIL can extend into and colonize endocervical glands, replacing benign endocervical mucosa. It can also undermine endocervical columnar mucosa, a feature that is generally considered a sign of benign squamous metaplasia, but it can be seen in a subset of HSIL that is associated with metaplastic changes. (Fig. 52.10) This type of HSIL can be difficult to distinguish from immature squamous metaplasia, and p16 immunohistochemistry can be helpful with block-positive p16 supporting a diagnosis of HSIL. (See “Molecular Markers.”) HSIL can also exhibit focal mucinous differentiation with scattered mucin-producing cells in the lower layers or near the epithelial surface, especially within endocervical gland crypts (25,26).
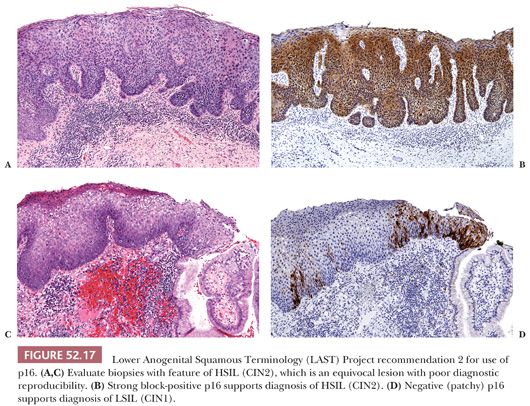
Diagnostic Reproducibility and Prognosis. The ALT study found good reproducibility for the histologic diagnosis of CIN3 with concordance in 72.8% of cases (27). However, the reproducibility between the clinical center pathologists and quality control pathologists for the diagnosis of CIN2 was significantly lower at 43.4%; a similar number of cases were downgraded or upgraded. A large population-based National Cancer Institute study of women from Guanacaste, Costa Rica also found the diagnosis of CIN2 to be significantly less reproducible than CIN3 (28). Two review pathologists agreed with 13% to 31% of CIN2 diagnoses versus 81% to 84% of CIN3 diagnoses. Recognizing that CIN2 is an equivocal diagnosis that encompasses both CIN1 and CIN3 as well as benign lesions, the CAP-ASCCP LAST Project recommends the use of p16 immunohistochemistry when considering a diagnosis of CIN2. (See “Molecular Markers.”)
Based on data from a retrospective cohort study from New Zealand where treatment for CIN3 was inappropriately withheld from a large number of women from 1965–1974, the incidence of invasive cervical cancer in women with untreated CIN3 is estimated to be 31.3% over the course of 30 years versus 0.7% for those with adequate treatment (29).
Differential Diagnosis
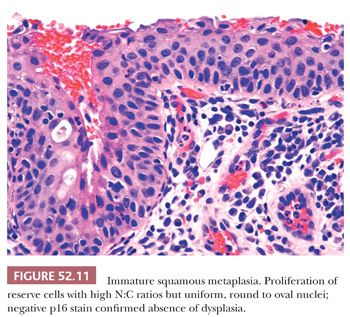
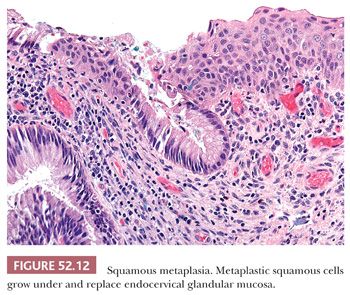
SQUAMOUS METAPLASIA. Immature squamous metaplasia represents a proliferation of reserve, basal-type cells that replace and undermine endocervical columnar mucosa. It occurs in the transformation zone (squamocolumnar junction) and can extend into endocervical gland necks. The cells are not crowded or disorganized. Although there is loss of maturation to the surface and the cells have high N:C ratios, the nuclei are uniform, bland, and round to oval in shape. Prominent nucleoli may be present and are a sign of reactive changes (Fig. 52.11). Mitotic activity may be present but no abnormal mitotic figures should be identified (Table 52.3). The presence of mucinous endocervical epithelium on the surface is a helpful indicator of benign squamous metaplasia but can also be seen in rare cases of HSIL (Fig. 52.12). The presence of hyperchromasia, nuclear pleomorphism, loss of polarity, and high or abnormal mitotic figures are atypical features that preclude a definitive diagnosis of benign squamous metaplasia. In the past, these types of lesions have been classified as atypical squamous metaplasia, but with the advent of biomarkers such as p16, immunohistochemical stains can be used to help determine whether these equivocal lesions are reactive or dysplastic. (See “Molecular Markers.”)
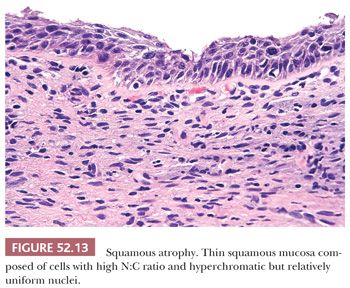
ATROPHY. Atrophy can be difficult to distinguish from thin HSIL as both can have cells with high N:C ratios and hyperchromatic nuclei. However, with atrophy, the nuclei should be relatively uniform with even chromatin and smooth nuclear contours (Fig. 52.13). Mitotic activity should be absent. In cases where the distinction cannot be confidently made based on histologic features alone, p16 and Ki-67 immunohistochemistry can be helpful. (See “Molecular Markers.”)
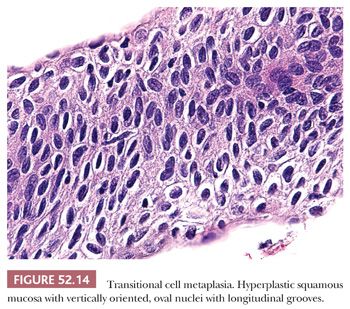
TRANSITIONAL METAPLASIA. Transitional metaplasia is associated with atrophy and most commonly seen in postmenopausal women but can also be found in premenopausal women. Similar to squamous metaplasia, transitional metaplasia occurs in the transformation zone and can extend into endocervical gland necks. The hyperplastic mucosa consists of basal and parabasal cells that morphologically resemble urothelium with vertically oriented, oval nuclei that often have longitudinal nuclear grooves (Fig. 52.14). Along the surface, the nuclei may have a horizontal orientation and streaming appearance, similar to umbrella cells. Perinuclear halos are common but in contrast to koilocytic halos, the halos are uniform and the nuclei are centrally located. The cells have high N:C ratios and lack maturation, but the oval nuclei are bland and uniform. Although the nuclei may be hyperchromatic, the chromatin is fine. Mitoses are rare (Table 52.3).
LOW-GRADE SQUAMOUS INTRAEPITHELIAL LESION. The distinction of LSIL from HSIL is based on the N:C ratios of the dysplastic cells and extent of mucosal involvement (Table 52.4). The nuclei in LSIL can be markedly enlarged and pleomorphic but have low N:C ratios, whereas the nuclei in HSIL are more uniform but with irregular nuclear contours and high N:C ratios. LSIL tends to involve the superficial layers of the mucosa, whereas high N:C ratio cells that extend upwards from the basal layer to at least one-third of the mucosal thickness characterize HSIL. Koilocytosis and parakeratosis can occur on the surface of HSIL so it is important to evaluate the lower part of the mucosa to determine if the changes represent HSIL or LSIL. In some cases for various reasons (e.g., tangential sectioning, small dissociated fragment, cautery artifact, etc.), a distinction cannot be made between LSIL and HSIL. These cases are best characterized as SIL of indeterminate grade.
Management. HSIL (CIN2) is the consensus threshold for treatment in the United States, except for young women. However, it is recognized that HSIL (CIN2) represents an equivocal diagnostic category that includes a mixture of LSIL (CIN1), HSIL (CIN3), and benign mimics. This is reflected in the higher regression rates for HSIL (CIN2) and lower rates of progression to cancer as compared with HSIL (CIN3).
In the general population, excision or ablation are both acceptable initial treatments for women with a histologic diagnosis of HSIL (CIN2 or CIN3) and adequate colposcopy (23). Ablation is unacceptable if colposcopy was inadequate. Hysterectomy is unacceptable as primary management of HSIL detected on biopsy. Given the significant risk for progression to invasive squamous cell carcinoma, observation with colposcopy and cytology is unacceptable except in young or pregnant women.
Because posttreatment management changes if HSIL (CIN2 or CIN3) is identified at margin or in a concurrent endocervical sample, it is essential that this information is clearly communicated in the pathology report. Immunohistochemical stain for p16 can be helpful in assessing margin status when the morphology is equivocal (CIN2 vs. CIN1) or when interpretation is hampered by cautery artifact. Women with positive excision margins can be managed with earlier follow-up with endocervical sampling at 4 to 6 months posttreatment (preferred) or repeat excision (acceptable) (23).
Conservative management with colposcopy and cytology at 6-month intervals for 12 months is preferred for young women with a histologic diagnosis of HSIL (CIN2) and satisfactory colposcopy, although treatment is also acceptable. If a distinction is not made between CIN2 and CIN3, it is acceptable to either treat or manage young women conservatively if colposcopy is adequate. Excision is recommended if the HSIL is classified as CIN3 (23).
Pregnant women can be followed with colposcopy and cytology at intervals of no more than every 12 weeks if the pregnancy is not advanced and there is no evidence of invasive disease. Postponing further evaluation until 6 weeks postpartum is also acceptable. Excision is unacceptable, unless invasive disease is suspected.
MOLECULAR MARKERS
Molecular markers for HPV have been increasingly used as an aid in the evaluation and diagnosis of cervical squamous dysplasias. Several different markers (e.g., p16, ProEx C, Ki-67, telomerase/TERC, cyclin E, HPV-16/-18, L1, etc.) and methodologies (i.e., immunohistochemistry, in situ hybridization, polymerase chain reaction [PCR]) are discussed in the literature but the quality of the studies varies widely. The LAST Project performed a comprehensive literature search and review of high-quality articles evaluating molecular markers (16). p16 was the only marker with sufficient data to allow full analysis of its utility in the evaluation of lower anogenital tract lesions. ProEx C and Ki-67 showed similar trending data to p16, but there was insufficient data for independent analysis. The data supports the use of p16 immunohistochemistry (IHC) in specific situations when evaluating squamous lesions of the anogenital tract. The evidence did not support the use of IHC markers in combination. Specifically, the addition of Ki-67 did not significantly improve performance over p16 IHC alone. Although use of Ki-67 and/or ProEx C IHC may be considered in cases where p16 IHC is inconclusive, this practice is not based on evidence. The term recommend was used if the recommendations are unlikely to change based on further evidence. Overall, the quality of the evidence supporting improved diagnostic reproducibility with hematoxylin and eosin (H&E)/p16 was rated as high and the evidence supporting improved sensitivity of H&E/p16 for the diagnosis of a precancerous lesion, high-moderate. The quality of the evidence for the test characteristics of H&E/p16 was rated as moderate to high.
p16 Immunohistochemistry
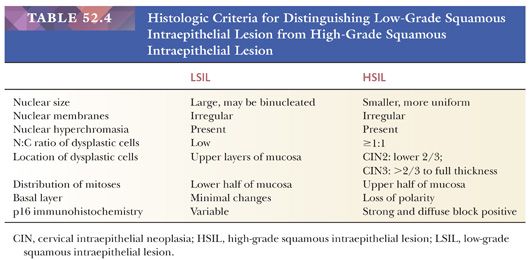
The presence of strong and diffuse block-positive p16 staining has been shown by many studies to correlate with the presence of high-risk HPV that has integrated into the host genome and supports a diagnosis of SIL (30–33). The CAP-ASCCP LAST Project defines a positive p16 stain as continuous strong nuclear or nuclear with cytoplasmic staining of the basal layer extending up at least one-third of the mucosal thickness (16) (Fig. 52.15A). Cytoplasmic only or patchy, focal staining should be interpreted as negative (Fig. 52.15B).
The CAP-ASCCP LAST Project recommends the use of p16 IHC in the following specific settings:
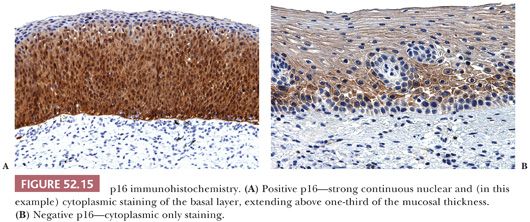
1. When the H&E morphologic differential diagnosis is between HSIL (CIN2 or CIN3) and a mimic such as immature squamous metaplasia, atrophy, reparative changes, or tangential sectioning. Strong and diffuse block-positive p16 results support a diagnosis of HSIL, whereas negative p16 results support a non-HPV–associated lesion (Fig. 52.16).
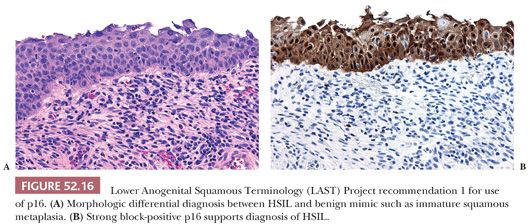
2. When the lesion shows morphologic features of HSIL (CIN2), which is a biologically equivocal lesion with poor diagnostic reproducibility. This recommendation is targeted at improving the diagnostic reproducibility and accuracy of a diagnosis of HSIL (CIN2). Strong and diffuse block-positive p16 results would support classification of the lesion as HSIL (CIN2), whereas negative or non–block-positive results strongly favor LSIL (CIN1) or a non–HPV-associated process such as immature squamous metaplasia (Fig. 52.17). However, p16 should not be used when the morphologic differential diagnosis is between LSIL (CIN1) and negative because not all cases of LSIL are associated with high-risk HPV. Overexpression of p16 is only seen when HPV has integrated into the host genome and this does not occur with low-risk HPV types. With LSIL, a negative p16 stain does not exclude the possibility of dysplasia.
3. As an objective adjudication tool when there is lack of consensus among pathologists in the diagnosis of HSIL (CIN2 or CIN3). The use of p16 substantially improves interobserver variability in the interpretation of HPV-related lower anogenital tract squamous lesions.
4. As an adjunct to H&E morphology in the special circumstance where a patient with a prior cytologic diagnosis of HSIL, ASC-H, AGC-not otherwise specified (NOS), or ASCUS/HPV-16+ has a biopsy where a precancerous lesion is not identified (Fig. 52.18). These women are at high-risk for missed high-grade disease, and p16 IHC can help to draw attention to small lesions. The p16-positive area must also meet morphologic criteria for a diagnosis of precancer before issuing a diagnosis of HSIL (CIN2 or CIN3). In a retrospective evaluation of negative cervical biopsies following high-risk Pap test results over a 7-year period at Stanford Hospital & Clinics, p16 detected missed foci of SIL where the patient was found to have HSIL on follow-up in ~3% of cases (34).
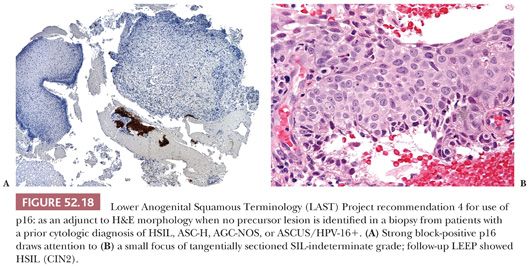
The LAST Project also specifically recommends against the routine use of p16 where the morphology is unequivocally negative or diagnostic of LSIL (CIN1) or HSIL (CIN3). This specific recommendation is to discourage overuse of p16 IHC and to minimize misinterpretation of cases based on p16 results. At this time, there is insufficient data in the literature to indicate that the natural history of p16-positive morphologically unequivocal LSIL (CIN1) differs from p16-negative LSIL (CIN1), although there is some evidence to suggest that it represents a higher risk lesion. p16 IHC is positive in the vast majority of HSIL (CIN3) (>99%), which argues against its utility in cases with morphologically unequivocal HSIL (CIN3). Because LSIL can exhibit block-positive p16 expression, grading of dysplasia should be based primarily on H&E morphology. Further studies will be required to address unanswered questions such as the natural history of p16-positive LSIL and whether these women should be managed differently than those with p16-negative LSIL, potential overutilization of p16 IHC and impact on patient management, and the utility of markers other than p16 IHC whether used singly or in combination.
ProEx C Immunohistochemistry
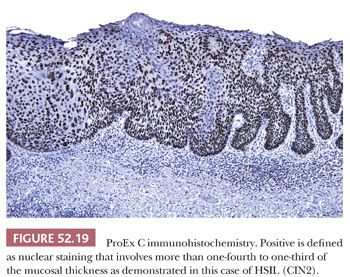
ProEx C is an assay composed of monoclonal antibodies directed against topoisomerase II-alpha and minichromosome maintenance protein-2. It detects aberrant S-phase induction, and a limited number of studies have shown that it performs similarly to p16 IHC (35,36). Interpretation of ProEx C relies on determining the extent of nuclear reactivity within the vertical layers of the mucosa because normal ectocervix is characterized by diffuse staining of one or two cell layers of the basal layer. The definition of positive ProEx C varies from greater than 25% to greater than one-third of the mucosal thickness (36–38). Extent of staining loosely correlates with grade of dysplasia; CIN3 typically shows full-thickness or near full-thickness staining of the mucosa (Fig. 52.19).
Ki-67 Immunohistochemistry
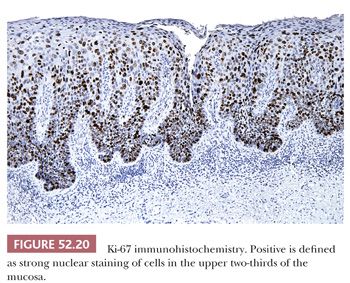
Various studies have advocated the use of immunohistochemical stain for the proliferation marker Ki-67 as an aid in the diagnosis of cervical dysplasia (31,35,39–42). Although there is general agreement that only strong nuclear staining of cells in the upper two-thirds of the mucosa should be interpreted as positive, some studies specify a requirement for a cluster of two or more positive cells (Fig. 52.20). Accurate interpretation of Ki-67 stain requires well-oriented specimens because tangential sectioning can make it difficult to determine the location of the positive cells. If used, Ki-67 is best as an adjunct to p16 because false-positive Ki-67 results can occur in the setting of benign reactive or reparative conditions (31,40,42).
Human Papillomavirus In Situ Hybridization
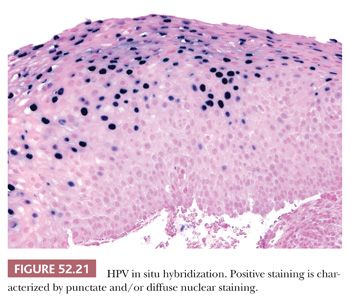
The advantage of HPV in situ hybridization (ISH) is high specificity and the ability to determine viral integration status. Nonintegrated virus appears as diffuse nuclear staining, whereas integrated virus exhibits punctate nuclear staining (Fig. 52.21). However, high viral load can cause integrated virus to appear as diffuse nuclear staining. The drawback of HPV ISH is the relatively low sensitivity of the assay in comparison with p16 IHC (30). It is also less widely available.
SQUAMOUS CELL CARCINOMA
In the United States, the incidence and mortality rates of cervical cancer have significantly decreased since the introduction of the Papanicolaou smear (Pap test, cervical cytology) as a screening test in 1941. However, it continues as the eighth most common cancer for women of Hispanic descent and the ninth for women of African American or American Indian descent (43). Worldwide, cervical carcinoma is the second most common cancer and the third leading cause of cancer-related deaths (44). The discrepancy in rates worldwide and within the United States is largely due to differences in availability of cervical cancer screening programs.
Squamous cell carcinomas comprise the majority of the cancers of the cervix (~80%) and are the type that has benefitted most from cervical cytology screening. Infection with high-risk types of HPV has been shown to be a necessary but not sufficient cause of cervical cancer (11). Although 13 HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66) have been identified as high-risk, HPV types 16 and 18 are considered the most carcinogenic and associated with the greatest risk of CIN3 or higher (19,45,46).
The median age for the diagnosis of cervical squamous cell carcinoma and adenocarcinoma is 48 years (47). Risk factors include increased number of sexual partners, earlier age of first intercourse, earlier age at first birth, and increase in duration of oral contraceptive use. Most of the risk factors are related to a woman’s chance of exposure to high-risk HPV types. Immunocompromised patients, such as those with HIV and transplant recipients, are also at increased risk for HPV-related dysplasia (48–50). Smoking appears to increase risk for squamous cell carcinoma only and not for adenocarcinoma (47). Although cervical cancer is frequently asymptomatic, the most common symptoms include abnormal vaginal bleeding, postcoital bleeding, and vaginal discharge.
Cervical squamous cell carcinomas can be subdivided into two main groups: superficially invasive carcinomas and invasive carcinomas. Historically, superficially invasive carcinoma has been referred to as microinvasive squamous cell carcinoma, early invasive carcinoma, or microcarcinoma. The term superficially invasive squamous cell carcinoma (SISCCA) is recommended by the CAP-ASCCP LAST Project for use across the lower anogenital tract for minimally invasive carcinomas that have been completely excised and are candidates for conservative management (16).
Gross Pathology
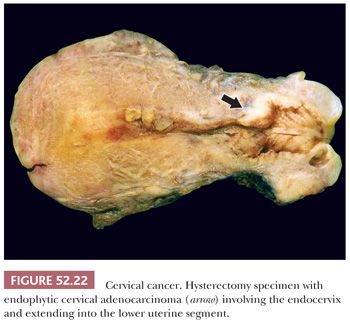
By definition, superficially invasive carcinomas are not grossly visible but may be visible by colposcopy after application of 3% to 5% acetic acid to the cervix. More advanced invasive carcinomas can be either exophytic, endophytic, or ulcerative (Fig. 52.22). On hysterectomy specimens, care should be taken to determine macroscopic tumor size, depth of invasion, and if present, involvement of vaginal, endometrial, and parametrial tissue.
Superficially Invasive Squamous Cell Carcinoma
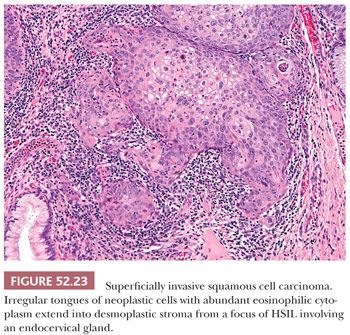
Histology. SISCCAs arise in the background of HSIL (CIN3) and can be recognized from low power as a break in the usual smooth contours of the basement membrane. Irregular tongues of epithelium with more abundant eosinophilic cytoplasm extend into the submucosa where they are associated with a chronic inflammatory response and loose, fibroblastic stroma (i.e., desmoplasia) (Fig. 52.23). Often at the point of invasion, the neoplastic cells become more differentiated and exhibit evidence of maturation with keratinization or keratin pearl formation (Fig. 52.23). HSIL features that are strongly associated with superficial invasion include extensive involvement of the surface mucosa, expansion of deep endocervical crypts, and intraepithelial squamous maturation or necrosis (51).
Diagnostic Criteria. The CAP-ASCCP LAST Project defines cervical SISCCA as an invasive carcinoma that meets four criteria: not grossly visible, 3.0 mm or less depth of invasion, 7.0 mm or less maximal horizontal extent, and completely excised. This corresponds to Federation Internationale de Gynecologie et d’Obstetrique (FIGO) stage IA1 or American Joint Commission on Cancer (AJCC) T1a1.
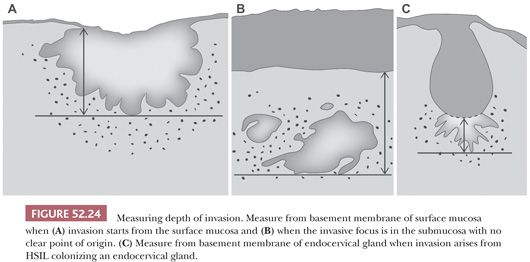
Measurement for depth of invasion should start at the basement membrane of the mucosa at the point of invasion. This can either be from the surface or from an endocervical gland (Fig. 52.24). In cases where the origin is indeterminate, it is best to measure from the basement membrane of the surface mucosa. Measurement of horizontal extent is simplest when the maximal extent is present within a single cross section. However, assessment of horizontal extent should take into account invasion that extends across multiple cross sections. In these cases, maximal horizontal extent can be estimated by multiplying the number of cross sections involved by the thickness of the tissue sections (usually 2 to 3 mm). If discontinuous foci are present, the LAST Project recommends reporting the number and size of each focus after excluding the possibility of a single lesion (16). The diagnosis of SISCCA is applicable if each focus meets size criteria and the lesion is completely excised.
Before defining a lesion as SISCCA, it is essential that the invasive component is completely excised. The presence of LSIL or HSIL at margin does not exclude a diagnosis of SISCCA but it does correlate with increased risk of persistent SIL and multifocal invasive squamous carcinoma (16). If invasive carcinoma is present at margin, the LAST Project recommends stating whether the invasive tumor exceeds size criteria for SISCCA, or if it meets size criteria, the lesion can be diagnosed as “at least a superficially invasive squamous carcinoma.”
The presence or absence of lymphatic–vascular invasion does not change the FIGO stage but may change management with conservative treatment reserved for patients without lymphatic–vascular invasion. Although lymphatic–vascular invasion is acknowledged as a poor prognostic factor, more recent studies have not found it to be an independent prognostic indicator (52,53).
Differential Diagnosis
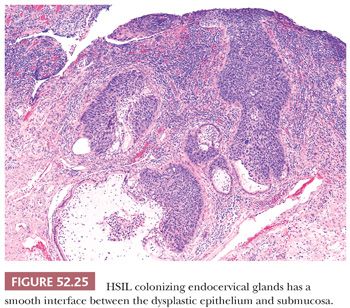
HIGH-GRADE SQUAMOUS INTRAEPITHELIAL LESION WITH ENDOCERVICAL GLAND EXTENSION. HSIL with extensive involvement of endocervical glands can be difficult to distinguish from SISCCA (Fig. 52.25). Retention of nuclear polarity, lack of confluent growth, and a smooth interface between the epithelium and submucosa supports endocervical gland extension of HSIL.
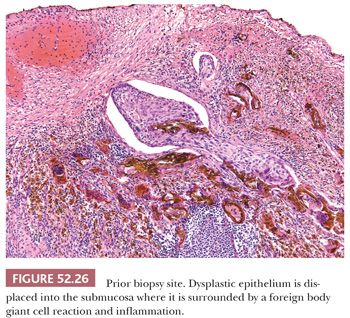
PRIOR BIOPSY SITE. Prior biopsy sites can exhibit changes such as displacement of epithelium into the submucosa or a jagged epithelial–stromal interface that can cause concern for superficial invasion (Fig. 52.26). In cases where the displaced or disrupted epithelium is clearly benign, it becomes relatively straightforward to recognize the changes as secondary to a prior biopsy. However, in cases where the epithelium is dysplastic, identifying fibrin around the displaced nests or granulation tissue at the epithelial–stromal interface would support prior biopsy site changes. Obtaining level sections can be helpful in difficult cases.
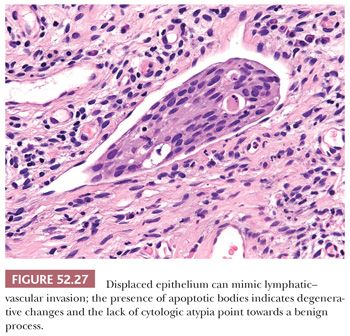
DISPLACED EPITHELIUM. Displacement of fragments of benign or dysplastic squamous epithelium into lymphatic–vascular spaces can also mimic lymphatic–vascular invasion (Fig. 52.27). The lack of cytologic atypia and degenerative changes are clues to this artifact. With excision specimens, sectioning of the gross specimen can also displace fragments of dysplastic or malignant epithelium into lymphatic–vascular spaces. This can be harder to discern because the fragments will appear abnormal. If the cluster within the space is free-floating and not associated with fibrin, it is best to avoid a definitive diagnosis of lymphatic–vascular space invasion. Immunohistochemical stains for lymphatic and vascular markers such as D2-40 and ERG can be helpful for confirming the presence of lymphatic–vascular space invasion.
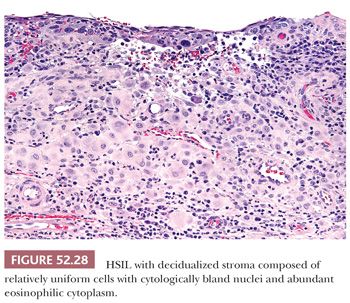
DECIDUALIZED STROMA. In pregnant patients, decidualization of the cervical stroma underlying HSIL can lead to a mistaken impression of invasion. The abundant eosinophilic cytoplasm of the decidualized stromal cells can mimic malignant squamous cells with keratinizing cytoplasm (Fig. 52.28).
Invasive Squamous Cell Carcinoma
Invasive squamous cell carcinomas of the cervix can be classified by grade and/or morphology. However, grade and type have not been found to be prognostically significant. Instead, depth of invasion, lymphatic or vascular invasion, and size are important prognostic variables.
Histology. Invasive squamous cell carcinomas are graded as well-differentiated (grade 1), moderately differentiated (grade 2), or poorly differentiated (grade 3). Well-differentiated squamous cell carcinomas are composed of cells with uniform nuclei, eosinophilic cytoplasm, and readily identifiable evidence of squamous differentiation such as keratin formation and intercellular bridges. Poorly differentiated carcinomas are composed of malignant cells with enlarged, hyperchromatic nuclei and high N:C ratios with little evidence of squamous differentiation. They may be difficult to distinguish from other types of poorly differentiated carcinomas (e.g., adenocarcinoma). Moderately differentiated carcinomas fall in between well- and poorly differentiated carcinomas by exhibiting some evidence of squamous differentiation.
The World Health Organization classifies invasive cervical squamous cell carcinomas into eight subtypes based on morphologic appearance: keratinizing, nonkeratinizing, basaloid, warty, papillary, lymphoepithelioma-like, squamotransitional cell, and verrucous (54). Except for verrucous carcinoma, all the subtypes are associated with high-risk HPV.
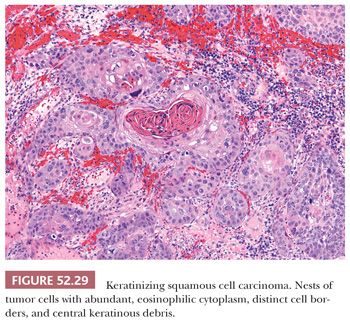
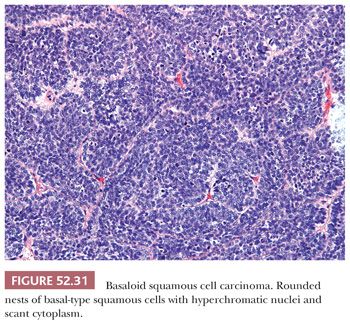
CONVENTIONAL SQUAMOUS CELL CARCINOMA. Conventional squamous cell carcinomas are keratinizing, nonkeratinizing, or basaloid. These subtypes reflect the histologic grade. Keratinizing squamous cell carcinomas are well-differentiated tumors characterized by keratin pearls (concentric whorls of squamous cells) and tumor cells with abundant, dense, eosinophilic cytoplasm surrounding central nests of keratin. Jagged nests of malignant cells and individual keratinizing cells invade into surrounding desmoplastic stroma (Fig. 52.29). In contrast, nonkeratinizing carcinomas lack keratin pearl formation and appear less well differentiated with higher N:C ratios, increased nuclear pleomorphism, and more frequent mitotic figures (Fig. 52.30). The cells continue to exhibit evidence of squamous differentiation with dense eosinophilic cytoplasm and intercellular bridges. Nonkeratinizing carcinomas are typically moderately differentiated. Basaloid squamous cell carcinomas are poorly differentiated with rounded nests of basal-type squamous cells with hyperchromatic nuclei, inconspicuous nucleoli, and scant cytoplasm (Fig. 52.31). Comedo-type necrosis or keratinization can also occur within the nests.
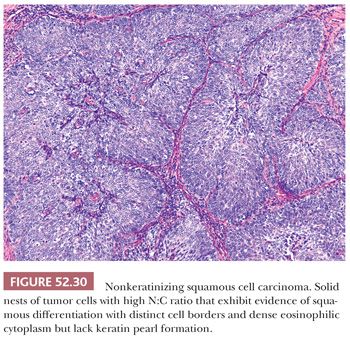
WARTY AND PAPILLARY SQUAMOUS CELL CARCINOMA. The warty and papillary subtypes are less common and refer to the architectural pattern of the invasive squamous cell carcinoma. Warty carcinomas superficially resemble a condyloma with koilocytes and hyperparakeratosis, whereas papillary carcinomas exhibit fibrovascular cores lined by cells resembling HSIL.
LYMPHOEPITHELIOMA-LIKE CARCINOMA. Lymphoepithelioma-like carcinoma resembles undifferentiated nasopharyngeal carcinoma and consists of syncytial nests of poorly differentiated epithelial cells with vesicular nuclei and prominent nucleoli in a background of an intense lymphocytic infiltrate. Although some studies have shown an association with HPV and no association with the Epstein-Barr virus (EBV), others from Asia have detected EBV in a significant subset of cases (55–57). A more recent study by Chao et al. (58) found low copy numbers of EBV DNA in 9/9 cases of lymphoepithelioma-like carcinoma and 7/25 squamous cell carcinoma (no special type); high-risk HPV was detected in 8/9 cases of lymphoepithelioma-like carcinoma and all cases of squamous cell carcinoma. Given the low copy numbers of EBV, it was postulated that the EBV was present in tumor-infiltrating lymphocytes and not in the tumor cells, arguing against an etiologic role for EBV.
SQUAMOTRANSITIONAL CELL CARCINOMA. Squamotransitional cell carcinoma is a rare variant that resembles transitional cell carcinoma of the urinary tract but is associated with high-risk HPV. A bladder primary needs to be excluded before making this diagnosis. Inverted transitional cell papilloma is a benign counterpart that can be distinguished from squamotransitional cell carcinoma by its small size, circumscription, and bland cytology (59).
VERRUCOUS CARCINOMA. Verrucous carcinoma is a rare special type of very well-differentiated squamous cell carcinoma that more commonly occurs in the vulva. The tumor is characterized by an exophytic warty growth that is mirrored by a well-circumscribed endophytic component. The exophytic component consists of hyperkeratotic, warty fronds of cytologically bland squamous epithelium (“church spires”). The interface with the underlying stroma consists of a pushing border with associated chronic inflammation. It lacks the jagged infiltration of conventional invasive squamous cell carcinomas. Verrucous carcinomas are slow-growing with minimal metastatic potential, although they can recur locally.
Differential Diagnosis
SQUAMOUS INTRAEPITHELIAL LESION. Superficial biopsies of the warty or papillary subtypes of squamous cell carcinoma can lead to a mistaken impression of preneoplastic process such as LSIL or HSIL. The warty subtype of squamous cell carcinoma resembles a condyloma on the surface with invasive squamous cell carcinoma apparent at the base of the lesion. Superficial biopsies can lead to an underdiagnosis of LSIL. If condylomatous changes are seen in a biopsy of a large mass and the junction with the submucosa cannot be evaluated, consideration should be given to the possibility of a warty squamous cell carcinoma. Similarly, consideration should be given to the papillary subtype if a biopsy shows what appears to be HSIL with papillary architecture because cervical SIL is typically flat and not exophytic.
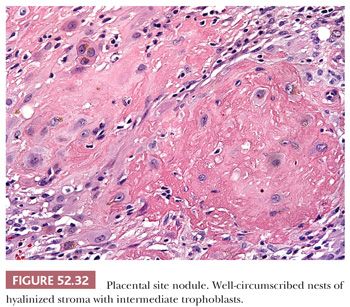
PLACENTAL SITE NODULE. Placental site nodules are benign remnants of placental implantation sites that are more common in the endometrium and myometrium but can also occur in the lower uterine segment and upper endocervix (60). Placental site nodules are well circumscribed and composed of intermediate trophoblasts with hyalinized intercellular stroma. The trophoblasts are large cells with hyperchromatic irregular nuclei, prominent nucleoli, and abundant eosinophilic to amphophilic cytoplasm (61) (Fig. 52.32). Mitotic figures are rare, and the Ki-67 proliferation rate should not exceed 10% to 15%. If the trophoblastic nature of the cells is not recognized, the cytologic atypia and eosinophilic cytoplasm can lead to a mistaken diagnosis of squamous cell carcinoma. Immunohistochemical stain for p16 can be helpful as placental site nodules exhibit no or focal weak staining, whereas most squamous cell carcinomas are diffusely positive (62). Placental site nodules are positive for inhibin-α (cytoplasmic), placental alkaline phosphatase, and human placental lactogen (focal), which can also be helpful because these stains are negative in squamous cell carcinoma (63).
EPITHELIOID TROPHOBLASTIC TUMOR. Epithelioid trophoblastic tumor represents the neoplastic form of placental site nodules. They similarly occur more commonly in the uterine corpus but can also occur in the cervix where they can mimic invasive squamous cell carcinoma. They grow as nodules of epithelioid tumor cells with central acellular, eosinophilic material that is often frankly necrotic but may also resemble keratin. Colonization of the overlying cervical mucosa can give the mistaken impression of squamous carcinoma in situ. Immunohistochemical stain for p16 is typically negative in epithelioid trophoblastic tumors in contrast with the diffuse block-positive staining seen with the majority of squamous cell carcinomas (62). Epithelioid trophoblastic tumors are focally positive for human placental lactogen and human chorionic gonadotropin (64).
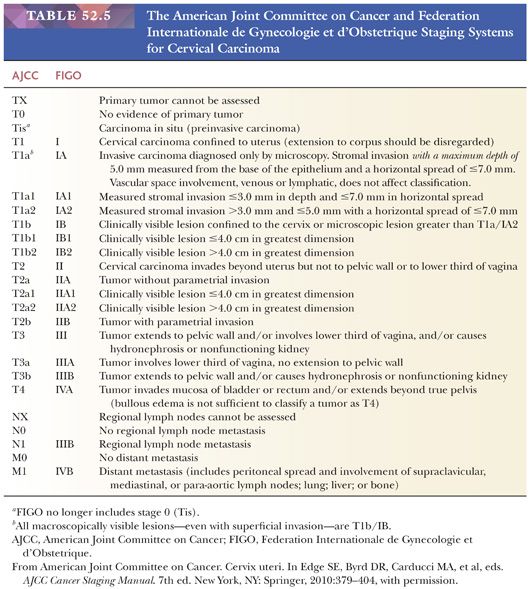
Tumor Staging. Cervical carcinomas are clinically staged according to the AJCC tumor-node-metastasis classification system and the FIGO system (65) (Table 52.5). Staging is based on physical examination, diagnostic procedures (e.g., colposcopy, hysteroscopy, cystoscopy, proctoscopy, intravenous urography), and radiologic studies. If grossly visible disease is present, the tumor is at least stage T1b, regardless of the pathologic findings. Depth of invasion and horizontal extent of tumor becomes important in cases with low-stage disease where the tumor is not grossly visible. The risk for lymph node involvement strongly correlates with stage. The obturator and iliac lymph nodes are most commonly involved by metastatic carcinoma (66–68). The pattern of drainage leads to spread to the pelvic sidewall lymph nodes followed by common iliac then para-aortic lymph nodes.
Management. The management of women with invasive squamous cell carcinoma depends on the FIGO stage at diagnosis (69). SISCCA (FIGO stage IA1) is associated with a negligible risk for lymph node metastasis (1% to 2%), risk of recurrence (0.9%), and risk of death (0.5%) (70,71). Consequently, it can be treated conservatively with cold knife conization or large loop excision in women who wish to preserve fertility, or with simple hysterectomy.
Because squamous cell carcinomas with a depth of invasion greater than 3 to less than or equal to 5 mm (FIGO stage IA2) are associated with a higher risk of lymph node metastasis (7.8%) and death (2.4%), local excision is considered inadequate, and these lesions are not classified as superficially invasive (16,70). Patients with FIGO stage IA2 tumors and above are often treated with modified radical hysterectomy and regional lymph node dissection. However, a subset of patients with FIGO stage IA2 and IB1 tumors can undergo fertility-preserving treatment with radical trachelectomy (72). Although this spares the uterus, the live birth rate for women after trachelectomy is often low (17%), and the rate of second trimester losses and preterm births can be high (72). FIGO stage IB to IIA tumors can be treated with radical hysterectomy or nonsurgically with radiation therapy. These two treatment options are equally effective and yield comparable 5-year survival rates. Late-stage FIGO IIB to IVB tumors are generally treated with radiation therapy and concurrent chemotherapy.
Prognosis. The overall 5-year survival for patients with squamous cell carcinoma is 70.5% (73). Although prognosis is excellent when detected early (FIGO stage IA1, IA2) with 5-year survival of 97.5%, patients presenting with distant metastasis (FIGO stage IVB) have a low 5-year survival rate of 9.3%. Tumor size and depth of tumor invasion are independent prognostic factors (74). In immunocompromised patients, cervical carcinoma is associated with a very aggressive course and poor outcome (75,76).
BENIGN ENDOCERVICAL GLANDULAR LESIONS
It is important to become familiar with the myriad of benign endocervical glandular lesions so that they are not mistaken for an endocervical adenocarcinoma. In general, these benign mimics are more common than invasive or in situ endocervical adenocarcinoma. Most are readily recognizable and the majority are incidental microscopic findings that rarely produce a mass lesion. However, these benign entities can cause diagnostic dilemmas, especially in small samples. It can be helpful to look for more typical areas that exhibit classic morphologic features of the benign glandular lesion. In especially challenging cases, immunohistochemical stains may be helpful, but it is important to clearly define the question and to fully understand the exceptions to the expected patterns of staining. Nonneoplastic endocervical glandular lesions can be broadly categorized as metaplasias or ectopias, “hyperplasias,” and reactive or inflammatory lesions.
METAPLASIAS AND ECTOPIAS
Tubal or Tuboendometrioid Metaplasia
The term tubal metaplasia is used when the glands exhibit overt tubal differentiation with terminal bars and cilia. However, the term tuboendometrioid metaplasia is preferred because there is often a mixture of tubal and endometrioid differentiation. Although ciliated endocervical cells are a normal finding at the junction of the cervix with the endometrium in the lower uterine segment, tuboendometrioid metaplasia occurs close to the transformation zone and is a common finding following biopsy, excision, or other procedure (77–79). Extensive tuboendometrioid metaplasia has also been described in association with in utero diethylstilbestrol (DES) exposure (80).
Tuboendometrioid metaplasia is typically composed of a mixture of cell types and can involve the surface glands or extend into deep endocervical glands. The cellular constituents consist of ciliated and nonciliated low columnar to cuboidal mucin-depleted epithelial cells. The nonciliated cells may have apical snouts or clear cytoplasm, resembling peg cells in the fallopian tube (Fig. 52.33). Although the nuclei are often bland and oval with minimal nuclear overlap, they can exhibit more pronounced cytologic atypia with nuclear hyperchromasia, elongation, and crowding. Mitotic figures are uncommon but can be prominent, especially when the glands exhibit endometrioid differentiation (Fig. 52.34A). The stroma surrounding the glands varies from edematous to hypercellular. When tuboendometrioid metaplasia occurs in deep endocervical glands, it can be associated with periglandular edema, which should not be mistaken for a desmoplastic reaction. The stroma may also become condensed and hypercellular, resembling endometrial-type stroma. The glands usually conform to normal endocervical glandular architecture, but tuboendometrioid metaplasia with a pseudoinfiltrative appearance has been reported in association with in utero exposure to DES (80).
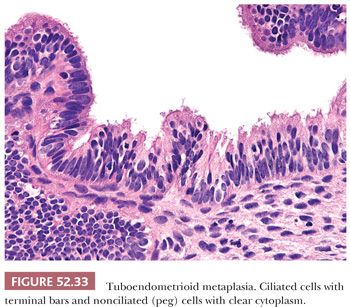
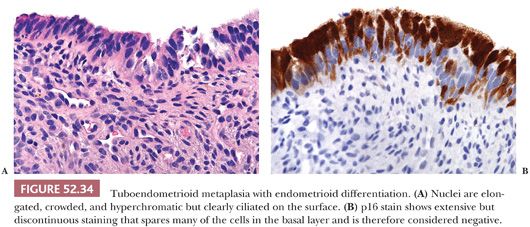
The challenge of tuboendometrioid metaplasia is distinguishing it from endocervical adenocarcinoma in situ. The presence of mitotic figures, nuclear atypia, nuclear stratification, and mucin depletion may raise concern for the possibility of malignancy. Intraepithelial lymphocytes with a surrounding halo can also mimic apoptotic bodies, and periglandular edema can be mistaken for desmoplasia. Features pointing to a benign metaplastic process include identification of cilia, mixture of cell types, few or absent mitotic figures and no abnormal forms, lack of apoptotic bodies, and absence of marked nuclear atypia. Although cilia is considered a hallmark of a benign process, a rare ciliated or tubal variant of endocervical adenocarcinoma in situ has been described (81). Immunohistochemical stain for p16 can be helpful in these cases (Fig. 52.34B). (See “Molecular Markers” later.)
Endometriosis
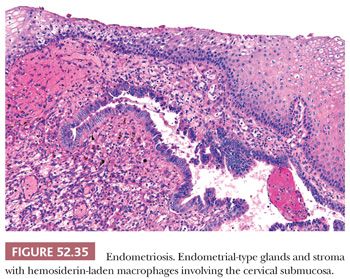
Endometriosis can occur on the mucosal surface of the surface or deep in the cervical stroma. Superficial endometriosis is usually not associated with pelvic endometriosis and occurs as part of a spectrum of tubal and tuboendometrioid metaplasia. In some cases, endometrial-type stroma without glands may be present. This is characterized by small superficial nodules or plaques of hypercellular stroma with prominent small vessels and extravasation of erythrocytes. In contrast, deep endometriosis is associated with pelvic endometriosis and occurs deep within the cervical stroma, often as an extension from the external surface of the cervix or paracervical connective tissue. The morphologic features are similar to endometriosis in other locations, that is, endometrial-type glands and stroma, and hemosiderin-laden macrophages (Fig. 52.35). Superficial endometriosis may be mistaken for endocervical adenocarcinoma in situ, whereas deep endometriosis can be confused with invasive adenocarcinoma. Although the glands are often pseudostratified and mitotically active, the nuclei are cytologically bland and relatively uniform. Identifying endometrial-type stroma and hemosiderin-laden macrophages can be especially helpful in establishing a diagnosis of endometriosis. Immunohistochemical stain for p16 can show extensive but noncontinuous reactivity. In contrast, endocervical adenocarcinoma should show continuous staining of virtually all the cells. PAX2 may be helpful with positive staining of endometriosis and lack of reactivity in endocervical adenocarcinoma (82). Ki-67 (MIB-1) is less useful as endometriosis can exhibit a high proliferation rate.
Endocervicosis
Endocervicosis is a rare condition that is characterized by a proliferation of bland endocervical glands occurring on the outer aspect of the cervix (usually anterior wall), vagina, bladder, peritoneum, and pelvic lymph nodes (83,84). It can be viewed as the mucinous counterpart of endometriosis and can be found in association with endometriosis and/or endosalpingiosis, which together are collectively known as müllerianosis (85). When it occurs in the cervix, it may be associated with a prior caesarean section and can present as a mass lesion. The glands are variably sized and lined by bland mucinous cells that exhibit minimal cytologic atypia. Mucin extravasation can cause a focal stromal reaction that should not be mistaken for a desmoplastic stromal response in the setting of invasion. Mitotic figures are rare or absent. Because endocervicosis involves the outer wall of the cervix and paracervical tissue, there should be a gap between the normal endocervical glands and the endocervicosis. Minimal deviation adenocarcinoma (adenoma malignum) is the main consideration in the differential diagnosis and can be distinguished by glands extending continuously from the mucosal surface into the deep aspect of the cervical wall. Perineural invasion has been described with endocervicosis (83), and although it is considered a benign lesion, a single vaginal case with malignant transformation has been reported (86).
Endosalpingiosis
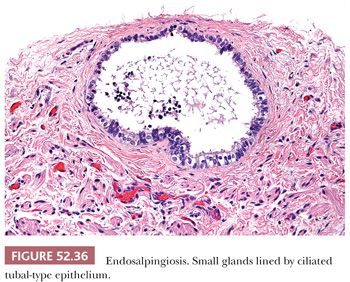
Endosalpingiosis is commonly seen as an incidental finding involving the pelvic and lower abdominal peritoneum, and pelvic and para-aortic lymph nodes. It can also occur in the cervix where it involves the paracervical soft tissue in contrast to tuboendometrioid metaplasia, which involves the superficial mucosal aspect of the cervix. Endosalpingiosis is characterized by small glands lined by ciliated tubal-type epithelial cells (Fig. 52.36). In rare instances, it can form a cystic mass with transmural involvement of the cervix and lower uterine segment, as well as involvement of the serosal surfaces of the uterus, adnexa, and intestine (87). In these cases of florid cystic endosalpingiosis, the glands vary markedly in size ranging from small to as large as 3 cm. It can be distinguished from minimal deviation adenocarcinoma by noting the presence of tubal-type epithelium with a mixture of cell types, that is, ciliated cells, nonciliated secretory cells with apical snouts, and scattered peg cells. There should be no mitotic activity and minimal nuclear atypia. However, growth around nerves mimicking perineural invasion can occur.
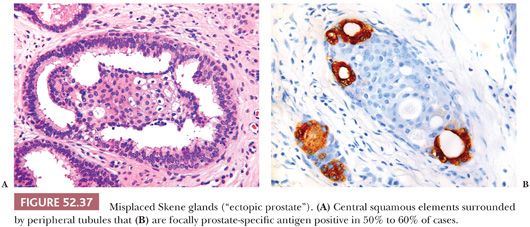
Misplaced Skene Glands (So-Called Ectopic Prostate)
Ectopic prostatic tissue is an uncommon incidental finding in the cervix that in rare cases can form a mass lesion (88–90). It is thought to represent a developmental anomaly that is derived from paraurethral Skene glands, which are the female equivalent of prostatic glands in men (90). Similar lesions also occur in the vagina. The ectopic prostatic tissue is typically located in the ectocervix (superficial or deep) but can occasionally involve the transformation zone. It consists of both glandular and squamous elements with tubules present around the periphery of the squamous elements (Fig. 52.37). In some cases, the glandular elements focally form a double cell layer. Cribriform and papillary patterns are common. Sebaceous glands, basaloid formations resembling hair follicles, and microglandular proliferations resembling nephrogenic adenoma are uncommon findings that can be seen in some cases of ectopic prostatic tissue. Focal prostate-specific antigen and prostatic acid phosphatase expression is identified in approximately 50% to 60% of cases tested (90).
Atypical Oxyphilic Metaplasia
Atypical oxyphilic metaplasia is a rare incidental finding characterized by superficial endocervical glands that are focally lined by large cuboidal or polygonal cells with abundant dense eosinophilic cytoplasm and variable nuclear atypia (91). The cytoplasmic appearance is the characteristic morphologic feature of this lesion. The nuclei are frequently multilobated or multinucleated but lack stratification or mitotic activity. The changes are not extensive, involving only a few glands or even a single gland. Because the features resemble radiation atypia, a prior history of pelvic radiation should be excluded.
ENDOCERVICAL GLANDULAR “HYPERPLASIAS”
Deep Endocervical Glands and Nabothian Cysts
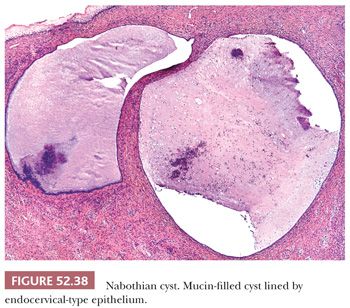
A nabothian cyst forms when squamous metaplasia replaces endocervical mucosa and occludes the endocervical gland opening on the surface of the cervix; the gland cystically dilates as a result of entrapped mucin (Fig. 52.38). Nabothian cysts and normal endocervical glands can occasionally be located deep within the cervical stroma and extend close to the paracervical connective tissues (92,93). The glands and cysts are lined by focally or extensively attenuated bland mucinous endocervical-type epithelium that can be occasionally ciliated. The lack of cytologic atypia and absence of a surrounding stromal reaction are further evidence of a benign process.
Microglandular Hyperplasia
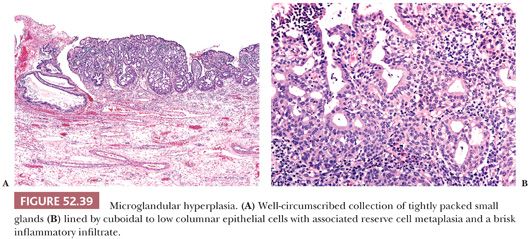
Microglandular hyperplasia commonly occurs in reproductive age women and was first reported in women taking oral contraception as a potential mimic of endocervical adenocarcinoma (94). However, it is not invariably associated with a history of hormone use or pregnancy and has also been reported in postmenopausal women (95,96). It is typically an incidental finding but can become florid and clinically present as an endocervical polyp or cervical erosion.
Morphologically, it is characterized by tightly packed small glands lined by flattened to cuboidal or low columnar epithelial cells with cytoplasmic vacuoles and a brisk inflammatory infiltrate, including neutrophils and plasma cells (Fig. 52.39). Associated reserve cells and squamous metaplasia is also commonly present and can exhibit maturation to small nests of squamous epithelium. The constituent cells are uniform with inconspicuous nucleoli, fine chromatin, and absent or rare mitotic activity. Atypical features such as signet ring cells, pseudoinfiltrative pattern of growth, myxoid stroma, hobnail cells, and mild to moderate nuclear atypia can lead to consideration of malignancy (97). When florid and present in small biopsy specimens, these features can result in an overdiagnosis of a microglandular variant of adenocarcinoma or clear cell carcinoma. In such cases, the presence of more typical areas of microglandular hyperplasia should point towards the correct diagnosis. Microglandular hyperplasia exhibits a low proliferation index and lacks extensive staining for p16, BCL2, and carcinoembryonic antigen (CEA) (98,99).
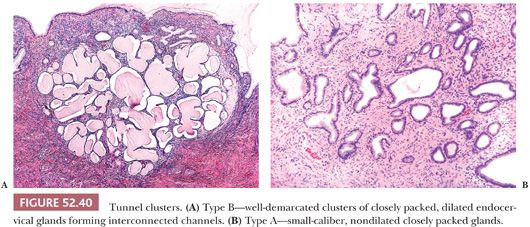
Tunnel Clusters
Tunnel clusters are a common incidental microscopic finding that can occasionally be seen on gross examination. In rare cases, they are associated with a mucoid vaginal discharge. Tunnel clusters occur in the superficial endocervix and on occasion can extend deep into the cervical stroma. Type B (cystic) tunnel clusters are more common than type A (noncystic), although there is some degree of overlap between the two types with some cases exhibiting a mixture of patterns. Type B tunnel clusters have a characteristic low-power appearance with well-demarcated clusters of closely packed, dilated endocervical glands that appear to be forming interconnected tunnels (100) (Fig. 52.40). The clusters may be multifocal. The glands are lined by low cuboidal to flattened epithelial cells with minimal intracytoplasmic mucin and little or no nuclear atypia or mitotic activity. Type A tunnel clusters are characterized by small-caliber, nondilated closely packed glands lined by cuboidal to tall columnar mucinous cells arranged around a central primary or secondary endocervical cleft (101) (Fig. 52.40). Most type A tunnel clusters are well-circumscribed but may occasionally exhibit a slightly irregular border with a pseudoinfiltrative appearance. Focal reactive nuclear atypia with enlargement and prominent nucleoli, as well as degenerative nuclear changes are commonly seen.
Lobular Endocervical Glandular Hyperplasia
Lobular endocervical glandular hyperplasia is an uncommon incidental finding that can rarely result in the formation of a mass lesion (102–104). It is a well-demarcated lesion that occurs in the inner half of the cervical wall and appears architecturally similar to the terminal duct lobular unit of the breast: large central duct surrounded by lobules of small- to medium-sized endocervical glands. The columnar mucinous epithelial cells are typically bland with basally located round to oval nuclei and pale eosinophilic cytoplasm (Fig. 52.41). The glands are surrounded by unremarkable cervical stroma. In rare cases, atypical features such as mild nuclear atypia, occasional mitotic activity, and focal intraglandular bridging or cribriform architecture may be present.
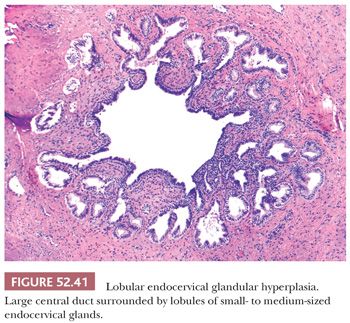
Lobular endocervical glandular hyperplasia, as well as type A tunnel clusters, contain neutral mucins and express HIK1083 and MUC6, markers of pyloric gland differentiation (105–107). It has been proposed as a precursor of cervical adenocarcinomas exhibiting gastric differentiation, such as mucinous variant of minimal deviation adenocarcinoma (108–110). Lobular endocervical glandular hyperplasia can usually be distinguished by the lack of a mass lesion, superficial location, circumscription, and absence of nuclear atypia. In rare cases where atypical features are present, immunohistochemical stains may be helpful. Based on a limited number of cases, lobular endocervical glandular hyperplasia has been shown to be negative for CEA and positive for PAX2, in contrast to minimal deviation adenocarcinoma, which exhibits the opposite phenotype (82,111). Estrogen receptor (ER) and smooth muscle actin (SMA) stains may also be helpful with ER expression in the periglandular stroma of lobular endocervical glandular hyperplasia and SMA stromal expression in minimal deviation adenocarcinoma (112).
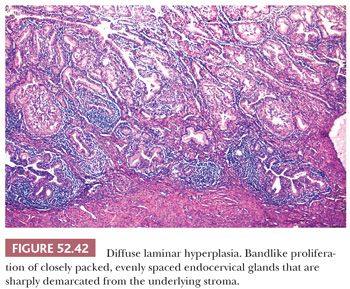
Diffuse Laminar Endocervical Glandular Hyperplasia
Diffuse laminar endocervical glandular hyperplasia is a rare incidental finding in premenopausal women (113). Although it is usually asymptomatic, it can be associated with copious watery or mucoid discharge. Morphologically, it is characterized by a bandlike proliferation of endocervical glands that is usually confined to the inner third of the cervix and sharply demarcated from the underlying stroma such that a straight line can almost be drawn under the lesion (Fig. 52.42). The small- to medium-sized endocervical glands are often closely packed and evenly spaced. The architecture varies from simple, round glands to branched or tufting within glands. Mild nuclear atypia and an inflammatory or edematous stromal reaction may be present. Features that are helpful in distinguishing diffuse laminar endocervical glandular hyperplasia from minimal deviation adenocarcinoma include the lack of a mass lesion, absence of marked atypia, and the sharp linear demarcation from the underlying cervical stroma. The latter feature may not be apparent in small, superficial biopsy specimens, and a larger excision may be necessary to exclude the possibility of malignancy.
Nonspecific Endocervical Hyperplasia
Hyperplastic endocervical glands that do not fit the criteria for tunnel clusters, lobular endocervical glandular hyperplasia, or diffuse laminar endocervical hyperplasia can be referred to as nonspecific endocervical hyperplasia. Similar to the other benign hyperplasias, these lesions are typically well-demarcated and cytologically bland.
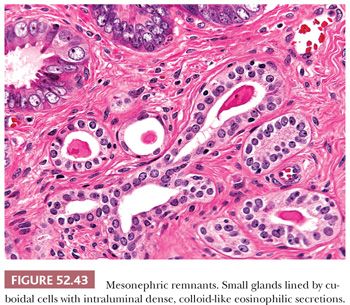
Mesonephric Remnants and Mesonephric Gland Hyperplasia
Mesonephric remnants are located deep within the stroma of the lateral walls of the cervix and can rarely be found in the vagina. On low power, they exhibit a linear growth pattern and occur parallel to the mucosal surface but deep to the normal endocervical glands. They consist of small glands lined by cuboidal cells with characteristic dense, colloid-like eosinophilic secretions within the lumen that are PAS-positive and diastase-resistant (Fig. 52.43). Architecturally, mesonephric glands range from elongated ducts with retiform, cystic, or angulated patterns of growth to clusters of small tubules arranged around central ducts. Mitotic activity is rare, and the cells are bland with minimal nuclear stratification or pleomorphism. Because the distinction between mesonephric remnants and mesonephric gland hyperplasia is somewhat arbitrary, a 6-mm-size cutoff has been proposed (114).
Hyperplastic mesonephric glands can exhibit a lobular, diffuse, or ductal pattern of growth and approach the mucosal surface by 1 mm where they are more likely to be sampled by punch biopsy (114,115). Lobular mesonephric hyperplasia consists of clusters of mesonephric glands that in some cases surround a central duct. With diffuse hyperplasia, the lack of clustering can lead to an infiltrative appearance, and angulated glands can appear to melt through the stroma. The uncommon ductal variant consists of somewhat linear ductlike structures without tubules, but small papillary invaginations may be present. In cases of florid mesonephric hyperplasia, mesonephric glands can extensively involve the wall of the cervix and intermix with normal endocervical glands (116). Rarely, transmural extension into the paracervical connective tissues may be seen. These cases can be very difficult to distinguish from mesonephric adenocarcinoma. However, mesonephric adenocarcinoma usually presents as a mass lesion, whereas mesonephric hyperplasia is typically an incidental finding. It is also helpful to identify foci of cytologically malignant cells, destructive stromal invasion, or lymphovascular invasion to support a diagnosis of malignancy.
Distinction of mesonephric remnants/hyperplasia from other endocervical glandular lesions is chiefly based on morphology, especially the identification of the characteristic intraluminal dense PAS-positive secretions. In challenging cases, immunohistochemical stains may be helpful, but it is important to clearly delineate the question being asked. CD10, epithelial membrane antigen, vimentin, and calretinin are consistently positive, whereas CEA and ER are typically negative (117). Luminal expression of CD10 is characteristic of mesonephric glands but the staining is often focal and can be negative (118,119). It can be used to distinguish mesonephric remnants/hyperplasia from other benign endocervical glandular lesions, which are typically negative for CD10. However, because endocervical adenocarcinoma (invasive and in situ) and endometrial adenocarcinoma can also express CD10, it cannot be used to support mesonephric origin in malignant cases or in cases where the differential diagnosis is between florid mesonephric hyperplasia and endocervical adenocarcinoma. In the latter case, PAX2 may be helpful because it is strongly positive in mesonephric remnants/hyperplasia but typically negative in endocervical adenocarcinomas (82). Mesonephric remnants/hyperplasia can also express BCL2, PAX8, p16 (focal), and TTF-1 (82,117,120).
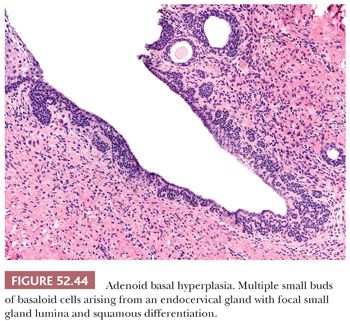
Adenoid Basal Hyperplasia
Adenoid basal hyperplasia occurs most commonly in postmenopausal women and is an uncommon incidental finding usually seen in hysterectomy specimens. It is characterized by multiple small buds of basaloid cells arising from the overlying normal squamous or endocervical mucosa or occurring as detached nests within the cervical stroma (121). The cells exhibit minimal nuclear atypia and no mitotic activity or stromal reaction. Focal squamous differentiation or small gland lumina may be seen (Fig. 52.44). Evaluation of the entire lesion and thorough sectioning of excision or hysterectomy specimens is required to exclude the presence of a more severe lesion before making a diagnosis of adenoid basal hyperplasia. The main entity to consider in the differential diagnosis is adenoid basal carcinoma from which it can be distinguished by its small size, superficial location, and continuity with the overlying epithelium. Deep stromal involvement and destructive stromal invasion are considered features of adenoid basal carcinoma. However, because metastasis is rare in cases of adenoid basal carcinoma that lack destructive stromal invasion, the term adenoid basal epithelioma has been proposed to reflect the clinically benign course of these lesions (121). The presence of concomitant HSIL would favor adenoid basal carcinoma because premalignant squamous lesions are usually not seen in association with adenoid basal hyperplasia. In practice, the distinction between adenoid basal hyperplasia and adenoid basal carcinoma can be difficult and somewhat arbitrary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


