•Transmembrane proteins
•Have one or more portions that span the phospholipid bilayer
•This group includes receptor molecules linked to intracellular enzymes, transport molecules, and ion channels
•Most are linked to the bilayer through covalent bonds
•While the majority of transmembrane proteins are in an α-helical configuration, porins are transmembrane proteins composed of antiparallel β-strands arranged in a barrel shape
•Specific transmembrane proteins provide hydrophilic paths for ions involved in electrical signaling, primarily Na+, K+, Ca2+, and Cl−
•The amino acid sequence in specific regions of these proteins determines the selectivity for specific ions
•Peripheral proteins
•Do not interact with the hydrophobic core of the bilayer; instead, they are usually bound to the membrane indirectly by interactions with other transmembrane proteins or directly with lipid polar head groups
•Examples include proteins that link the cytoskeleton to membrane junctions (e.g., actin, protein kinase C)
•Interactions take place through non-covalent bonds
Cell Transport and Electrolyte Concentrations
•There are two basic types of cell transport: diffusion and active transport
•Diffusion is driven by concentration gradients (does not require energy in the form of ATP)
•Simple diffusion: Usually hydrophobic substances such as oxygen, carbon dioxide, and urea
•Facilitated diffusion: Carrier-mediated, thus limited by the number of carrier proteins
•Active transport requires ATP
•There are three major types of transport proteins
•ATP-powered pump are ATPases that use the energy of ATP hydrolysis to move ions across a membrane against their electrochemical gradient
•The Na+/K+-ATPase pumps 3 Na+ ions out and 2 K+ ions into the cell per ATP hydrolyzed
•The Ca2+-ATPase pumps 2 Ca2+ ions out of the cell (or into the sarcoplasmic reticulum in muscle cells) per ATP hydrolyzed
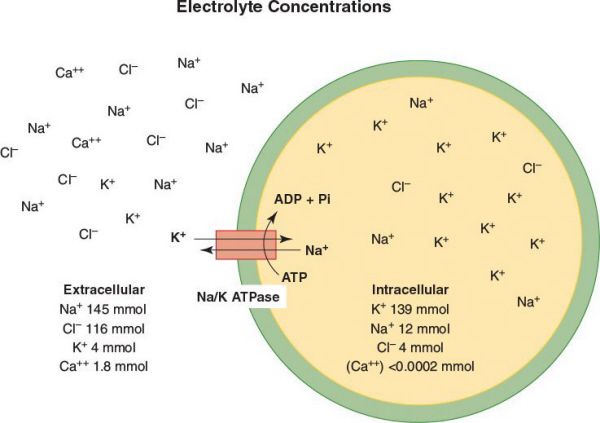
•These pumps create an intracellular ion milieu of high K+, low Na+, and low Ca2+ that is very different from the extracellular fluid milieu of low K+, high Na+, and high Ca2+
•Ion channels: Catalyze movement of specific ions down their electrochemical gradient
•An example is the potassium channel which allows K+ to move across the membrane down its concentration gradient
•While the Na+/K+-ATPase pumps K+ ions into the cytosol from the extracellular medium and thus generates the K+ concentration gradient, it is the movement of K+ ions down their concentration gradient from the cytosol outward through the “resting K+ channels” that generates the negative membrane potential of about −70 Mv
•Transporters: Facilitate movement of specific small molecules or ions, such as glucose, amino acids, Na+, and H+
•Uniporters transport a single type of molecule down a concentration gradient
•Symporters transport one molecule against a concentration gradient, driven by movement of one or more ions down an electrochemical gradient in the same direction
•Antiporters transport one molecule against a concentration gradient, driven by movement of one or more ions down an electrochemical gradient in the opposite direction
An 80-year-old man is rushed to the emergency room as a trauma patient after falling down a flight of stairs. On arrival, his EKG demonstrates cardiac bigeminy with normal sinus complexes followed by wide complexes. He also has nausea, vomiting, and is disoriented. The paramedics in the field report that he has a history of diabetes, hypertension, and congestive heart failure. On arrival, the nurse asks you which lab tests to order.
In addition to head trauma, one must consider digitalis toxicity in this patient. Thus, in addition to obtaining a full electrolyte panel, it may be helpful to send a digoxin level.
Digoxin Toxicity
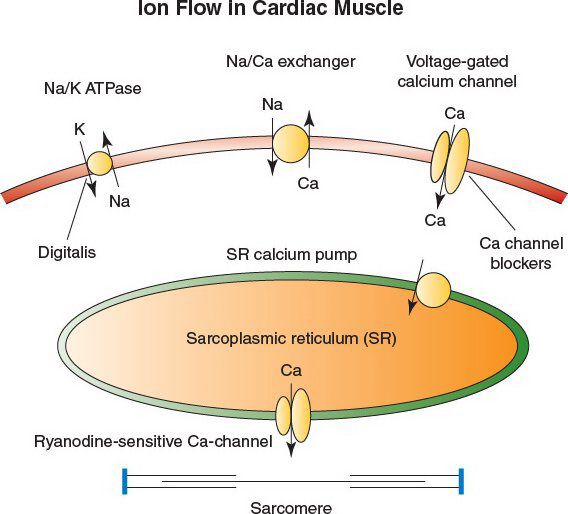
•The cardiac glycosides digoxin and digitoxin inhibit the Na/K ATPase, known as the “sodium pump”
•Inhibition of the pump leads to positive inotropy by (1) increased intracellular sodium and (2) reduced calcium expulsion through a calcium-sodium exchanger
•The increase in free calcium leads to greater contractility in the cardiac sarcomere
•Digoxin is toxic at levels greater than 2 ng/mL, and toxicity is usually manageable by withholding further drug administration
•Signs and symptoms of digitalis toxicity include visual and GI disturbances, and cardiac arrhythmias such as premature ventricular contractions (PVCs) and bigeminy
•For these patients, electrolytes, especially potassium, calcium, and magnesium, must be closely monitored along with withdrawal of glycoside therapy
•For treatment of more serious arrhythmias, lidocaine is preferred
•Severe digitalis intoxication is usually accompanied by hyperkalemia and depressed automaticity
•The administration of antiarrhythmics such as quinidine and procainamide are contraindicated in digoxin toxicity, but short-acting beta-adrenergic blockers such as esmolol can be considered for supraventricular tachyarrhythmias
•Calcium channel blockers are contraindicated as they inhibit renal excretion of digoxin
•Likewise, cardioversion should be reserved for ventricular fibrillation because glycoside-induced arrhythmias can be made worse
•Administration of digitalis-specific antibodies (DSFab; commercially: Digibind or DigiFab) is first line therapy for cardiac manifestations of digoxin toxicity
•Lidocaine and phenytoin are preferred for ventricular tachycardia and/or fibrillation
•Insertion of a temporary pacemaker catheter is viable for severe digitalis toxicity, but should be carefully considered as the threshold for ventricular arrhythmias may be lowered
You see a 56-year-old woman in clinic after a right hemicolectomy in which 2 of 17 lymph nodes are positive for colonic adenocarcinoma. You tell her that she needs chemotherapy and that 5-fluorouracil will likely be one of the agents used to treat her. What is the mechanism of action of 5-fluorouracil?
A metabolite of 5-fluorouracil (5-FU) called 5-fluoro-2′-deoxyuridine-5′-phosphate (FdUMP) complexes with thymidylate synthase, an enzyme required for the synthesis of thymine nucleotides. This results in inhibition of DNA synthesis. 5-FU also interferes with RNA processing and function. Thus, cytotoxicity of 5-FU is based on both DNA and RNA effects. Its main side effects are myelosuppression and mucositis. It is a cell cycle-dependent anticancer drug.
Which is the most variable phase of the cell cycle?
G1 is a part of the cell cycle in interphase, and it is the most variable phase.
The Cell Cycle
•The cell cycle is composed of a long interphase (G1, S, G2) and a short mitosis (M) phase
•Interphase:
•G1 phase prepares cell for entry into S phase, or the cell enters G0 if cellular conditions are not right
•G0 may be irreversible (terminal differentiation) or reversible
•The restriction point, R, is a point in G1 after which the cell is committed to DNA synthesis
•S phase: DNA synthesis occurs and two sister chromosomes are generated
•G2 phase: a quiescent phase in which the cell prepares for entry into mitosis
•RNA and protein synthesis occur during both the G1 and G2 phases
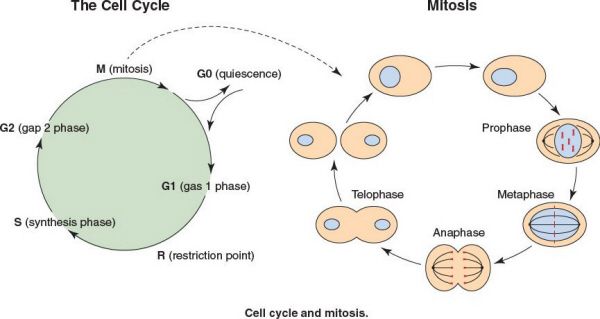
•Mitosis: Cell division
•Prophase
•The chromosomes shorten, the nucleolus disappears, the nuclear envelope disappears, and the spindle apparatus forms
•The centrioles replicate and move toward opposite ends of cell
•The shortened chromosomes, known as identical chromatids, are held together by the centromere
•Metaphase
•The chromosomes move toward the center of the cell and the centromeres align at the equatorial plate
•The centromeres attach to spindle fibers from the spindle apparatus
•The centromeres of each chromatid duplicate
•Anaphase
•The chromatids migrate to opposite poles
•The chromatids are now referred to as chromosomes
•Telophase
•The chromosomes decondense
•Each cell forms a nucleolus and nuclear envelope
The M phase of the cell cycle is most susceptible to radiation therapy.
A 46-year-old woman has blood streaked bowel movements. Her mother and two siblings have had colon cancer, all before 50 years of age. A colonoscopy 7 years prior was negative for tumor or polyps. Which hereditary trait does this patient most likely have?
Lynch I syndrome is one of the two main types of hereditary nonpolyposis colorectal carcinoma (HNPCC). HNPCC families carry mutations in the genes responsible for a mismatch repair. This leads to the accumulation of mutations and microsatellite instability, both of which contribute to the rapid progression of tumors in HNPCC.
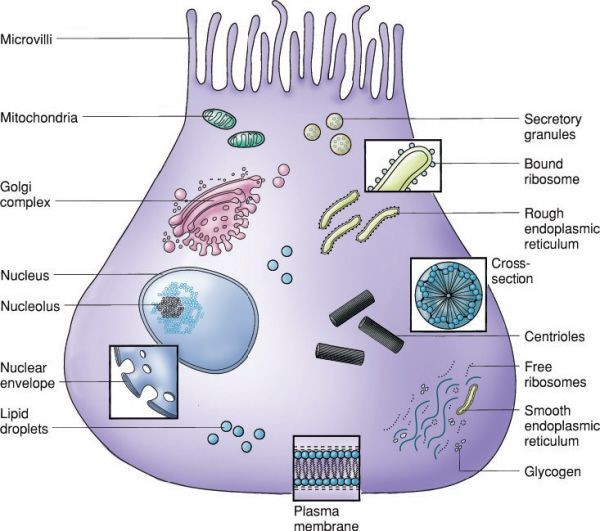
Typical epithelial cell. (With permission from Mulholland MW, Lillemoe KD, Doherty GM, Maier RV, Upchurch GR, eds. Greenfield’s Surgery. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.)
Components of the Nucleus
•Nuclear envelope
•Composed of a double-membrane
•The outer membrane of the nuclear envelope sometimes has ribosomes attached that may be continuous with the endoplasmic reticulum
•Traffic in and out of the nucleus is regulated by pore complexes composed of a cylindrical structure called the annulus, which forms the rim, and an inner central granule
•Nucleolus
•Primary function of the nucleolus is the generation of ribosomes, composed of proteins and ribosomal RNA
•The fibrillar zone is where ribosomal RNA is transcribed
•The granular zone has sites where the ribosomal RNA is condensed with ribosomes and proteins in order to form pre-ribosomal subunits
•The subunits are transported to the cytoplasm, where they are assembled into free ribosomes or membrane-bound ribosomes, which become attached to the rough endoplasmic reticulum involved in the protein synthesis
•Ribosomes are composed of 50S and 30S subunits
•Chromatin: DNA and protein complex
•Matrix: Involved in replication, transcription, and posttranscriptional processing and transport
•DNA replication and transcription take place in the nucleus
•Translation and posttranslational processing occur in the rough endoplasmic reticulum, Golgi complex, or free ribosomes in the cytoplasm
A 15-year-old woman develops a urinary tract infection and is given a course of ciprofloxacin. Where does this medication have its action?
Ciprofloxacin inhibits DNA gyrase, which is involved in DNA replication.
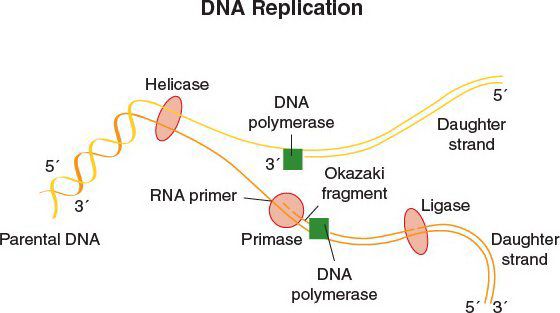
DNA Replication
•DNA replication begins at the origin of replication
•DNA gyrase, a topoisomerase, relieves supercoiling in DNA by creating a transient break in the double helix
•The leading strand provides the backbone for continuous DNA synthesis
•The lagging strand provides for discontinuous, Okazaki fragment production
•DNA polymerase synthesizes DNA in the 5′→3′ direction of both strands
•DNA ligase seals the Okazaki fragments
•Transcription of messenger RNA from DNA occurs by assembly of complementary base pairs on the DNA template one nucleotide at a time, which is catalyzed by RNA polymerase
•Still in the nucleus, the initial transcript then undergoes posttranscriptional processing
•This consists of capping of the 5′ end and polyadenylation of the 3′ end
•The product is a stretch of RNA with both introns (noncoding segments) and exons (which contain the coding for proteins)
•Introns are removed from the RNA transcript by splicing
•The resulting messenger RNA is moved to the cytoplasm where it binds to ribosomes to begin translation
Which amino acids are essential for a postoperative, critically ill patient who has not eaten for 1 week?
The essential amino acids are so named because humans cannot synthesize them and they must be provided in the diet. They are arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
Translation
•Messenger RNA (mRNA) is transcribed from DNA and is exported to the ribosomes, either in cytosol or in the endoplasmic reticulum (ER)
•The ribosomes begin the process of translation, in which the mRNA acts as a template for the production of proteins from amino acids
(With permission from Mulholland MW, Lillemoe KD, Doherty GM, Maier RV, Upchurch GR, eds. Greenfield’s Surgery. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.)
The Genetic Code
•Amino acids are synthesized and attached to transfer RNAs (tRNA) which pair specific amino acids with specific mRNA sequences
•Sequences occur in triplets called codons
•Any given string of ribonucleotides, therefore, gives rise to three reading frames which specify how the codons are “read”
•The codon AUG is an initiation codon, and it encodes the amino acid methionine.
•Codons UAA, UAG, and UGA are stop codons
•An mRNA sequence with 50 or more codons without a stop codon is known as an open reading frame
•The three ribonucleotide sequence on the tRNA that recognizes and binds to the codon on the mRNA is known as the anticodon
•This binding occurs by the formation of hydrogen bonds between complementary nucleotides
•The process of coupling amino acids with appropriate tRNAs occurs by a process known as activation of amino acids
•Aminoacyl-tRNA synthetases couple tRNAs with amino acids in an ATP-dependent enzymatic process that is catalyzed by magnesium
On postoperative day 7 after undergoing a low anterior colon resection, a 63-year-old male tests positive for vancomycin-resistant enterococcus (VRE) upon surveillance culture of his stool. He is started on oral linezolid. What is the mechanism of action of this medication?
Linezolid inhibits the 50S ribosomal subunit involved in protein synthesis.
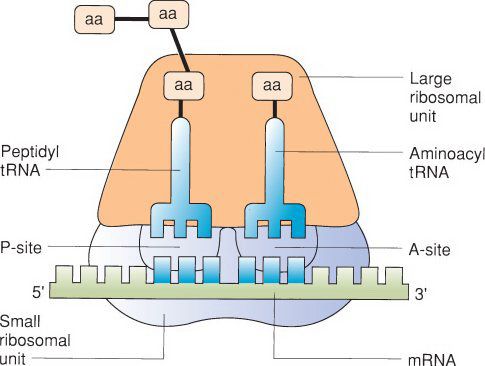
The Ribosome
•In the next stage of translation, initiation occurs when a ribosomal subunit binds to the mRNA
•Ribosomes are generally composed of 60S and 40S subunits
•The first subunit bound to mRNA is the smaller 40S subunit
•This is followed by binding of the tRNA corresponding to the initiation codon AUG, which encodes the amino acid methionine
•The 60S subunit then binds the mRNA to begin the process of polypeptide synthesis
•These steps require energy, in the form of GTP, and the assistance of proteins known as initiation factors
•Of note, the corresponding prokaryotic ribosomal components are 50S and 30S, which are the targets of some antibiotics
•The initial methionine amino acid begins the polypeptide
•Polypeptide synthesis progresses in the N to C-terminal direction when referring to amino acid orientation
•The subsequent elongation phase consists of the sequential recruitment of activated amino acids to the ribosome-mRNA complex and the formation or peptide bonds, a process that is catalyzed by ribosomal RNA, or a ribozyme
•This process also requires GTP and protein elongation factors
•Translation is terminated when the complex encounters one of the three stop codons
•Protein release factors assist in the cleavage of the final amino acid-tRNA bond, the dissociation of the ribosomal complex, and the release of the generated polypeptide
•The process of translation often takes place with clusters of ribosomes known as polysomes simultaneously translating the same mRNA strand
A nurse comes to your office concerned that a colleague has been having hypoglycemic spells. She suspects that her colleague is injecting herself with insulin. What study can you use to elucidate this situation?
Insulin is initially synthesized as a single polypeptide chain (preproinsulin) that has an N-terminal signal sequence that directs its processing once secreted. This signal sequence is cleaved off and the resulting proinsulin is stored in secretory granules. Disulfide bonds form between the A and B peptide portions of the precursor molecule. When insulin secretion is triggered by elevated blood glucose, the molecule is cleaved such that the C peptide is removed and the active insulin molecule, consisting of A and B peptides linked by disulfide bonds, is released. The proinsulin concentration is less than 20% of total immunoreactive insulin, while in patients who have an insulinoma, this rises to 30% to 60%. Exogenous insulin administration is diagnosed when a patient presents with the triad of hypoglycemia, a low plasma C-peptide level, and high insulin levels.
Post-Translational Modification
•Polypeptide chains synthesized by ribosomes assume a natural, energy-conserving three-dimensional shape by folding to maximize hydrogen bonds, ionic and van der Waals interactions, and hydrophobic interactions
•However, many will be further processed by cleavage, modification of targeting sequences, modification of amino- and carboxy terminals, and addition of side chains that ultimately change the shape of the protein
•These modifications have effects on targeting, protein activation and function, protein–protein interactions, and protein degradation
Protein Targeting
•In the endoplasmic reticulum (ER) of human cells, proteins destined for membranes or secretion are designated by a signal sequence, usually consisting of 15 to 30 amino acids at the amino-terminal end recognized by the signal recognition particle
•This results in the temporary halting of translation as the peptide emerges from the ribosomal complex, and brings the nascent peptide to receptors in the ER membrane and the peptide translocation complex
•This complex guides the peptide into the lumen of the ER in an ATP-dependent process
•Here, glycosylation, removal of signal sequences, and other modifications occur
•Two classic biochemical ways of adding carbohydrate side chains are via N-linked (to asparagine) and O-linked (to serine or threonine) glycosylation
•N-linked carbohydrates are added in the ER
•O-linked carbohydrates are added in the Golgi complex
•Cysteine residues in polypeptide chains are often linked covalently to other cysteine residues located elsewhere in the peptide sequence via disulfide bonds in such a way that they stabilize the tertiary structure of a protein molecule
•Proteins in eukaryotic cells are targeted for destruction by the covalent attachment of ubiquitin to the protein in an ATP-dependent manner
•A determinant of protein half-life is the specific amino-terminal amino acid on the protein (after the amino-terminal end has been processed)
The Cytoskeleton
•Cell shape and activity are supported by a dynamic network of protein filaments constituting the cytoskeleton
•The cytoskeleton is an important component of cell–cell interaction
•The three main types of filaments are actin, intermediate filaments, and microtubules
Actin
•Globular subunits make up filaments that have polarity
•Free monomeric and filamentous actin are in a dynamic equilibrium, constantly adding to one end and being degraded off of the other end of the actin chain
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


