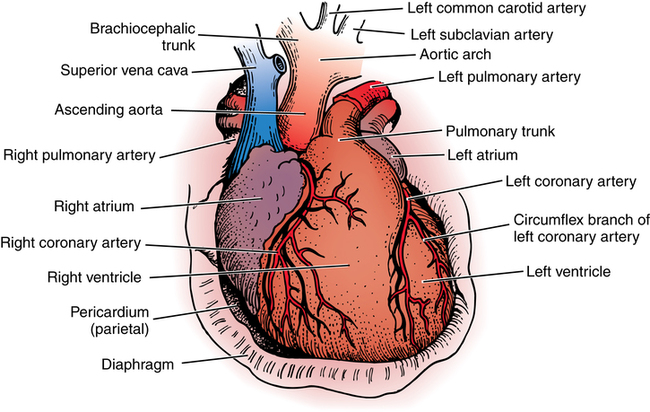
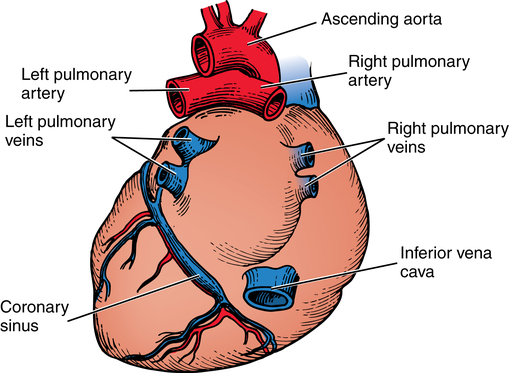
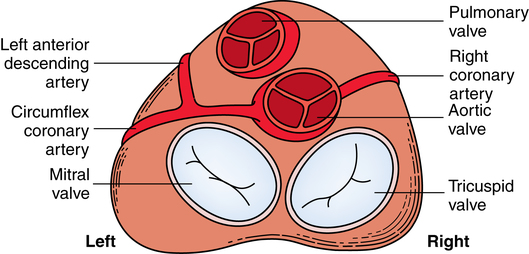
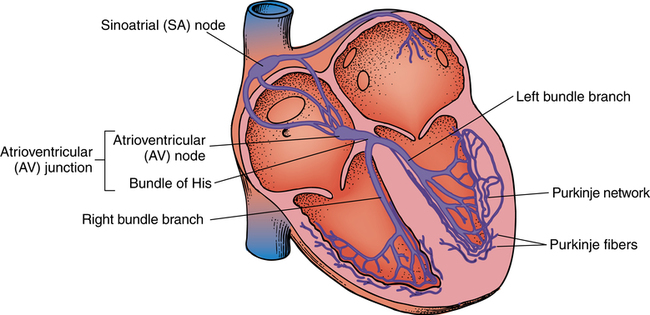
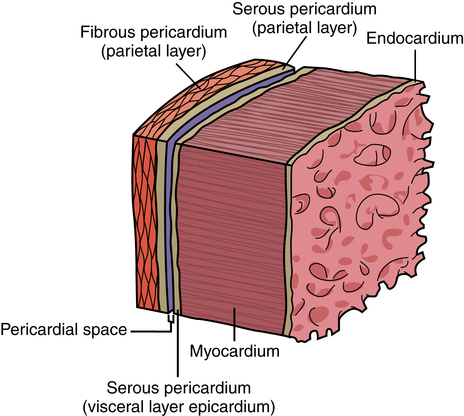
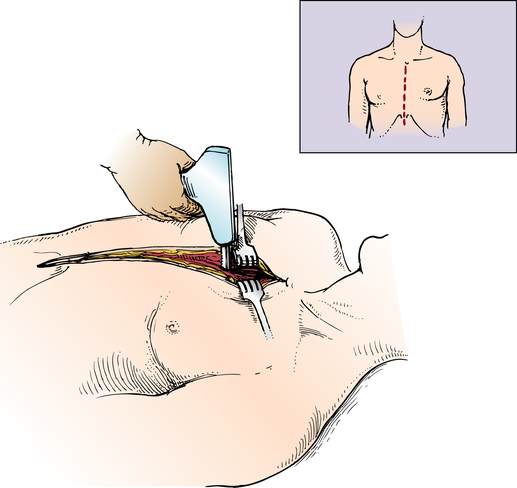
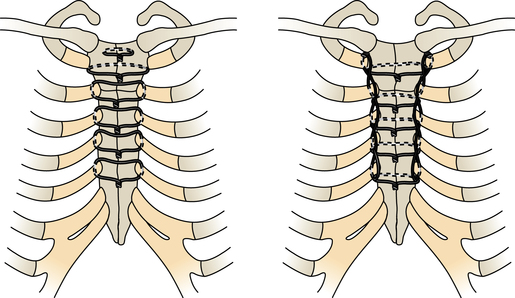
Chapter 43 After studying this chapter, the learner will be able to: • Identify the pertinent anatomy of the heart and great vessels. • List the types of conduits used for coronary artery bypass grafting. Cardiac procedures involve the heart and associated great vessels. To understand diagnostic procedures, hemodynamic monitoring, myocardial preservation techniques, and CPB used in conjunction with cardiac surgery requires knowledge of the normal anatomy and physiology of the heart. The heart is located in the middle mediastinum slightly left of midline. The heart is a four-chambered muscular “pump” enveloped by a closed, double-layered fibroserous sac—the pericardium. The outer parietal layer forms the sac that contains a small amount of clear serous fluid that lubricates the heart’s moving surfaces. The base of the pericardium is attached to the diaphragm; the apex surrounds the great vessels arising from the base of the heart (Figs. 43-1 and 43-2). The layers of the heart are the epicardium (outer visceral pericardium), myocardium (muscle fibers), and endocardium (inner membrane lining) (Fig. 43-3). Divided into right and left halves by an oblique longitudinal septum, each half of the heart has two chambers: a thin-walled upper atrium and a thick-walled lower ventricle. The right side of the heart pumps the pulmonary circulation, and the left side of the heart pumps blood into the systemic circulation. The right atrium receives desaturated blood from the inferior and superior venae cavae and the coronary veins. Four heart valves promote unobstructed unidirectional blood flow through the chambers (Fig. 43-4). These valves are of two types: 1. Atrioventricular (AV) valves: The bases of the cusps (the endocardial leaflets) of these valves attach to the fibrous ring that surrounds their opening between the atrium and ventricle on each side of the heart. a. The right tricuspid valve has three cusps, and lies between the right atrium and ventricle. b. The left mitral valve has two cusps, and lies between the left atrium and ventricle. 2. Semilunar valves: These valves open to allow blood to flow from the heart chambers into the great vessels. 1. The anterior descending or interventricular branch courses toward the apex of the heart. Its branches distribute over the anterolateral wall of the left ventricle. Septal branches supply the anterior interventricular septum. 2. The circumflex branch passes posteriorly. Following the AV groove and passing under the left atrial appendage, it meets the right coronary artery at the base of the junction of both ventricles. The conduction system of the heart (Fig. 43-5) permits synchronous contraction of the atria followed by contraction of the ventricles. The right and left sides of the heart function simultaneously but independently. Muscular contractions of the atria and ventricles are controlled by an electrical impulse that originates in the sinoatrial (SA) node. This “pacemaker” is a dense network of specialized Purkinje fibers that begin at the junction of the right atrium and superior vena cava. These fibers become continuous with muscle fibers of the atrium at the node’s periphery. The stimulus is passed to the smaller AV node beneath the endocardium in the interatrial septum. A mass of interwoven conductive tissue, this node’s specialized fibers are continuous with atrial muscle fibers and the AV bundle of His. Myocardial contraction is referred to as systole; cardiac relaxation is referred to as diastole. Venous blood from the entire body enters the right atrium via the superior and inferior venae cavae and passes through the tricuspid valve to the right ventricle, from which it is ejected through the pulmonary valve into the pulmonary arterial trunk (Fig. 43-6). Right and left pulmonary arteries originating from the trunk carry the blood to the lungs, where it takes up oxygen and gives off carbon dioxide. Oxygenated blood is transported from the lungs to the left atrium by the pulmonary veins and enters the left ventricle through the mitral valve. Contraction of the left ventricle propels blood through the aortic valve into the aorta, from which it is carried to all parts of the body by arterial branches. Congenital malformations corrected in infancy or early childhood are discussed in Chapter 8. This chapter focuses on surgical procedures performed for acquired heart diseases in adults. The principles of general and thoracic surgery apply to cardiac surgery, but several factors require emphasis: • Extra minutes are not available, and seconds save lives. • The team concept is of utmost importance. An experienced team working together can handle emergencies expeditiously. • Comprehensive physical and psychological preparation of the patient precedes a surgical procedure. Postoperatively the patient is taken to an intensive care unit (ICU). The patient is continuously monitored intraoperatively and postoperatively, including during transport to the ICU. 1. The OR for cardiovascular surgery should be equipped with the following: a. Cardiac defibrillator, pacemaker, and intraaortic counterpulsation devices. b. Cardioplegia (to induce cardiac arrest) and inotropic (to modify cardiac muscle contractility) drugs, including but not limited to the following: c. Laboratory facilities for blood gas, acid-base balance determinations, potassium, glucose, and hematocrit. Modern analyzers have microprocessors to determine values. 2. The basic thoracic setup is used with the addition of cardiovascular instruments (i.e., various noncrushing vascular and anastomosis clamps, cardiotomy suction tips and sump tubes, and cardiovascular sutures). 3. Prosthetic devices are sterilized by the manufacturer. Care is taken to maintain sterility during placement in the patient. Many types of valves, patches, grafts, and catheters are available. They should be biocompatible, nonthrombogenic, and nonbiodegradable. Valves come made of either a bioprosthetic component or metal. Bioprosthetic valves are preserved with glutaraldehyde. They are rinsed in fresh sterile saline three times to remove the preservative. Some surgeons culture the valve before implantation. 4. Local and/or systemic hypothermia may be used intraoperatively to reduce the body’s need for oxygen and to preserve myocardial function. Commercial preparations of sterile slush for local hypothermia are convenient. 5. Intraoperative autotransfusion is often used for blood volume replacement. Blood substitutes such as hetastarch, an albumin substitute for plasma expansion, may be administered. Properly crossmatched blood should be available for transfusion in the event of excessive blood loss. Platelets, stored at room temperature, may be given after CPB to enhance clotting. Often little or no blood is needed for transfusion in many procedures when CPB is used. Also, medications such as aprotinin (Trasylol) may be infused to protect platelet function during CPB. 6. Closed water-seal drainage or suction drainage is used postoperatively to drain the mediastinum and/or pleural space(s) (Fig. 43-7). 7. Many devices are available for cardiac pacing, ventricular support, and treatment of cardiogenic shock. A portable cardiopulmonary support system, external pulsatile pump, and other devices are used. It is critical that these devices be properly sterilized and handled. Read package labels and inserts for the specific manufacturer’s instructions. The circulating nurse should affix labels and record serial numbers and identifying data in the patient’s chart. In the event of a mechanical failure, this information becomes important. Lot numbers are logged according to facility policy and procedure. 8. The scrub person should set up a separate table for assembling devices. Check to be certain that all parts are available and functional. A missing component could be catastrophic. Several different approaches can be used for entering the chest cavity for cardiac surgery (Fig. 43-8). A vertical incision extends through the midline from the suprasternal notch to approximately 2 inches below the xiphoid process (Fig. 43-9). Retrosternal tissue is dissected. The bony sternum is split (divided) with a powered sternal saw. Caution is used to avoid injury to underlying mediastinal structures, especially if the chest has been opened before. The blade has a safety guard to prevent penetration into the mediastinum. At closure, heavy-gauge stainless steel wires are placed around or through the sternum, tightly pulled together, and twisted (Fig. 43-10). The ends are buried in the sternum. Other nonabsorbable sutures may be used to provide firm fixation. The linea alba, subcutaneous tissue, and skin are sutured. Although placement of invasive pressure monitoring lines is not their responsibility, perioperative nurses should be aware of the implications of data and the potential for complications, such as thrombus, dysrhythmias, embolus, cardiac arrest, and postoperative infection. For assessment of tissue perfusion, invasive hemodynamic monitoring is used to determine blood pressures in major arteries, veins, and the heart chambers.2,4,5,11 Indwelling catheter lines are inserted to measure the following: • Radial and femoral artery pressures • Central venous pressure (CVP) • Pulmonary artery pressure. The Swan-Ganz catheter line determines right atrial, right ventricular, and pulmonary capillary wedge pressures of left ventricular function. It is also used to determine cardiac function as it measures cardiac output, index, oxygen saturation of the central venous system and systemic vascular resistance that, along with pulmonary artery pressures, helps to determine if a patient is hypovolemic, hypervolemic, or euvolemic. • Transesophageal echocardiography (TEE): A transesophageal ultrasound probe is used for assessment of graft patency, myocardial perfusion, adequacy of valve replacement, or ventricular function.1,11,12 A Doppler color flow probe also may be useful to quantitatively assess other repairs. • Electrophysiologic measurements: A computerized mapping system is used to identify the focus of dysrhythmias. • Near-infrared reflectance spectroscopy: A device equipped with a sensor is attached to the patient’s head. Light transmitted through the skin and skull to the brain is reflected to light detectors in the sensor. Changes in oxygen levels in the brain change light absorption. These changes may alert the anesthesia provider and surgeon to a developing oxygen deficit. CPB is the technique of oxygenating and perfusing blood by means of a mechanical pump-oxygenator system. This apparatus temporarily substitutes for the function of the patient’s heart and lungs during cardiac surgery. CPB is used for most intracardiac (open heart) and coronary artery procedures. Venous blood is diverted from the body to the machine for oxygenation (extracorporeal circulation) and is pumped back to the patient (Fig. 43-11). • Bubble: Bubbles of oxygen are supplied to the blood by direct blood/gas contact. • Membrane: Oxygen and carbon dioxide diffuse through a permeable Teflon or polyethylene membrane that contains the blood. This method diminishes blood/gas interface. Several types of disposable membrane oxygenators are available. • Microporous membrane: Blood film is separated from ventilating gas by a microporous polypropylene membrane folded like an accordion and operated like a bubble oxygenator. Blood pressure in this oxygenator exceeds gas pressure at all times, thus precluding gas bubbles passing through the microporous membrane. Deliberate cardiac arrest may be effected by one or a combination of the following methods: 1. Aortic cross-clamping: The aorta is occluded with a vascular clamp proximal to the aortic cannula to block systemic circulation. Ischemic (anoxic) cardiac arrest occurs as the blocked systemic blood within the heart becomes deoxygenated and cardiac metabolic needs are depleted. This technique can be maintained for only a limited period, because myocardial damage and necrosis will occur when the oxygen supply and energy required to maintain the subcellular system are depleted. Cerebrospinal fluid pressure may increase. 2. Cardioplegia: Most cardiac surgery is based on the use of cardioplegic solutions used alone or in combination with other techniques discussed. These are preparations of a small amount of potassium in crystalloid, blood, or other solution. Cardioplegia is delivered into the aortic root through the coronary ostia or into the coronary sinus through the right atrium. • Antegrade infusion: A catheter is placed into the coronary ostia via the ascending aorta above the aortic valve and below the aortic cross-clamp (Fig. 43-12). Cardioplegia is infused and flows into the coronary circulation. It is kept out of the left ventricle by a competent aortic valve and systemically by the aortic cross-clamp. Between 500 and 1000 mL of solution at 70 mm Hg pressure is initially used. Additional amounts are administered with each anastomosis. Severe coronary disease may cause uneven doses of cardioplegia and interfere with perfusion.
Cardiac surgery
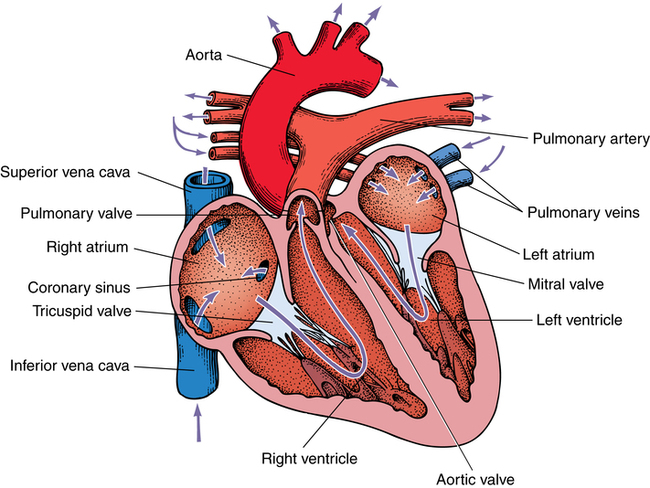
Anatomy of the heart and great vessels
Heart
Valves
Coronary circulation
Physiology of the heart
Electrical conduction system
Cardiac cycle
Special features of cardiac surgery
General considerations
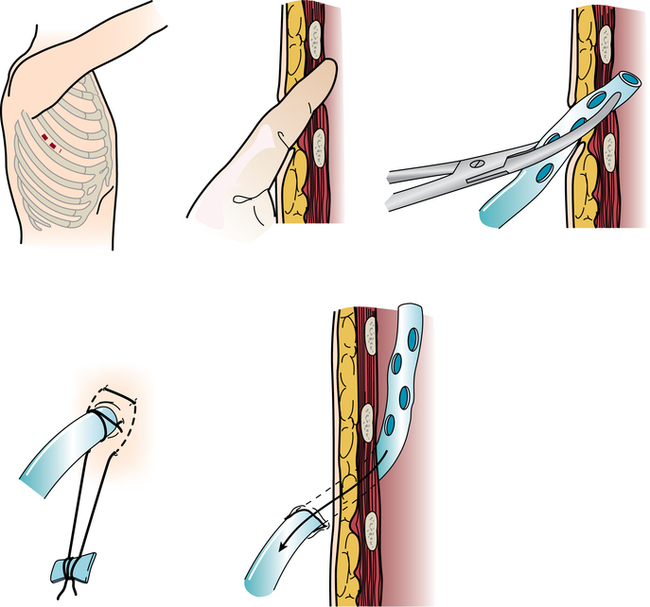
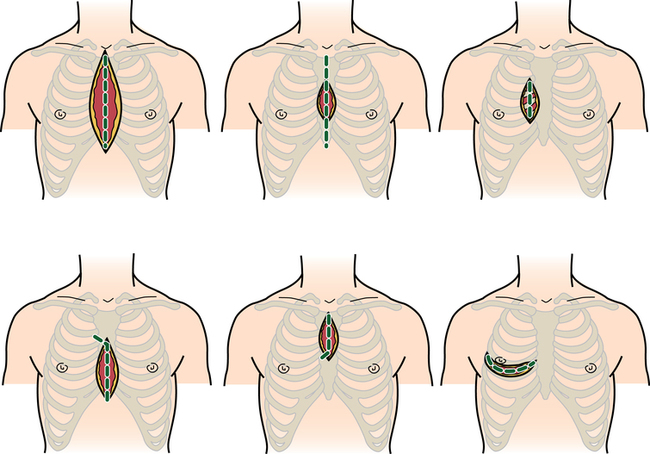
Commonly used incisions for cardiac surgery
Median sternotomy
Invasive hemodynamic monitoring
Intraoperative monitoring
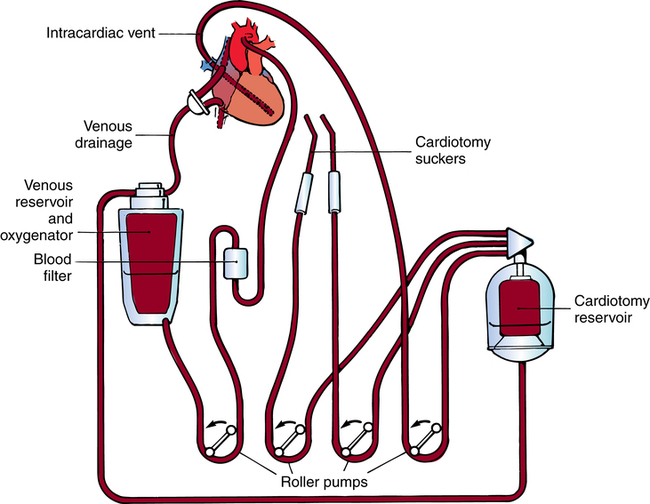
Cardiopulmonary bypass
Components of a bypass system
Oxygenator.
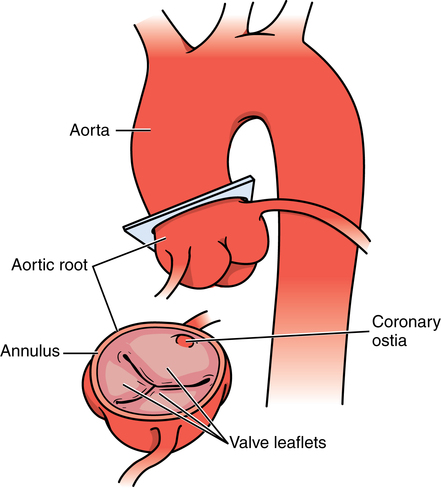
Myocardial preservation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



 Website
Website