310 | Hypersensitivity Pneumonitis and Pulmonary Infiltrates with Eosinophilia |
HYPERSENSITIVITY PNEUMONITIS
INTRODUCTION AND DEFINITION
Hypersensitivity pneumonitis (HP), also referred to as extrinsic allergic alveolitis, is a pulmonary disease that occurs due to inhalational exposure to a variety of antigens leading to an inflammatory response of the alveoli and small airways. Systemic manifestations such as fever and fatigue can accompany respiratory symptoms. Although sensitization to an inhaled antigen as manifested by specific circulating IgG antibodies is necessary for the development of HP, sensitization alone is not sufficient as a defining characteristic, because many sensitized individuals do not develop HP. The incidence and prevalence of HP are variable, depending on geography, occupation, avocation, and environment of the cohort being studied. As yet unexplained is the decreased risk of developing HP in smokers.
OFFENDING ANTIGENS
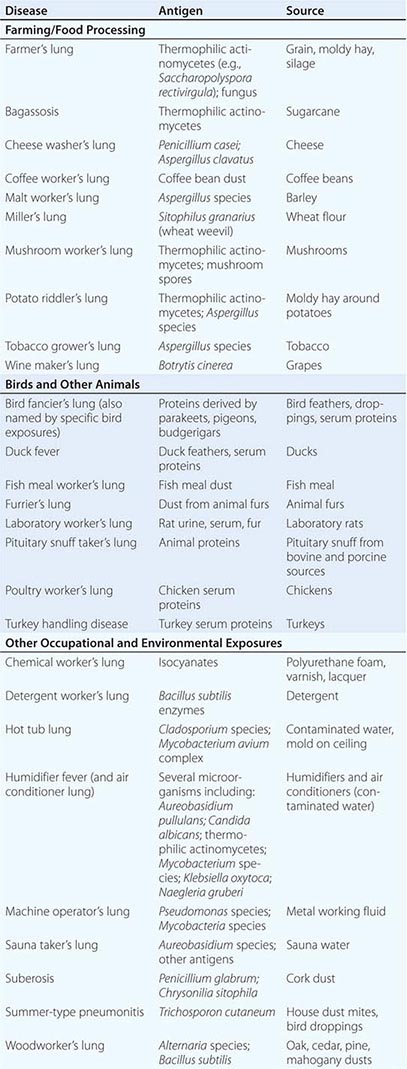
HP can be caused by any of a large list of potential offending inhaled antigens (Table 310-1). The various antigens and environmental conditions described to be associated with HP give rise to an expansive list of monikers given to specific forms of HP. Antigens derived from fungal, bacterial, mycobacterial, bird-derived, and chemical sources have all been implicated in causing HP.
EXAMPLES OF HYPERSENSITIVITY PNEUMONITIS |
Categories of individuals at particular risk in the United States include farmers, bird owners, industrial workers, and hot tub users. Farmer’s lung occurs as a result of exposure to one of several possible sources of bacterial or fungal antigens such as grain, moldy hay, or silage. Potential offending antigens include thermophilic actinomycetes or Aspergillus species. Bird fancier’s lung (also referred to by names corresponding to specific birds) must be considered in patients who give a history of keeping birds in their home and is precipitated by exposure to antigens derived from feathers, droppings, and serum proteins. Occupational exposure to birds may also cause HP, as is seen in poultry worker’s lung. Chemical worker’s lung is provoked by exposure to occupational chemical antigens such as diphenylmethane diisocyanate and toluene diisocyanate. Mycobacteria may cause HP rather than frank infection, a phenomenon observed in hot tub lung and in HP due to metalworking fluid.
PATHOPHYSIOLOGY
The pathophysiology of HP has not been characterized in depth on an immunologic level, although it has been established that HP is an immune-mediated condition that occurs in response to inhaled antigens that are small enough to deposit in distal airways and alveoli. From a lymphocyte perspective, HP has been categorized as a condition with a TH1 inflammatory pattern. However, emerging evidence suggests that TH17 lymphocyte subsets may be involved in the pathogenesis of the disease as well. Although the presence of precipitating IgG antibodies against specific antigens in HP suggests a prominent role for adaptive immunity in the pathophysiology of HP, innate immune mechanisms may also make an important contribution. This is highlighted by the observation that Toll-like receptors and downstream signaling proteins such as MyD88 are activated in HP. Although no clear genetic basis for HP has been established, in specific cohorts, polymorphisms in genes involved in antigen processing and presentation, including TAP1 and major histocompatibility complex type II, have been observed.
CLINICAL PRESENTATION
Given the heterogeneity among patients, variability in offending antigens, and differences in the intensity and duration of exposure to antigen, the presentation of HP is accordingly variable. Although these categories are not fully satisfactory in capturing this variability, HP has been traditionally categorized as having acute, subacute, and chronic forms. Acute HP usually manifests itself 4–8 h following exposure to the inciting antigen, often intense in nature. Systemic symptoms, including fevers, chills, and malaise, are prominent and are accompanied by dyspnea. Symptoms resolve within hours to days if no further exposure to the offending antigen occurs. In subacute HP resulting from ongoing antigen exposure, the onset of respiratory and systemic symptoms is typically more gradual over the course of weeks. A similar presentation may occur as a culmination of intermittent episodes of acute HP. Although respiratory impairment may be quite severe, antigen avoidance generally results in resolution of the symptoms, although with a slower time course, on the order of weeks to months, than that seen with acute HP. Chronic HP can present with an even more gradual onset of symptoms than subacute HP, with progressive dyspnea, cough, fatigue, weight loss, and clubbing of the digits. The insidious onset of symptoms and frequent lack of an anteceding episode of acute HP make diagnosing chronic HP a challenge. Unlike with the other forms of HP, there can be an irreversible component to the respiratory impairment that is not responsive to removal of the responsible antigen from the patient’s environment. The disease progression to hypoxemic respiratory failure can mirror that seen in idiopathic pulmonary fibrosis (IPF). Fibrotic lung disease is a potential feature of chronic HP due to exposure to bird antigens, whereas an emphysematous phenotype may be seen in farmer’s lung.
The categories of acute, subacute, and chronic HP are not completely sufficient in classifying HP. The HP Study Group found on cluster analysis that a cohort of HP patients is best described in bipartite fashion, with one group featuring recurrent systemic signs and symptoms and the other featuring more severe respiratory findings.
Concordant with the variability in the presentation of HP is the observed variability in outcome. HP that has not progressed to chronic lung disease has a more favorable outcome with likely resolution if antigen avoidance can be achieved. However, chronic HP resulting in lung fibrosis has a poorer prognosis, with patients with chronic pigeon breeder’s lung having demonstrated a similar mortality as seen in IPF.
DIAGNOSIS
Although there is no set of universally accepted criteria for arriving at a diagnosis of HP, diagnosis depends foremost on establishing a history of exposure to an offending antigen that correlates with respiratory and systemic symptoms. A careful occupational and home exposure history should be taken and may be supplemented if necessary by a clinician visit to the work or home environment. Specific inquiries will be influenced by geography and the occupation of the patient. When HP is suspected by history, the additional workup is aimed at establishing an immunologic and physiologic response to inhalational antigen exposure with chest imaging, pulmonary function testing, serologic studies, bronchoscopy, and, on occasion, lung biopsy.
Chest Imaging Chest x-ray findings in HP are nonspecific and can even lack any discernible abnormalities. In cases of acute and subacute HP, findings may be transient and can include ill-defined micronodular opacities or hazy ground-glass airspace opacities. Findings on chest x-ray will often resolve with removal from the offending antigen, although the time course of resolution may vary. With chronic HP, the abnormalities seen on the chest radiograph are frequently more fibrotic in nature and may be difficult to distinguish from IPF.
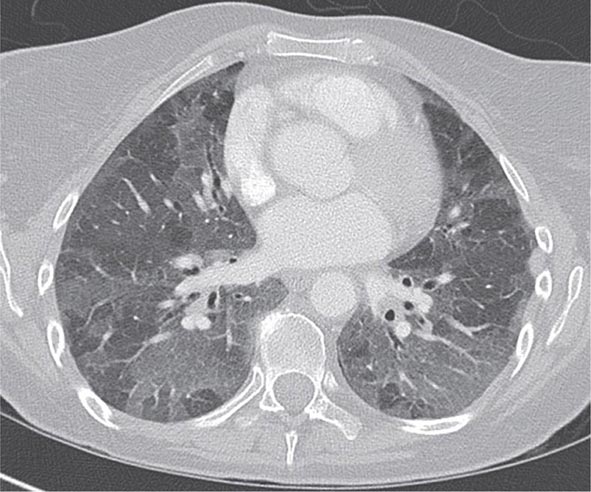
With the wide availability of high-resolution computed tomography (HRCT), this modality has become a common component in the diagnostic workup for HP. Although the HRCT may be normal in acute forms of HP, this may be due to lack of temporal correlation between exposure to the offending antigen and obtaining the imaging. Additionally, because of the transient nature of acute HP, HRCT is not always performed. In subacute forms of the disease, ground-glass airspace opacities are characteristic, as is the presence of centrilobular nodules. Expiratory images may show areas of air trapping that are likely caused by involvement of the small airways (Fig. 310-1). Reticular changes and traction bronchiectasis can be observed in chronic HP. Subpleural honeycombing similar to that seen in IPF may be present in advanced cases, although unlike in IPF, the lung bases are frequently spared.
FIGURE 310-1 Chest computed tomography scan of a patient with subacute hypersensitivity pneumonitis in which scattered regions of ground-glass infiltrates in a mosaic pattern consistent with air trapping are seen bilaterally. This patient had bird fancier’s lung. (Courtesy of TJ Gross; with permission.)
Pulmonary Function Testing (PFT) Either restrictive or obstructive PFTs can be present in HP, so the pattern of PFT change is not useful in establishing the diagnosis of HP. However, obtaining PFTs is of use in characterizing the physiologic impairment of an individual patient and in gauging the response to antigen avoidance and/or corticosteroid therapy. Diffusion capacity for carbon monoxide may be significantly impaired, particularly in cases of chronic HP with fibrotic pulmonary parenchymal changes.
Serum Precipitins Assaying for precipitating IgG antibodies against specific antigens can be a useful adjunct in the diagnosis of HP. However, the presence of an immunologic response alone is not sufficient for establishing the diagnosis, because many asymptomatic individuals with high levels of exposure to antigen may display serum precipitins, as has been observed in farmers and in pigeon breeders. It should also be noted that panels that test for several specific serum precipitins often provide false-negative results, because they represent an extremely limited proportion of the universe of potential offending environmental antigens.
Bronchoscopy Bronchoscopy with bronchoalveolar lavage (BAL) may be used in the evaluation of HP. Although not a specific finding, BAL lymphocytosis is characteristic of HP. However, in active smokers, a lower threshold should be used to establish BAL lymphocytosis, because smoking will result in lower lymphocyte percentages. Most cases of HP have a CD4+/CD8+ lymphocyte ratio of less than 1, but again, this is not a specific finding and has limited utility in the diagnosis of HP.
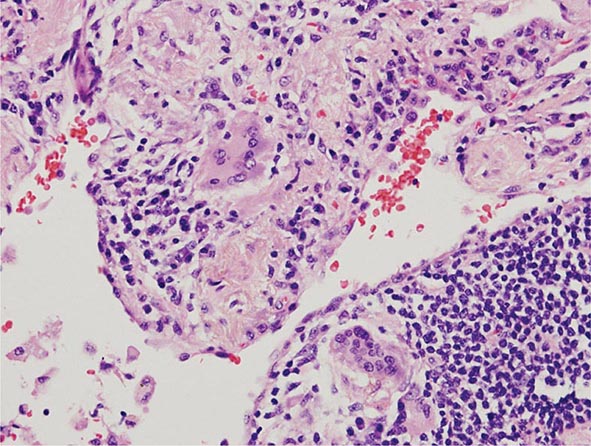
Lung Biopsy Tissue samples may be obtained by a bronchoscopic approach using transbronchial biopsy, or more architecturally preserved specimens may be obtained by a surgical approach (video-assisted thoracoscopy or open approach). As is the case with BAL, histologic specimens are not absolutely necessary to establish the diagnosis of HP, but they can be useful in the correct clinical context. A common histologic feature in HP is the presence of noncaseating granulomas in the vicinity of small airways (Fig. 310-2). As opposed to pulmonary sarcoidosis, in which noncaseating granulomas are well defined, the granulomas seen in HP are loose and poorly defined in nature. Within the alveolar spaces and in the interstitium, a mixed cellular infiltrate with a lymphocytic predominance is observed that is frequently patchy in distribution. Bronchiolitis with the presence of organizing exudate is also often observed. Fibrosis may be present as well, particularly as the disease progresses to its chronic form. Fibrotic changes may be focal but can be diffuse and severe with honeycombing in advanced cases, similar to findings in IPF.
FIGURE 310-2 Open-lung biopsy from a patient with subacute hypersensitivity pneumonitis demonstrating a loose, nonnecrotizing granuloma made up of histiocytes and multinucleated giant cells. Peribronchial inflammatory infiltrate made up of lymphocytes and plasma cells is also seen. (Courtesy of TJ Gross; with permission.)
Clinical Prediction Rule Although not meant as a set of validated diagnostic criteria, a clinical prediction rule for predicting the presence of HP has been published by the HP Study Group. They identified six statistically significant predictors for HP, the strongest of which was exposure to an antigen known to cause HP. Other predictive criteria were the presence of serum precipitins, recurrent symptoms, symptoms occurring 4–8 h after antigen exposure, crackles on inspiration, and weight loss.
DIFFERENTIAL DIAGNOSIS
Differentiating HP from other conditions that cause a similar constellation of respiratory and systemic symptoms requires an increased index of suspicion based on obtaining a history of possible exposure to an offending antigen. Presentations of acute or subacute HP can be mistaken for respiratory infection. In cases of chronic disease, HP must be differentiated from interstitial lung disease, such as IPF or nonspecific interstitial pneumonitis (NSIP); this can be a difficult task even with lung biopsy. Given the presence of pulmonary infiltrates and noncaseating granulomas on biopsy, sarcoidosis is also a consideration in the differential diagnosis of HP. Unlike in HP, however, hilar adenopathy may be prominent on chest x-ray, organs other than the lung may be involved, and noncaseating granulomas in pathologic specimens tend to be well formed. Other inhalational syndromes, such as organic toxic dust syndrome (OTDS), can be misdiagnosed as HP. OTDS occurs with exposure to organic dusts, including those produced by grains or mold silage, but neither requires prior antigen sensitization nor is characterized by positive serum precipitins.
GLOBAL CONSIDERATIONS
![]() As the ever-expanding list of antigens and exposures associated with the development of HP suggests, populations at risk for HP will vary globally based on specifics of local occupational, avocational, and environmental factors. Specific examples of geographically limited HP include summer-type pneumonitis seen in Japan and suberosis seen in cork workers in Portugal and Spain.
As the ever-expanding list of antigens and exposures associated with the development of HP suggests, populations at risk for HP will vary globally based on specifics of local occupational, avocational, and environmental factors. Specific examples of geographically limited HP include summer-type pneumonitis seen in Japan and suberosis seen in cork workers in Portugal and Spain.
PULMONARY INFILTRATES WITH EOSINOPHILIA
Although eosinophils are normal constituents of the lungs, there are several pulmonary eosinophilic syndromes that are characterized by pulmonary infiltrates on imaging along with an increased number of eosinophils in lung tissue, in sputum, and/or in BAL fluid, with resultant increased respiratory symptoms and the potential for systemic manifestations. Because the eosinophil plays such an important role in each of these syndromes, it is often difficult to distinguish between them, but there are important clinical and pathologic differences as well as differences in prognosis and treatment paradigms.
CLASSIFYING PULMONARY INFILTRATES WITH EOSINOPHILIA AND GENERAL APPROACH
Because there are so many different diagnoses associated with pulmonary infiltrates with eosinophilia, the first step in classifying pulmonary eosinophilic syndromes is distinguishing between primary pulmonary eosinophilic lung disorders and those with eosinophilia that are secondary to a specific cause such as a drug reaction, an infection, a malignancy, or another pulmonary condition such as asthma. Table 310-2 lists primary and secondary pulmonary eosinophilic disorders.
PULMONARY INFILTRATES WITH EOSINOPHILIA |
For each patient, a detailed history is of utmost importance and can help elucidate what the underlying disease is. Details regarding onset, timing, and precipitants of specific symptoms can help discern one diagnosis from another. History regarding pharmacologic, occupational, and environmental exposures is instructive, and family and travel history are crucial. In addition to details about the sinuses and lungs, it is important to inquire about systemic manifestations and assess for physical findings of cardiac, gastrointestinal (GI), neurologic, dermatologic, and genitourinary involvement, all of which may give clues to specific diagnoses. Once the details from history and physical are teased out, laboratory testing (including measurements of blood eosinophils, cultures, and markers of inflammation), spirometry and radiographic imaging can help distinguish between different diseases. Often, however, BAL, transbronchial, or open lung biopsies are required. In many cases, biopsies or noninvasive diagnostic studies of other organs (e.g., echocardiogram, electromyogram, or bone marrow biopsy) can be helpful.
PATHOPHYSIOLOGY
Pathologically, the pulmonary eosinophilic syndromes are characterized by tissue infiltration by eosinophils (Fig. 310-2). In eosinophilic granulomatosis with polyangiitis (EGPA), extravascular granulomas and necrotizing vasculitis may occur in the lungs, as well as in the heart, skin, muscle, liver, spleen, and kidneys, and may be associated with fibrinoid necrosis and thrombosis.
The exact etiology of the various pulmonary eosinophilic syndromes is unknown; however, it is felt that these syndromes result from dysregulated eosinophilopoiesis or an autoimmune process because of the prominence of allergic features and the presence of immune complexes, heightened T cell immunity, and altered humoral immunity as evidenced by elevated IgE and rheumatoid factor. Because of its integral involvement in eosinophilopoiesis, interleukin 5 (IL-5) has been hypothesized to play an etiologic role, and efforts to block this cytokine are being investigated. Antineutrophil cytoplasmic antibodies (ANCAs) are present in about half of patents with EGPA; binding of ANCAs to vascular walls likely contributes to vascular inflammation and injury as well as chemotaxis of inflammatory cells.
ACUTE EOSINOPHILIC PNEUMONIA
Acute eosinophilic pneumonia is a syndrome characterized by fevers, acute respiratory failure that often requires mechanical ventilation, diffuse pulmonary infiltrates, and pulmonary eosinophilia in a previously healthy individual (Table 310-3).
DIAGNOSTIC CRITERIA OF ACUTE EOSINOPHILIC PNEUMONIA |
Clinical Features and Etiology At presentation, acute eosinophilic pneumonia is often mistaken for acute lung injury or acute respiratory distress syndrome (ARDS), until a BAL is performed and reveals >25% eosinophils. Although the predominant symptoms of acute eosinophilic pneumonia are cough, dyspnea, malaise, myalgias, night sweats, and pleuritic chest pain, physical exam findings include high fevers, basilar rales, and rhonchi on forced expiration. Acute eosinophilic pneumonia most often affects males between age 20 and 40 with no history of asthma. Although no clear etiology has been identified, several case reports have linked acute eosinophilic pneumonia to recent initiation of tobacco smoking or exposure to other environmental stimuli including dust from indoor renovations.
In addition to a suggestive history, the key to establishing a diagnosis of acute eosinophilic pneumonia is the presence of >25% eosinophilia on BAL fluid. While lung biopsies show eosinophilic infiltration with acute and organizing diffuse alveolar damage, it is generally not necessary to proceed to biopsy to establish a diagnosis. Although patients present with an elevated white blood cell count, in contrast to other pulmonary eosinophilic syndromes, acute eosinophilic pneumonia is often not associated with peripheral eosinophilia upon presentation. However, between 7 and 30 days of disease onset, peripheral eosinophilia often occurs with mean eosinophil counts of 1700. Erythrocyte sedimentation rate (ESR), C-reactive protein, and IgE levels are high but nonspecific, whereas HRCT is always abnormal with bilateral random patchy ground-glass or reticular opacities, and small pleural effusions in as many as two-thirds of patients. Pleural fluid is characterized by a high pH with marked eosinophilia.
Clinical Course and Response to Therapy Although some patients improve spontaneously, most patients require admission to an intensive care unit and respiratory support with either invasive (intubation) or noninvasive mechanical ventilation. However, what distinguishes acute eosinophilic pneumonia from both other cases of acute lung injury as well as some of the other pulmonary eosinophilic syndromes is the absence of organ dysfunction or multisystem organ failure other than respiratory failure. One of the characteristic features of acute eosinophilic pneumonia is the high degree of corticosteroid responsiveness and the excellent prognosis. Another distinguishing feature of acute eosinophilic pneumonia is that complete clinical and radiographic recovery without recurrence or residual sequelae occurs in almost all patients within several weeks of initiation of therapy.
CHRONIC EOSINOPHILIC PNEUMONIA
In contrast to acute eosinophilic pneumonia, chronic eosinophilic pneumonia is a more indolent syndrome that is characterized by pulmonary infiltrates and eosinophilia in both the tissue and blood. Most patients are female nonsmokers with a mean age of 45, and patients do not usually develop the acute respiratory failure and significant hypoxemia appreciated in acute eosinophilic pneumonia. Similar to EGPA, a majority have asthma, with many having a history of allergies.
Patients present with a subacute illness over weeks to months, with cough, low-grade fevers, progressive dyspnea, weight loss, wheezing, malaise, and night sweats, and a chest x-ray with migratory bilateral peripheral or pleural-based opacities. Although this “photographic negative pulmonary edema” appearance on chest x-ray and chest CT is pathognomonic of chronic eosinophilic pneumonia, less than 25% of patients present with this finding. Other radiographic findings include atelectasis, pleural effusions, lymphadenopathy, and septal line thickening.
Almost 90% of patients have peripheral eosinophilia, with mean eosinophil counts of over 30% of total white blood cell count. BAL eosinophilia is also an important distinguishing feature with mean BAL eosinophil counts of close to 60%. Both peripheral and BAL eosinophilia are very responsive to treatment with corticosteroids. Other laboratory features of chronic eosinophilic pneumonia include increased ESR, C-reactive protein, platelets, and IgE. Lung biopsy is also often not required to establish a diagnosis, but may show accumulation of eosinophils and histiocytes in the lung parenchyma and interstitium, as well as cryptogenic organizing pneumonia, but with minimal fibrosis. Nonrespiratory manifestations are uncommon, but arthralgias, neuropathy, and skin and GI symptoms have all been reported; their presence may suggest EGPA or hypereosinophilic syndrome. Another similarity is the rapid response to corticosteroids with quick resolution of peripheral and BAL eosinophilia and improvement in symptoms. In contrast to acute eosinophilic pneumonia, though, over 50% of patients relapse, and many require prolonged courses of corticosteroids for months to years.
EOSINOPHILIC GRANULOMATOSIS WITH POLYANGIITIS (EGPA)
Previously known as allergic angiitis granulomatosis or Churg-Strauss syndrome, this complex syndrome is characterized by eosinophilic vasculitis that may involve multiple organ systems including the lungs, heart, skin, GI tract, and nervous system. Although EGPA is characterized by peripheral and pulmonary eosinophilia with infiltrates on chest x-ray, the primary features that distinguish EGPA from other pulmonary eosinophilic syndromes are the presence of eosinophilic vasculitis in the setting of asthma and involvement of multiple end organs (a feature it shares with hypereosinophilic syndrome). Although perceived to be quite rare, in the last few years, there has appeared to be an increased incidence of this disease, particularly in association with various asthma therapies.
The primary features of EGPA include asthma, peripheral eosinophilia, neuropathy, pulmonary infiltrates, paranasal sinus abnormality, and presence of eosinophilic vasculitis. It typically occurs in several phases. The prodromal phase is characterized by asthma and allergic rhinitis, and usually begins when the individual is in his or her twenties or thirties, typically persisting for many years. The eosinophilic infiltrative phase is characterized by peripheral eosinophilia and eosinophilic tissue infiltration of various organs including the lungs and GI tract. The third phase is the vasculitic phase and may be associated with constitutional signs and symptoms including fever, weight loss, malaise, and fatigue. The mean age at diagnosis is 48 years, with a range of 14 to 74 years; the average length of time between diagnosis of asthma and vasculitis is 9 years.
Similar to other pulmonary eosinophilic syndromes, constitutional symptoms are very common in EGPA and include weight loss of 10–20 lb, fevers, and diffuse myalgias and migratory polyarthralgias. Myositis may be present with evidence of vasculitis on muscle biopsies. In contrast to the eosinophilic pneumonias, EGPA involves many organ systems including the lungs, skin, nerves, heart, GI tract, and kidneys.
Symptoms and Clinical Manifestations • RESPIRATORY Most EGPA patients have asthma that arises later in life and in individuals who have no family history of atopy. The asthma can often be severe, and oral corticosteroids are often required to control symptoms but may lead to suppression of vasculitic symptoms. In addition to the more common symptoms of cough, dyspnea, sinusitis, and allergic rhinitis, alveolar hemorrhage and hemoptysis may also occur.
NEUROLOGIC Over three-fourths of EGPA patients have neurologic manifestations. Mononeuritis multiplex most commonly involves the peroneal nerve, but also involves the ulnar, radial, internal popliteal, and occasionally, cranial nerves. Cerebral hemorrhage and infarction may also occur and are important causes of death. Despite treatment, neurologic sequelae often do not completely resolve.
DERMATOLOGIC Approximately half of EGPA patients develop dermatologic manifestations. These include palpable purpura, skin nodules, urticarial rashes, and livedo.
CARDIOVASCULAR Granulomas, vasculitis, and widespread myocardial damage may be found on biopsy or at autopsy, and cardiomyopathy and heart failure may be seen in up to half of all patients but are often at least partially reversible. Acute pericarditis, constrictive pericarditis, myocardial infarction, and other electrocardiographic changes all may occur. The heart is a primary target organ in EGPA, and cardiac involvement often portends a worse prognosis.
GI GI symptoms are common in EGPA and likely represent an eosinophilic gastroenteritis characterized by abdominal pain, diarrhea, GI bleeding, and colitis. Ischemic bowel, pancreatitis, and cholecystitis have also been reported in association with EGPA and usually portend a worse prognosis.
RENAL Renal involvement is more common than once thought, and approximately 25% of patients have some degree of renal involvement. This may include proteinuria, glomerulonephritis, renal insufficiency, and rarely, renal infarct.
Lab Abnormalities Systemic eosinophilia is the hallmark laboratory finding in patients with EGPA and reflects the likely pathogenic role that the eosinophil plays in this disease. Eosinophilia greater than 10% is one of the defining features of this illness and may be as high as 75% of the peripheral white blood cell count. It is present at the time of diagnosis in over 80% of patients but may respond quickly (often within 24 h) to initiation of systemic corticosteroid therapy. Even in the absence of systemic eosinophilia, tissue eosinophilia may be present.
Although not specific to EGPA, ANCAs are present in up to two-thirds of patients, mostly with a perinuclear staining pattern. Nonspecific lab abnormalities that may be present in patients with EGPA include a marked elevation in ESR, a normochromic normocytic anemia, an elevated IgE, hypergammaglobulinemia, and positive rheumatoid factor and antinuclear antibodies (ANA). Although BAL often reveals significant eosinophilia, this may be seen in other eosinophilic lung diseases. Similarly, PFT often reveals an obstructive defect similar to asthma.
Radiographic Features Chest x-ray abnormalities are extremely common in EGPA and consist of bilateral, nonsegmental, patchy infiltrates that often migrate and may be interstitial or alveolar in appearance. Reticulonodular and nodular disease without cavitation can be seen, as can pleural effusions and hilar adenopathy. The most common CT findings include bilateral ground-glass opacity and airspace consolidation that is predominantly subpleural. Other CT findings include bronchial wall thickening, hyperinflation, interlobular septal thickening, lymph node enlargement, and pericardial and pleural effusions. Angiography may be used diagnostically and may show signs of vasculitis in the coronary, central nervous system, and peripheral vasculature.
Treatment and Prognosis of EGPA Most patients diagnosed with EGPA have previously been diagnosed with asthma, rhinitis, and sinusitis, and have received treatment with inhaled or systemic corticosteroids. Because these agents are also the initial treatment of choice for EGPA patients, institution of these therapies in patients with EGPA who are perceived to have severe asthma may delay the diagnosis of EGPA because signs of vasculitis may be masked. Corticosteroids dramatically alter the course of EGPA: up to 50% of those who are untreated die within 3 months of diagnosis, whereas treated patients have a 6-year survival of over 70%. Common causes of death include heart failure, cerebral hemorrhage, renal failure, and GI bleeding. Recent data suggest that clinical remission may be obtained in over 90% of patients treated; approximately 25% of those patients may relapse, often due to corticosteroid tapering, with a rising eosinophil count heralding the relapse. Myocardial, GI, and renal involvement most often portend a poor prognosis. In such cases, treatment with higher doses of corticosteroids or the addition of cytotoxic agents such as cyclophosphamide is often warranted. Although survival does not differ between those treated or untreated with cyclophosphamide, cyclophosphamide is associated with a reduced incidence of relapse and an improved clinical response to treatment. Other therapies that have been used successfully in the management of EGPA include azathioprine, methotrexate, intravenous gamma globulin, and interferon α. Plasma exchange has not been shown to provide any additional benefit. Recent studies examining the efficacy of anti-IL-5 therapy have shown promise.
HYPEREOSINOPHILIC SYNDROMES
Hypereosinophilic syndromes (HES) constitute a heterogeneous group of disease entities manifest by persistent eosinophilia >1500 eosinophils/μL in association with end organ damage or dysfunction, in the absence of secondary causes of eosinophilia. In addition to familial, undefined, and overlap syndromes with incomplete criteria, the predominant HES subtypes are the myeloproliferative and lymphocytic variants. The myeloproliferative variant may be divided into three subgroups: (1) chronic eosinophilic leukemia with demonstrable cytogenetic abnormalities and/or blasts on peripheral smear; (2) the platelet-derived growth factor receptor α (PDGFRα)–associated HES, attributed to a constitutively activated tyrosine kinase fusion protein (Fip1L1-PDGFRα) due to a chromosomal deletion on 4q12; this variant is often responsive to imatinib; and (3) the FIP1-negative variant associated with clonal eosinophilia and at least four of the following: dysplastic peripheral eosinophils, increased serum vitamin B12, increased tryptase, anemia, thrombocytopenia, splenomegaly, bone marrow cellularity >80%, spindle-shaped mast cells, and myelofibrosis.
Extrapulmonary Manifestations of HES More common in men than in women, HES occurs between the ages of 20 and 50 and is characterized by significant extrapulmonary involvement, including infiltration of the heart, GI tract, kidney, liver, joints, and skin. Cardiac involvement includes myocarditis and/or endomyocardial fibrosis, as well as a restrictive cardiomyopathy.
Pulmonary Manifestations of HES Similar to the other pulmonary eosinophilic syndromes, these HES are manifest by high levels of blood, BAL, and tissue eosinophilia. Lung involvement occurs in 40% of these patients and is characterized by cough and dyspnea, as well as pulmonary infiltrates. Although it is often difficult to discern the pulmonary infiltrates and effusions seen on chest x-ray from pulmonary edema resulting from cardiac involvement, CT scan findings include interstitial infiltrates, ground-glass opacities, and small nodules. HES are typically not associated with ANCA or elevated IgE.
Course and Response to Therapy Unlike the other pulmonary eosinophilic syndromes, less than half of patients with these HES respond to corticosteroids as first-line therapy. Although other treatment options include hydroxyurea, cyclosporine, and interferon, the tyrosine kinase inhibitor imatinib has emerged as an important therapeutic option for patients with the myeloproliferative variant. Anti-IL-5 therapy with mepolizumab also holds promise for these patients and is currently being investigated.
ALLERGIC BRONCHOPULMONARY ASPERGILLOSIS
Allergic bronchopulmonary aspergillosis (ABPA) is an eosinophilic pulmonary disorder that occurs in response to allergic sensitization to antigens from Aspergillus species fungi. The predominant clinical presentation of ABPA is an asthmatic phenotype, often accompanied by cough with production of brownish plugs of mucus. ABPA has also been well described as a complication of cystic fibrosis. A workup for ABPA may be beneficial in patients who carry a diagnosis of asthma but have proven refractory to usual therapy. ABPA is a distinct diagnosis from simple asthma, characterized by prominent peripheral eosinophilia and elevated circulating levels of IgE (>417 IU/mL). Establishing a diagnosis of ABPA also requires establishing sensitivity to Aspergillus antigens by skin test reactivity, positive serum precipitins for Aspergillus, and/or direct measurement of circulating specific IgG and IgE to Aspergillus. Central bronchiectasis is described as a classic finding on chest imaging in ABPA but is not necessary for making a diagnosis. Other possible findings on chest imaging include patchy infiltrates and evidence of mucus impaction.
Systemic glucocorticoids may be used in the treatment of ABPA that is persistently symptomatic despite the use of inhaled therapies for asthma. Courses of glucocorticoids should be tapered over 3–6 months, and their use must be balanced against the risks of prolonged steroid therapy. Antifungal agents such as fluconazole and voriconazole given over a 4-month course reduce the antigenic stimulus in ABPA and may therefore modulate disease activity in selected patients. The use of monoclonal antibody against IgE (omalizumab) has been described in treating severe ABPA, particularly in individuals with ABPA as a complication of cystic fibrosis.
ABPA-like syndromes have been reported as a result to sensitization to several non-Aspergillus species fungi. However, these conditions are substantially rarer than ABPA, which may be present in a significant proportion of patients with refractory asthma.
INFECTIOUS PROCESSES
Infectious etiologies of pulmonary eosinophilia are largely due to helminths and are of particular importance in the evaluation of pulmonary eosinophilia in tropical environments and in the developing world (Table 310-4). These infectious conditions may also be considered in recent travelers to endemic regions. Loffler syndrome refers to transient pulmonary infiltrates with eosinophilia that occurs in response to passage of helminthic larvae through the lungs, most commonly larvae of Ascaris species (roundworm). Symptoms are generally self-limited and may include dyspnea, cough, wheeze, and hemoptysis. Loffler syndrome may also occur in response to hookworm infection with Ancylostoma duodenale or Necator americanus. Chronic Strongyloides stercoralis infection can lead to recurrent respiratory symptoms with peripheral eosinophilia between flares. In immunocompromised hosts, including patients on glucocorticoids, a severe, potentially fatal, hyperinfection syndrome can result from Strongyloides infection. Paragonimiasis, filariasis, and visceral larval migrans can all cause pulmonary eosinophilia as well.
INFECTIOUS CAUSES OF PULMONARY EOSINOPHILIA |
Source: Adapted from P Akuthota, PF Weller: Clin Microbiol Rev 25:649, 2012.
DRUGS AND TOXINS
A host of medications are associated with the development of pulmonary infiltrates with peripheral eosinophilia. Therefore, drug reaction must always be included in the differential diagnosis of pulmonary eosinophilia. Although the list of medications associated with pulmonary eosinophilia is ever expanding, common culprits include nonsteroidal anti-inflammatory medications and systemic antibiotics, most specifically nitrofurantoin. Additionally, various and diverse environmental exposures such as particulate metals, scorpion stings, and inhalational drugs of abuse may also cause pulmonary eosinophilia. Radiation therapy for breast cancer has been linked with eosinophilic pulmonary infiltration as well. The mainstay of treatment is removal of the offending exposure, although glucocorticoids may be necessary if respiratory symptoms are severe.
GLOBAL CONSIDERATIONS
![]() In the United States, drug-induced eosinophilic pneumonias are the most common cause of eosinophilic pulmonary infiltrates. A travel history or evidence of recent immigration should prompt the consideration of parasite-associated disorders. Tropical eosinophilia is usually caused by filarial infection; however, eosinophilic pneumonias also occur with other parasites such as Ascaris spp., Ancylostoma spp., Toxocara spp., and Strongyloides stercoralis. Tropical eosinophilia due to Wuchereria bancrofti or Wuchereria malayi occurs most commonly in southern Asia, Africa, and South America and is treated successfully with diethylcarbamazine. In the United States, Strongyloides is endemic to the southeastern and Appalachian regions.
In the United States, drug-induced eosinophilic pneumonias are the most common cause of eosinophilic pulmonary infiltrates. A travel history or evidence of recent immigration should prompt the consideration of parasite-associated disorders. Tropical eosinophilia is usually caused by filarial infection; however, eosinophilic pneumonias also occur with other parasites such as Ascaris spp., Ancylostoma spp., Toxocara spp., and Strongyloides stercoralis. Tropical eosinophilia due to Wuchereria bancrofti or Wuchereria malayi occurs most commonly in southern Asia, Africa, and South America and is treated successfully with diethylcarbamazine. In the United States, Strongyloides is endemic to the southeastern and Appalachian regions.
ACKNOWLEDGMENTS
We acknowledge the contributions of Dr. Alicia K. Gerke and Dr. Gary W. Hunninghake to the previous edition of this chapter.
311 | Occupational and Environmental Lung Disease |
Occupational and environmental lung diseases are difficult to distinguish from those of nonenvironmental origin. Virtually all major categories of pulmonary disease can be caused by environmental agents, and environmentally related disease usually presents clinically in a manner indistinguishable from that of disease not caused by such agents. In addition, the etiology of many diseases may be multifactorial; occupational and environmental factors may interact with other factors (such as smoking and genetic risk). It is often only after a careful exposure history is taken that the underlying workplace or general environmental exposure is uncovered.
Why is knowledge of occupational or environmental etiology so important? Patient management and prognosis are affected significantly by such knowledge. For example, patients with occupational asthma or hypersensitivity pneumonitis often cannot be managed adequately without cessation of exposure to the offending agent. Establishment of cause may have significant legal and financial implications for a patient who no longer can work in his or her usual job. Other exposed people may be identified as having the disease or prevented from getting it. In addition, new associations between exposure and disease may be identified (e.g., nylon flock worker’s lung disease and diacetyl-induced bronchiolitis obliterans).
Although the exact proportion of lung disease due to occupational and environmental factors is unknown, a large number of individuals are at risk. For example, 15–20% of the burden of adult asthma and chronic obstructive pulmonary disease (COPD) has been estimated to be due to occupational factors.
HISTORY AND EXPOSURE ASSESSMENT
The patient’s history is of paramount importance in assessing any potential occupational or environmental exposure. Inquiry into specific work practices should include questions about the specific contaminants involved, the presence of visible dusts, chemical odors, the size and ventilation of workspaces, the use of respiratory protective equipment, and whether co-workers have similar complaints. The temporal association of exposure at work and symptoms may provide clues to occupation-related disease. In addition, the patient must be questioned about alternative sources of exposure to potentially toxic agents, including hobbies, home characteristics, exposure to secondhand smoke, and proximity to traffic or industrial facilities. Short-term and long-term exposures to potential toxic agents in the distant past also must be considered.
Workers in the United States have the right to know about potential hazards in their workplaces under federal Occupational Safety and Health Administration (OSHA) regulations. Employers must provide specific information about potential hazardous agents in products being used through Material Safety Data Sheets as well as training in personal protective equipment and environmental control procedures. However, the introduction of new processes and/or new chemical compounds may change exposure significantly, and often only the employee on the production line is aware of the change. For the physician caring for a patient with a suspected work-related illness, a visit to the work site can be very instructive. Alternatively, an affected worker can request an inspection by OSHA. If reliable environmental sampling data are available, that information should be used in assessing a patient’s exposure. Because many of the chronic diseases result from exposure over many years, current environmental measurements should be combined with work histories to arrive at estimates of past exposure.
PULMONARY FUNCTION TESTS AND CHEST IMAGING
Exposures to inorganic and organic dusts can cause interstitial lung disease that presents with a restrictive pattern and a decreased diffusing capacity (Chap. 306e). Similarly, exposures to a number of organic dusts or chemical agents may result in occupational asthma or COPD that is characterized by airway obstruction. Measurement of change in forced expiratory volume (FEV1) before and after a working shift can be used to detect an acute bronchoconstrictive response.
The chest radiograph is useful in detecting and monitoring the pulmonary response to mineral dusts, certain metals, and organic dusts capable of inducing hypersensitivity pneumonitis. The International Labour Organisation (ILO) International Classification of Radiographs of Pneumoconioses classifies chest radiographs by the nature and size of opacities seen and the extent of involvement of the parenchyma. In general, small rounded opacities are seen in silicosis or coal worker’s pneumoconiosis, and small linear opacities are seen in asbestosis. Although useful for epidemiologic studies and screening large numbers of workers, the ILO system can be problematic when applied to an individual worker’s chest radiograph. With dusts causing rounded opacities, the degree of involvement on the chest radiograph may be extensive, whereas pulmonary function may be only minimally impaired. In contrast, in pneumoconiosis causing linear, irregular opacities like those seen in asbestosis, the radiograph may lead to underestimation of the severity of the impairment until relatively late in the disease. For patients with a history of asbestos exposure, conventional computed tomography (CT) is more sensitive for the detection of pleural thickening, and high-resolution CT (HRCT) improves the detection of asbestosis.
Other procedures that may be of use in identifying the role of environmental exposures in causing lung disease include skin prick testing or specific IgE antibody titers for evidence of immediate hypersensitivity to agents capable of inducing occupational asthma (flour antigens in bakers), specific IgG precipitating antibody titers for agents capable of causing hypersensitivity pneumonitis (pigeon antigen in bird handlers), and assays for specific cell-mediated immune responses (beryllium lymphocyte proliferation testing in nuclear workers or tuberculin skin testing in health care workers). Sometimes a bronchoscopy to obtain transbronchial biopsies of lung tissue may be required for histologic diagnosis (chronic beryllium disease). Rarely, video-assisted thoracoscopic surgery to obtain a larger sample of lung tissue may be required to determine the specific diagnosis of environmentally induced lung disease (hypersensitivity pneumonitis or giant cell interstitial pneumonitis due to cobalt exposure).
DETERMINANTS OF INHALATIONAL EXPOSURE
The chemical and physical characteristics of inhaled agents affect both the dose and the site of deposition in the respiratory tract. Water-soluble gases such as ammonia and sulfur dioxide are absorbed in the lining fluid of the upper and proximal airways and thus tend to produce irritative and bronchoconstrictive responses. In contrast, nitrogen dioxide and phosgene, which are less soluble, may penetrate to the bronchioles and alveoli in sufficient quantities to produce acute chemical pneumonitis.
Particle size of air contaminants must also be considered. Because of their settling velocities in air, particles >10–15 μm in diameter do not penetrate beyond the nose and throat. Particles <10 μm in size are deposited below the larynx. These particles are divided into three size fractions on the basis of their size characteristics and sources. Particles ~2.5–10 μm (coarse-mode fraction) contain crustal elements such as silica, aluminum, and iron. These particles mostly deposit relatively high in the tracheobronchial tree. Although the total mass of an ambient sample is dominated by these larger respirable particles, the number of particles, and therefore the surface area on which potential toxic agents can deposit and be carried to the lower airways, is dominated by particles <2.5 μm (fine-mode fraction). These fine particles are created primarily by the burning of fossil fuels or high-temperature industrial processes resulting in condensation products from gases, fumes, or vapors. The smallest particles, those <0.1 μm in size, represent the ultrafine fraction and make up the largest number of particles; they tend to remain in the airstream and deposit in the lung only on a random basis as they come into contact with the alveolar walls. If they do deposit, however, particles of this size range may penetrate into the circulation and be carried to extrapulmonary sites. New technologies create particles of this size (“nanoparticles”) for use in many commercial applications. Besides the size characteristics of particles and the solubility of gases, the actual chemical composition, mechanical properties, and immunogenicity or infectivity of inhaled material determine in large part the nature of the diseases found among exposed persons.
OCCUPATIONAL EXPOSURES AND PULMONARY DISEASE
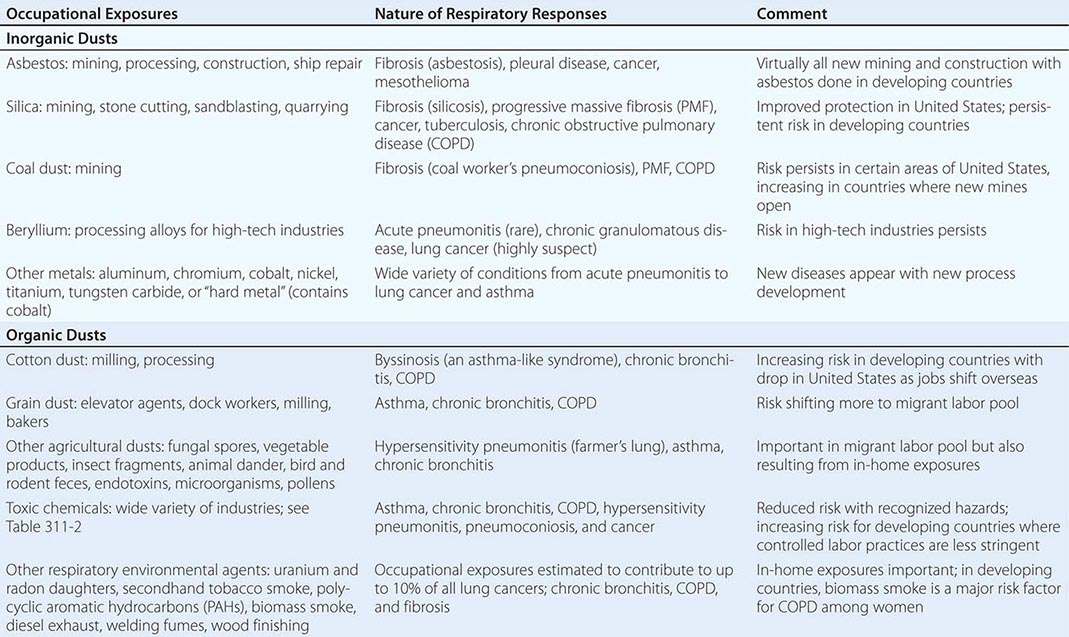
Table 311-1 provides broad categories of exposure in the workplace and diseases associated with chronic exposure in those industries.
CATEGORIES OF OCCUPATIONAL EXPOSURE AND ASSOCIATED RESPIRATORY CONDITIONS |
ASBESTOS-RELATED DISEASES
Asbestos is a generic term for several different mineral silicates, including chrysolite, amosite, anthophyllite, and crocidolite. In addition to workers involved in the production of asbestos products (mining, milling, and manufacturing), many workers in the shipbuilding and construction trades, including pipe fitters and boilermakers, were occupationally exposed because asbestos was widely used during the twentieth century for its thermal and electrical insulation properties. Asbestos also was used in the manufacture of fire-resistant textiles, in cement and floor tiles, and in friction materials such as brake and clutch linings.
Exposure to asbestos is not limited to persons who directly handle the material. Cases of asbestos-related diseases have been encountered in individuals with only bystander exposure, such as painters and electricians who worked alongside insulation workers in a shipyard. Community exposure resulted from the use of asbestos-containing mine and mill tailings as landfill, road surface, and playground material (e.g., Libby, MT, the site of a vermiculite mine in which the ore was contaminated with asbestos). Finally, exposure can occur from the disturbance of naturally occurring asbestos (e.g., from increasing residential development in the foothills of the Sierra Mountains in California).
Asbestos has largely been replaced in the developed world with synthetic mineral fibers such as fiberglass and refractory ceramic fibers, but it continues to be used in the developing world. The major health effects from exposure to asbestos are pleural and pulmonary fibrosis, cancers of the respiratory tract, and pleural and peritoneal mesothelioma.
Asbestosis is a diffuse interstitial fibrosing disease of the lung that is directly related to the intensity and duration of exposure. The disease resembles other forms of diffuse interstitial fibrosis (Chap. 315). Usually, exposure has taken place for at least 10 years before the disease becomes manifest. The mechanisms by which asbestos fibers induce lung fibrosis are not completely understood but are known to involve oxidative injury due to the generation of reactive oxygen species by the transition metals on the surface of the fibers as well as from cells engaged in phagocytosis.
Past exposure to asbestos is specifically indicated by pleural plaques on chest radiographs, which are characterized by either thickening or calcification along the parietal pleura, particularly along the lower lung fields, the diaphragm, and the cardiac border. Without additional manifestations, pleural plaques imply only exposure, not pulmonary impairment. Benign pleural effusions also may occur. The fluid is typically a serous or bloody exudate. The effusion may be slowly progressive or may resolve spontaneously.
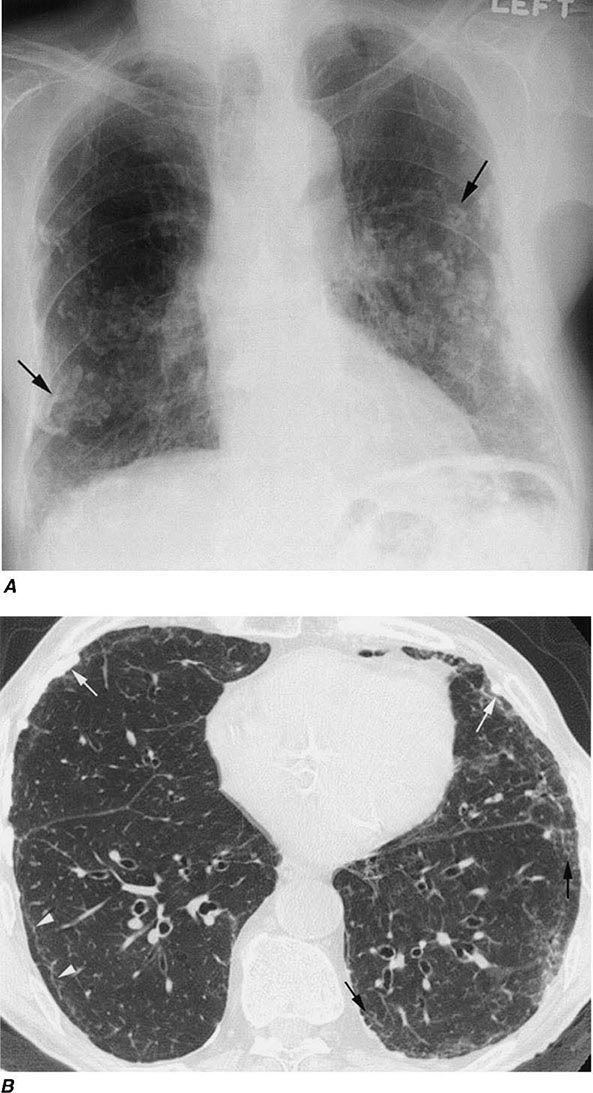
Irregular or linear opacities that usually are first noted in the lower lung fields are the chest radiographic hallmark of asbestosis. An indistinct heart border or a “ground-glass” appearance in the lung fields may be seen. HRCT may show distinct changes of subpleural curvilinear lines 5–10 mm in length that appear to be parallel to the pleural surface (Fig. 311-1).
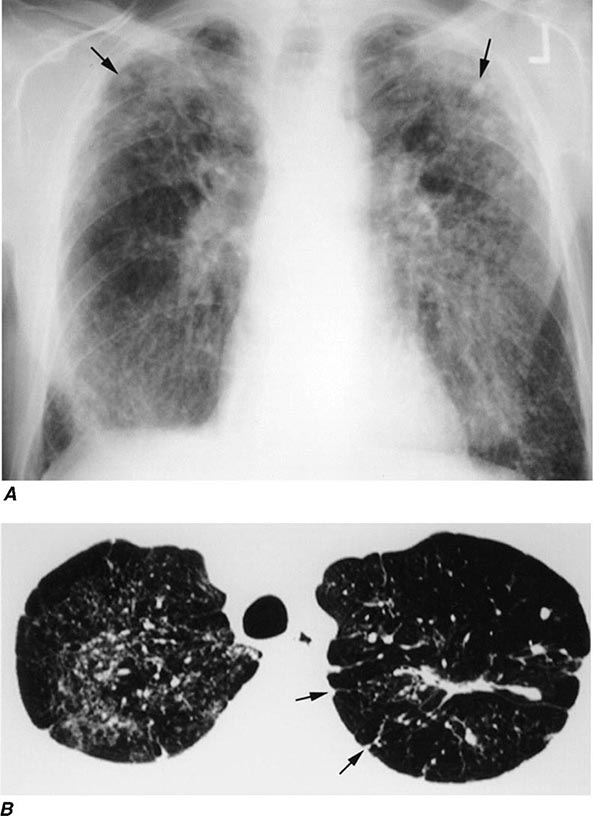
FIGURE 311-1 Asbestosis. A. Frontal chest radiograph shows bilateral calcified pleural plaques consistent with asbestos-related pleural disease. Poorly defined linear and reticular abnormalities are seen in the lower lobes bilaterally. B. Axial high-resolution computed tomography of the thorax obtained through the lung bases shows bilateral, subpleural reticulation (black arrows), representing fibrotic lung disease due to asbestosis. Subpleural lines are also present (arrowheads), characteristic of, though not specific for, asbestosis. Calcified pleural plaques representing asbestos-related pleural disease (white arrows) are also evident.
Pulmonary function testing in asbestosis reveals a restrictive pattern with a decrease in both lung volumes and diffusing capacity. There may also be evidence of mild airflow obstruction (due to peribronchiolar fibrosis).
Because no specific therapy is available for asbestosis, supportive care is the same as that given to any patient with diffuse interstitial fibrosis of any cause. In general, newly diagnosed cases will have resulted from exposures that occurred many years before.
Lung cancer (Chap. 107) is the most common cancer associated with asbestos exposure. The excess frequency of lung cancer (all histologic types) in asbestos workers is associated with a minimum latency of 15–19 years between first exposure and development of the disease. Persons with more exposure are at greater risk of disease. In addition, there is a significant interactive effect of smoking and asbestos exposure that results in greater risk than what would be expected from the additive effect of each factor.
Mesotheliomas (Chap. 316), both pleural and peritoneal, are also associated with asbestos exposure. In contrast to lung cancers, these tumors do not appear to be associated with smoking. Relatively short-term asbestos exposures of ≤1–2 years, occurring up to 40 years in the past, have been associated with the development of mesotheliomas (an observation that emphasizes the importance of obtaining a complete environmental exposure history). Although the risk of mesothelioma is much less than that of lung cancer among asbestos-exposed workers, over 2000 cases were reported in the United States per year at the start of the twenty-first century.
Because epidemiologic studies have shown that >80% of mesotheliomas may be associated with asbestos exposure, documented mesothelioma in a patient with occupational or environmental exposure to asbestos may be compensable.
SILICOSIS
Despite being one of the oldest known occupational pulmonary hazards, free silica (SiO2), or crystalline quartz, is still a major cause of disease. The major occupational exposures include mining; stonecutting; sand blasting; glass and cement manufacturing; foundry work; packing of silica flour; and quarrying, particularly of granite. Most often, pulmonary fibrosis due to silica exposure (silicosis) occurs in a dose-response fashion after many years of exposure.
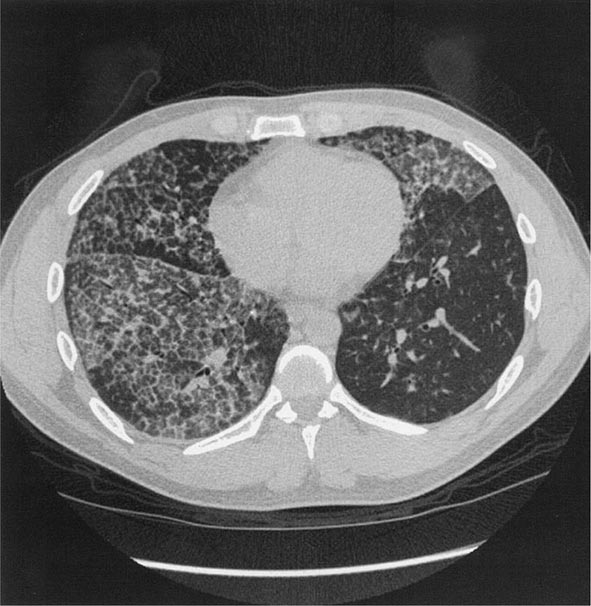
Workers heavily exposed through sandblasting in confined spaces, tunneling through rock with a high quartz content (15–25%), or the manufacture of abrasive soaps may develop acute silicosis with as little as 10 months of exposure. The clinical and pathologic features of acute silicosis are similar to those of pulmonary alveolar proteinosis (Chap. 315). The chest radiograph may show profuse miliary infiltration or consolidation, and there is a characteristic HRCT pattern known as “crazy paving” (Fig. 311-2). The disease may be quite severe and progressive despite the discontinuation of exposure. Whole-lung lavage may provide symptomatic relief and slow the progression.
FIGURE 311-2 Acute silicosis. This high-resolution computed tomography scan shows multiple small nodules consistent with silicosis but also diffuse ground-glass densities with thickened intralobular and interlobular septa producing polygonal shapes. This has been referred to as “crazy paving.”
With long-term, less intense exposure, small rounded opacities in the upper lobes may appear on the chest radiograph after 15–20 years of exposure, usually without associated impairment of lung function (simple silicosis). Calcification of hilar nodes may occur in as many as 20% of cases and produces a characteristic “eggshell” pattern. Silicotic nodules may be identified more readily by HRCT (Fig. 311-3). The nodular fibrosis may be progressive in the absence of further exposure, with coalescence and formation of nonsegmental conglomerates of irregular masses >1 cm in diameter (complicated silicosis). These masses can become quite large, and when this occurs, the term progressive massive fibrosis (PMF) is applied. Significant functional impairment with both restrictive and obstructive components may be associated with PMF.
FIGURE 311-3 Chronic silicosis. A. Frontal chest radiograph in a patient with silicosis shows variably sized, poorly defined nodules (arrows) predominating in the upper lobes. B. Axial thoracic computed tomography image through the lung apices shows numerous small nodules, more pronounced in the right upper lobe. A number of the nodules are subpleural in location (arrows).
Because silica is cytotoxic to alveolar macrophages, patients with silicosis are at greater risk of acquiring lung infections that involve these cells as a primary defense (Mycobacterium tuberculosis, atypical mycobacteria and fungi). Because of the increased risk of active tuberculosis, the recommended treatment of latent tuberculosis in these patients is longer. Another potential clinical complication of silicosis is autoimmune connective tissue disorders such as rheumatoid arthritis and scleroderma. In addition, there are sufficient epidemiologic data that the International Agency for Research on Cancer lists silica as a probable lung carcinogen.
Other, less hazardous silicates include fuller’s earth, kaolin, mica, diatomaceous earths, silica gel, soapstone, carbonate dusts, and cement dusts. The production of fibrosis in workers exposed to these agents is believed to be related either to the free silica content of these dusts or, for substances that contain no free silica, to the potentially large dust loads to which these workers may be exposed. Some silicates, including talc and vermiculite, may be contaminated with asbestos. Fibrosis of lung or pleura, lung cancer, and mesothelioma have been associated with chronic exposure to talc and vermiculite dusts.
COAL WORKER’S PNEUMOCONIOSIS (CWP)
Occupational exposure to coal dust can lead to CWP, which has enormous social, economic, and medical significance in every nation in which coal mining is an important industry. Simple radiographically identified CWP is seen in ~10% of all coal miners and in as many as 50% of anthracite miners with more than 20 years of work on the coal face. The prevalence of disease is lower in workers in bituminous coal mines.
With prolonged exposure to coal dust (i.e., 15–20 years), small, rounded opacities similar to those of silicosis may develop. As in silicosis, the presence of these nodules (simple CWP) usually is not associated with pulmonary impairment. In addition to CWP, coal dust can cause chronic bronchitis and COPD (Chap. 314). The effects of coal dust are additive to those of cigarette smoking.
Complicated CWP is manifested by the appearance on the chest radiograph of nodules ≥1 cm in diameter generally confined to the upper half of the lungs. As in silicosis, this condition can progress to PMF that is accompanied by severe lung function deficits and associated with premature mortality. Despite improvements in technology to protect coal miners, cases of PMF still occur in the United States at a disturbing rate.
Caplan syndrome (Chap. 380), first described in coal miners but subsequently in patients with silicosis, is the combination of pneumoconiotic nodules and seropositive rheumatoid arthritis. Silica has immunoadjuvant properties and is often present in anthracitic coal dust.
CHRONIC BERYLLIUM DISEASE
Beryllium is a lightweight metal with tensile strength, good electrical conductivity, and value in the control of nuclear reactions through its ability to quench neutrons. Although beryllium may produce an acute pneumonitis, it is far more commonly associated with a chronic granulomatous inflammatory disease that is similar to sarcoidosis (Chap. 390). Unless one inquires specifically about occupational exposures to beryllium in the manufacture of alloys, ceramics, or high-technology electronics in a patient with sarcoidosis, one may miss entirely the etiologic relationship to the occupational exposure. What distinguishes chronic beryllium disease (CBD) from sarcoidosis is evidence of a specific cell-mediated immune response (i.e., delayed hypersensitivity) to beryllium.
The test that usually provides this evidence is the beryllium lymphocyte proliferation test (BeLPT). The BeLPT compares the in vitro proliferation of lymphocytes from blood or bronchoalveolar lavage in the presence of beryllium salts with that of unstimulated cells. Proliferation is usually measured by lymphocyte uptake of radiolabeled thymidine.
Chest imaging findings are similar to those of sarcoidosis (nodules along septal lines) except that hilar adenopathy is somewhat less common. As with sarcoidosis, pulmonary function test results may show restrictive and/or obstructive ventilatory deficits and decreased diffusing capacity. With early disease, both chest imaging studies and pulmonary function tests may be normal. Fiberoptic bronchoscopy with transbronchial lung biopsy usually is required to make the diagnosis of CBD. In a beryllium-sensitized individual, the presence of noncaseating granulomas or monocytic infiltration in lung tissue establishes the diagnosis. Accumulation of beryllium-specific CD4+ T cells occurs in the granulomatous inflammation seen on lung biopsy. Susceptibility to CBD is highly associated with human leukocyte antigen DP (HLA-DP) alleles that have a glutamic acid in position 69 of the β chain.
OTHER METALS
Aluminum and titanium dioxide have been rarely associated with a sarcoid-like reaction in lung tissue. Exposure to dust containing tungsten carbide, also known as “hard metal,” may produce giant cell interstitial pneumonitis. Cobalt is a constituent of tungsten carbide and is the likely etiologic agent of both the interstitial pneumonitis and the occupational asthma that may occur. The most common exposures to tungsten carbide occur in tool and dye, saw blade, and drill bit manufacture. Diamond polishing may also involve exposure to cobalt dust. In patients with interstitial lung disease, one should always inquire about exposure to metal fumes and/or dusts. Especially when sarcoidosis appears to be the diagnosis, one should always consider possible CBD.
OTHER INORGANIC DUSTS
Most of the inorganic dusts discussed thus far are associated with the production of either dust macules or interstitial fibrotic changes in the lung. Other inorganic and organic dusts (see categories in Table 311-1), along with some of the dusts previously discussed, are associated with chronic mucus hypersecretion (chronic bronchitis), with or without reduction of expiratory flow rates. Cigarette smoking is the major cause of these conditions, and any effort to attribute some component of the disease to occupational and environmental exposures must take cigarette smoking into account. Most studies suggest an additive effect of dust exposure and smoking. The pattern of the irritant dust effect is similar to that of cigarette smoking, suggesting that small airway inflammation may be the initial site of pathologic response in those cases and continued exposure may lead to chronic bronchitis and COPD.
ORGANIC DUSTS
Some of the specific diseases associated with organic dusts are discussed in detail in the chapters on asthma (Chap. 309) and hypersensitivity pneumonitis (Chap. 310). Many of these diseases are named for the specific setting in which they are found, e.g., farmer’s lung, malt worker’s disease, and mushroom worker’s disease. Often the temporal relation of symptoms to exposure furnishes the best evidence for the diagnosis. Three occupational exposures are singled out for discussion here because they affect the largest proportions of workers.
Cotton Dust (Byssinosis) Workers occupationally exposed to cotton dust (but also to flax, hemp, or jute dust) in the production of yarns for textiles and rope making are at risk for an asthma-like syndrome known as byssinosis. Exposure occurs throughout the manufacturing process but is most pronounced in the portions of the factory involved with the treatment of the cotton before spinning, i.e., blowing, mixing, and carding (straightening of fibers). The risk of byssinosis is associated with both cotton dust and endotoxin levels in the workplace environment.
Byssinosis is characterized clinically as occasional (early-stage) and then regular (late-stage) chest tightness toward the end of the first day of the workweek (“Monday chest tightness”). Exposed workers may show a significant drop in FEV1 over the course of a Monday workshift. Initially the symptoms do not recur on subsequent days of the week. However, in 10–25% of workers, the disease may be progressive, with chest tightness recurring or persisting throughout the workweek. After >10 years of exposure, workers with recurrent symptoms are more likely to have an obstructive pattern on pulmonary function testing. The highest grades of impairment generally are seen in smokers.
Dust exposure can be reduced by the use of exhaust hoods, general increases in ventilation, and wetting procedures, but respiratory protective equipment may be required during certain operations. Regular surveillance of pulmonary function in cotton dust–exposed workers using spirometry before and after the workshift is required by OSHA. All workers with persistent symptoms or significantly reduced levels of pulmonary function should be moved to areas of lower risk of exposure.
Grain Dust Worldwide, many farmers and workers in grain storage facilities are exposed to grain dust. The presentation of obstructive airway disease in grain dust–exposed workers is virtually identical to the characteristic findings in cigarette smokers, i.e., persistent cough, mucus hypersecretion, wheeze and dyspnea on exertion, and reduced FEV1 and FEV1/FVC (forced vital capacity) ratio (Chap. 306e).
Dust concentrations in grain elevators vary greatly but can be >10,000 μg/m3 with many particles in the respirable size range. The effect of grain dust exposure is additive to that of cigarette smoking, with ~50% of workers who smoke having symptoms. Smoking grain dust–exposed workers are more likely to have obstructive ventilatory deficits on pulmonary function testing. As in byssinosis, endotoxin may play a role in grain dust–induced chronic bronchitis and COPD.
Farmer’s Lung This condition results from exposure to moldy hay containing spores of thermophilic actinomycetes that produce a hypersensitivity pneumonitis (Chap. 310). A patient with acute farmer’s lung presents 4–8 h after exposure with fever, chills, malaise, cough, and dyspnea without wheezing. The history of exposure is obviously essential to distinguish this disease from influenza or pneumonia with similar symptoms. In the chronic form of the disease, the history of repeated attacks after similar exposure is important in differentiating this syndrome from other causes of patchy fibrosis (e.g., sarcoidosis).
A wide variety of other organic dusts are associated with the occurrence of hypersensitivity pneumonitis (Chap. 310). For patients who present with hypersensitivity pneumonitis, specific and careful inquiry about occupations, hobbies, and other home environmental exposures is necessary to uncover the source of the etiologic agent.
TOXIC CHEMICALS
Exposure to toxic chemicals affecting the lung generally involves gases and vapors. A common accident is one in which the victim is trapped in a confined space where the chemicals have accumulated to harmful levels. In addition to the specific toxic effects of the chemical, the victim often sustains considerable anoxia, which can play a dominant role in determining whether the individual survives.
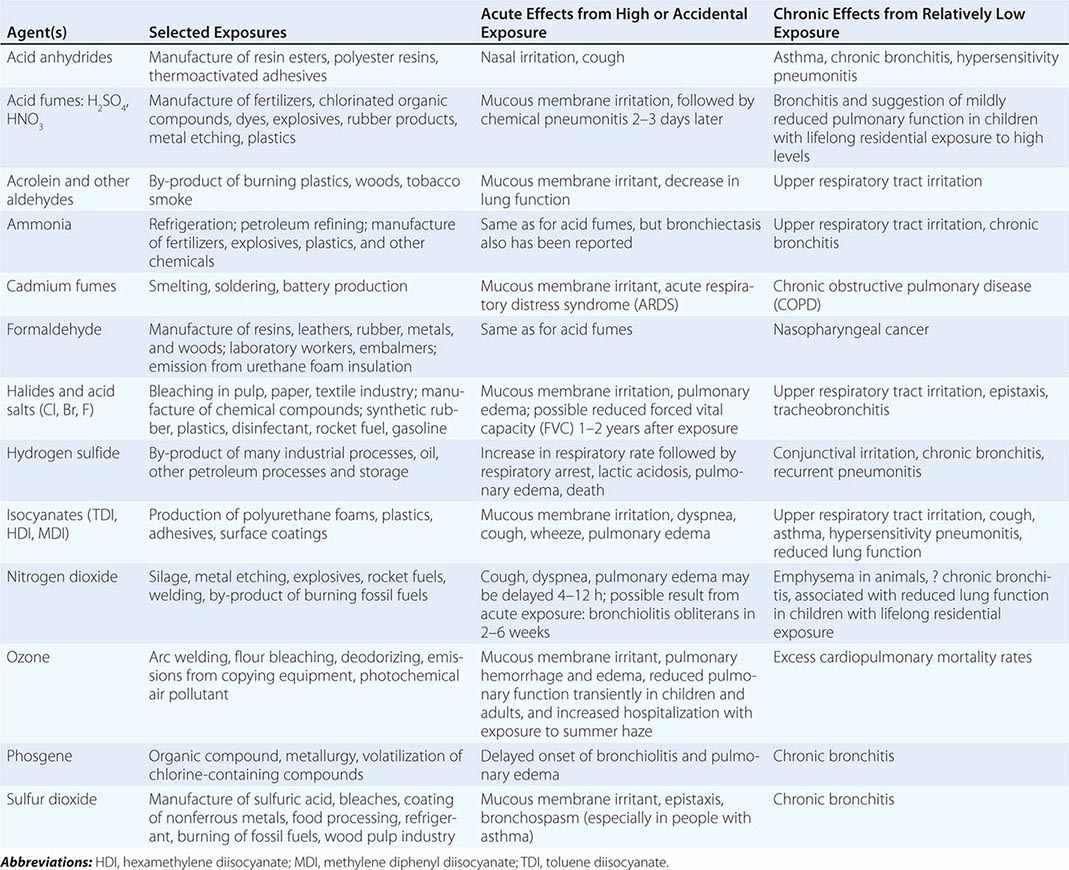
Table 311-2 lists a variety of toxic agents that can produce acute and sometimes life-threatening reactions in the lung. All these agents in sufficient concentrations have been demonstrated, at least in animal studies, to affect the lower airways and disrupt alveolar architecture, either acutely or as a result of chronic exposure. Some of these agents may be generated acutely in the environment (see below).
SELECTED COMMON TOXIC CHEMICAL AGENTS THAT AFFECT THE LUNG |
Firefighters and fire victims are at risk of smoke inhalation, an important cause of acute cardiorespiratory failure. Smoke inhalation kills more fire victims than does thermal injury. Carbon monoxide poisoning with resulting significant hypoxemia can be life-threatening (Chap. 473e). Synthetic materials (plastic, polyurethanes), when burned, may release a variety of other toxic agents (such as cyanide and hydrochloric acid), and this must be considered in evaluating smoke inhalation victims. Exposed victims may have some degree of lower respiratory tract inflammation and/or pulmonary edema.
Exposure to certain highly reactive, low-molecular-weight agents used in the manufacture of synthetic polymers, paints, and coatings (diisocyanates in polyurethanes, aromatic amines and acid anhydrides in epoxies) is associated with a high risk of occupational asthma. Although this occupational asthma manifests clinically as if sensitization has occurred, an IgE antibody–mediated mechanism is not necessarily involved. Hypersensitivity pneumonitis–like reactions also have been described in diisocyanate and acid anhydride–exposed workers.
Fluoropolymers such as Teflon, which at normal temperatures produce no reaction, become volatilized upon heating. The inhaled agents cause a characteristic syndrome of fever, chills, malaise, and occasionally mild wheezing, leading to the diagnosis of polymer fume fever. A similar self-limited, influenza-like syndrome—metal fume fever—results from acute exposure to fumes containing zinc oxide, typically from welding of galvanized steel. These inhalational fever syndromes may begin several hours after work and resolve within 24 h, only to return on repeated exposure.
Two other agents have been associated with potentially severe lung disease. Occupational exposure to nylon flock has been shown to induce a lymphocytic bronchiolitis, and workers exposed to diacetyl, which is used to provide “butter” flavor in the manufacture of microwave popcorn and other foods, have developed bronchiolitis obliterans (Chap. 315).
World Trade Center Disaster A consequence of the attack on the World Trade Center (WTC) on September 11, 2001, was relatively heavy exposure of a large number of firefighters and other rescue workers to the dust generated by the collapse of the buildings. Environmental monitoring and chemical characterization of WTC dust has revealed a wide variety of potentially toxic constituents, although much of the dust was pulverized cement. Possibly because of the high alkalinity of WTC dust, significant cough, wheeze, and phlegm production occurred among firefighters and cleanup crews. New cough and wheeze syndromes also occurred among local residents. Heavier exposure to WTC dust among New York City firefighters was associated with accelerated decline of lung function over the first year after the disaster. More recently, concerns have been raised about risk of interstitial lung disease, especially of a granulomatous nature.
OCCUPATIONAL RESPIRATORY CARCINOGENS
Exposures at work have been estimated to contribute to 10% of all lung cancer cases. In addition to asbestos, other agents either proven or suspected to be respiratory carcinogens include acrylonitrile, arsenic compounds, beryllium, bis(chloromethyl) ether, chromium (hexavalent), formaldehyde (nasal), isopropanol (nasal sinuses), mustard gas, nickel carbonyl (nickel smelting), polycyclic aromatic hydrocarbons (coke oven emissions and diesel exhaust), secondhand tobacco smoke, silica (both mining and processing), talc (possible asbestos contamination in both mining and milling), vinyl chloride (sarcomas), wood (nasal cancer only), and uranium. Workers at risk of radiation-related lung cancer include not only those involved in mining or processing uranium but also those exposed in underground mining operations of other ores where radon daughters may be emitted from rock formations.
ASSESSMENT OF DISABILITY
Disability is the term used to describe the decreased ability to work due to the effects of a medical condition. Physicians are generally able to assess physiologic dysfunction, or impairment, but the rating of disability for compensation of loss of income also involves nonmedical factors such as the education and employability of the individual. The disability rating scheme differs with the compensation-granting agency. For example, the U.S. Social Security Administration requires that an individual be unable to do any work (i.e., total disability) before he or she will receive income replacement payments. Many state workers’ compensation systems allow for payments for partial disability. In the Social Security scheme, no determination of cause is done, whereas work-relatedness must be established in workers’ compensation systems.
For respiratory impairment rating, resting pulmonary function tests (spirometry and diffusing capacity) are used as the initial assessment tool, with cardiopulmonary exercise testing (to assess maximal oxygen consumption) used if the results of the resting tests do not correlate with the patient’s symptoms. Methacholine challenge (to assess airway reactivity) can also be useful in patients with asthma who have normal spirometry when evaluated. Some compensation agencies (e.g., Social Security) have proscribed disability classification schemes based on pulmonary function test results. When no specific scheme is proscribed, the Guidelines of the American Medical Association should be used.
GENERAL ENVIRONMENTAL EXPOSURES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree