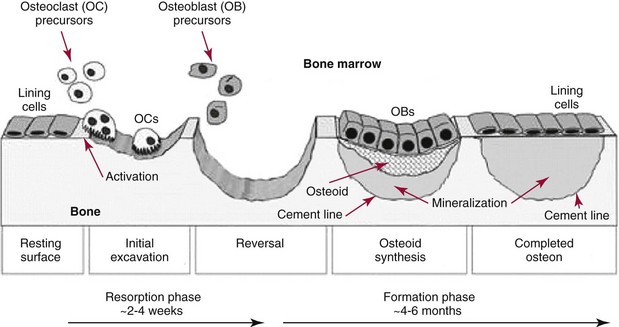
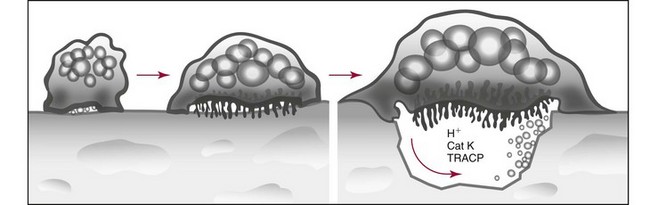
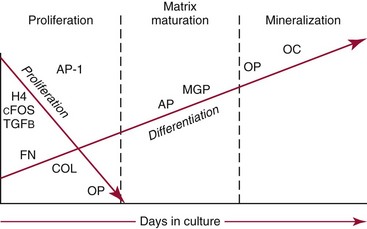
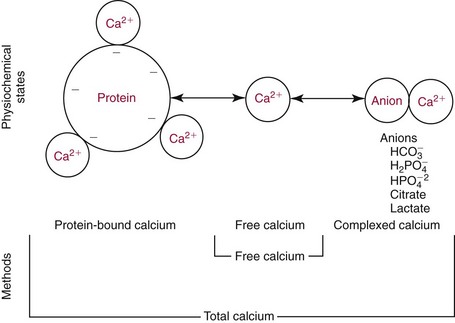
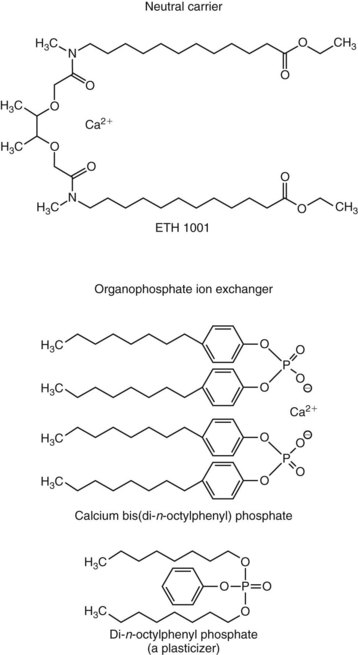
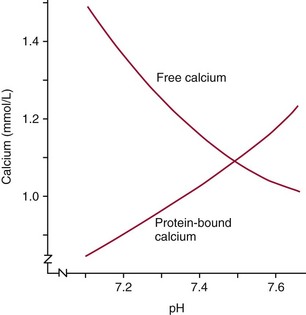
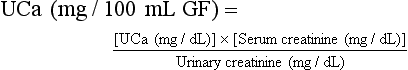Chapter 52 Juha Risteli, M.D., Ph.D., F.E.B.M.B., William E. Winter, M.D., Michael Kleerekoper, M.D., F.A.C.B., M.A.C.E. and Leila Risteli, M.D., Ph.D., M.A., F.E.B.M.B.* Bone is composed primarily of an extracellular mineralized matrix with a smaller cellular fraction. Bone is a dynamic tissue that is under continuous turnover or remodeling, which enables bone to repair damage and adjust strength. Osteoclasts and osteoblasts, the two main types of bone cells, are located on bone surfaces and are responsible for bone resorption and formation, respectively. Osteoclasts resorb bone, osteoblasts lay down new bone at a site of previous bone resorption, and osteocytes nourish the skeleton (Figure 52-1). Bone remodeling does not occur at random, but occurs instead in discrete packets known as bone remodeling units. Bone resorption and bone formation normally are coupled, with synthesis of new bone following the resorption of old. During menopause, the remodeling rate is often increased, but with excess resorption (a negative bone balance). The remodeling cycle can be divided into activation, resorption, reversal, formation, and termination phases. Circulating mononuclear osteoclast precursors are recruited, proliferate, and fuse to form osteoclasts. These giant multinucleated cells resorb bone by producing hydrogen ions to mobilize minerals and lysosomal enzymes to digest the organic matrix. Deep foldings of their plasma membrane (ruffled border) are in contact with the bone surface, forming the osteoclastic bone-resorbing compartment (Figure 52-2). The resorption lacuna contains degradative enzymes such as cathepsin K and several matrix metalloproteinases (MMPs). After resorption ceases, a cement line is deposited in the resorption cavity, probably by mononucleated cells. Stromal lining cells differentiate to osteoblasts. Osteoblasts form bone by synthesizing the organic matrix, including type I collagen, and participating in the mineralization of newly synthesized matrix. The development of the osteoblast phenotype has been divided into three consecutive phases, each with its typical gene expression patterns (Figure 52-3).181 Remodeling is followed by a quiescent or termination phase. Over the past few years, much new information about control of the bone remodeling cycle has become available. Understanding of signaling pathways has led to the development of potential new therapies.157 Two key signaling pathways are known as RANK/RANKL/OPG and Wnt. RANK (receptor activator of nuclear factor κ B) is a membrane protein expressed on the surface of osteoclasts. Its ligand (RANK ligand, or RANKL) is found on the surface of osteoblasts (also on stromal and T cells). Binding of RANK to RANKL activates osteoclasts (see Figure 52-2). Osteoprotegerin (OPG) is a cytokine [a member of the tumor necrosis factor (TNF) receptor superfamily] and a RANK homolog that can inhibit the production and maturation of osteoclasts by blocking the interaction of RANK with its ligand RANKL. OPG production is stimulated by estrogen, and the marked decrease in estrogen at menopause diminishes OPG production, leaving the way open for increased activation of osteoclasts and the resultant accelerated bone loss that accompanies menopause. The Wnt signaling pathway is far more complex and is involved in many physiologic systems beyond the skeleton. Key components that have been best studied thus far with respect to skeletal physiology include (1) the Frizzled family of G protein–coupled receptor proteins, (2) low-density lipoprotein receptor–related protein 5 encoded by the LRP5 gene and associated with high bone mass in affected families, (3) cathepsin K, (4) Dickkopf-related protein 1 (DKK1), and (5) sclerostin.238 An estimated 10 to 30% of the skeleton is remodeled each year, with wide variation among individuals. Bone growth and turnover are influenced by the metabolism of calcium, phosphate, and magnesium and several hormones, the primary ones being parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D [1,25(OH)2D]. Bone formation and resorption are affected, however, by a large number of other hormones and factors, including thyroid hormones, estrogens, androgens, cortisol, insulin, growth hormone, insulin-like growth factors (IGF-I and IGF-II), transforming growth factor-β (TGF-β), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF). Numerous cytokines alter bone remodeling primarily by stimulating resorption; these factors include interleukins (IL)-1, -4, -6 and -11; macrophage and granulocyte/macrophage colony-stimulating factors; and tumor necrosis factor-α (TNF-α). Findings suggest that leptin, which is secreted by adipocytes, can negatively regulate bone formation by osteoblasts.304,305 Calcium is the fifth most common element in the body and the most prevalent cation. An average human body contains about 1 kg, or ≈25 mol, of calcium (Table 52-1). The skeleton contains ≈99% of the body’s calcium, predominantly as extracellular crystals of unknown structure and a composition approaching that of hydroxyapatite [Ca10(PO4)6(OH)2]. Soft tissues and extracellular fluid contain about 1% of the body’s calcium. TABLE 52-1 Distribution of Calcium, Phosphate, and Magnesium in the Body Modified from Aurbach GD, Marx SJ, Speigel AM. Parathyroid hormone, calcitonin, and the calciferols. In: Wilson JD, Foster DW, eds. Williams textbook of endocrinology, 8th edition. Philadelphia, Pa: WB Saunders, 1992:1397-476. In blood, virtually all of the calcium is found in the plasma, which has a mean calcium concentration of ≈9.5 mg/dL (2.38 mmol/L). Calcium exists in three physicochemical states in plasma (Table 52-2): ≈50% is free (ionized), 40% is bound to plasma proteins, and 10% is complexed with small diffusible inorganic and organic anions, including bicarbonate, lactate, phosphate, and citrate.249 TABLE 52-2 Physicochemical States of Calcium, Phosphate, and Magnesium in Human Plasma Modified from Marshall RW. Plasma fractions. In: Nordin BEC, ed. Calcium, phosphate and magnesium metabolism. London: Churchill Livingstone, 1976:162-85. About 80% of protein-bound calcium is associated with albumin,88,168 with the remaining 20% associated with globulins. Because calcium binds to negatively charged sites on proteins, its binding is pH dependent. Alkalosis leads to an increase in negative charge and binding and a decrease in free calcium; conversely, acidosis leads to a decrease in negative charge and binding and an increase in free calcium. In vitro, for each 0.1 unit change in pH, approximately 0.2 mg/dL (0.05 mmol/L) of inverse change occurs in the serum free calcium concentration. Calcium can be redistributed among the three plasma pools, acutely or chronically, by alterations in the concentrations of protein and small anions, changes in pH, or changes in the quantities of free calcium and total calcium in the serum (Figure 52-4). Physiologically, calcium may be classified as intracellular or extracellular. Intracellular calcium has key roles in many important physiologic functions, including muscle contraction, hormone secretion, glycogen metabolism, and cell division.239 The intracellular concentration of calcium in the cytosol of unstimulated cells is around 0.1 µmol/L, which is less than Low total serum calcium (hypocalcemia) may be due to a reduction in albumin-bound calcium, the free fraction of calcium, or both (Box 52-1).272 Hypoalbuminemia is the most common cause of apparent hypocalcemia on a standard biochemical profile, particularly in hospitalized patients, because 1 g/dL of albumin binds approximately 0.8 mg/dL of calcium. Common clinical conditions associated with low serum albumin include chronic liver disease, nephrotic syndrome, congestive heart failure, malnutrition, and postsurgical volume replacement with saline or colloidal solutions. In these conditions, the concentration of free calcium typically is maintained in its physiologic reference interval. Common causes of true hypocalcemia are chronic renal failure and hypomagnesemia. In chronic renal failure, hypoproteinemia, hyperphosphatemia, low serum 1,25(OH)2D (caused by reduced renal synthesis), and/or skeletal resistance to PTH contribute to hypocalcemia. Magnesium deficiency also can lead to hypocalcemia through several mechanisms, including impairment of PTH secretion and decreased responsiveness of target organs to PTH action (end-organ resistance). Less common causes of hypocalcemia include hypoparathyroidism, pseudohypoparathyroidism, and activating mutations of the calcium-sensing receptor (CaSR). Hypoparathyroidism is due most commonly to parathyroid gland destruction during neck surgery (90%), and less commonly is associated with autoimmune endocrine disorders. Pseudohypoparathyroidism is biochemically similar to hypoparathyroidism, but these patients have (1) an inherited resistance to PTH, and (2) increased concentrations of PTH.190 The molecular basis for the most common form, pseudohypoparathyroidism type I [Albright’s hereditary osteodystrophy (AHO)], is an inactivating mutation in the gene coding for the stimulatory guanine nucleotide-binding protein in the adenylate cyclase complex. The causes of hypercalcemia are listed in Box 52-2. Primary hyperparathyroidism is the most common pathologic cause in outpatients, whereas malignancy is more common in hospitalized patients. Together, these two disorders account for 90 to 95% of all cases of hypercalcemia. PHPT is characterized by excessive secretion of PTH that results in hypercalcemia.282 It is most often due to a solitary adenoma (80 to 85% of cases), less frequently (about 15%) to hyperplasia involving all glands, and infrequently to parathyroid carcinoma (<1%).207 More than 80% of hyperparathyroid patients in developed countries are free of overt symptoms on presentation because of early detection of this disorder through the widespread use of chemistry panels that include calcium.282 The most common signs and symptoms of hypercalcemia are nonspecific and are related to the neuromuscular system. They include fatigue, malaise, and weakness with mild hypercalcemia (calcium <12 mg/dL or <3 mmol/L); depression, apathy, and inability to concentrate may be present at higher calcium concentrations. Hypercalcemia may induce mild nephrogenic diabetes insipidus with thirst, polydipsia, and polyuria. Renal colic caused by kidney stones can result from chronic hypercalcemia and hypercalciuria. Nephrocalcinosis can lead to slowly developing renal failure. Most patients with primary hyperparathyroidism (>60%) are postmenopausal women. Symptomatic patients with PHPT should undergo parathyroid surgery. If the patient is asymptomatic, guidelines have been established recommending surgery over monitoring depending on serum calcium concentration, creatinine clearance, urine calcium, bone mineral density, and age.282 In the “Guidelines for Management of Asymptomatic Primary Hyperparathyroidism” from the Third International Workshop on the topic,20A a serum calcium concentration greater than 1 mg/dL (0.25 mmol/L) above the reference interval was considered an indication for surgical intervention, as was a calculated creatinine clearance less than 60 mL/min. For patients who were to be managed without surgery, annual measurements of serum calcium and creatinine were recommended. Hypercalcemia occurs in 5 to 30% of individuals with cancer.188 Solid tissue malignancies commonly produce parathyroid hormone–related peptide (PTHrP), which is secreted into the circulation and stimulates bone resorption. PTHrP binds to the PTH receptor and is the principal mediator of humoral hypercalcemia of malignancy (HHM).294 Skeletal metastases from cancer also can produce hypercalcemia, but this is a late manifestation, reflecting a large metastatic burden, and less often presents a diagnostic problem. Cytokines such as lymphotoxin, interleukin-1, tumor necrosis factor, and PTHrP appear to be important mediators of hypercalcemia in multiple myeloma and other hematologic malignancies. Some lymphomas associated with acquired immunodeficiency syndrome or human T-lymphotropic virus type 1 (HTLV-1) infection cause hypercalcemia by producing 1,25(OH)2D. It is estimated that less than 5% of patients with hypercalcemic cancer have coexisting primary hyperparathyroidism.188 Laboratory test selection is similar to that in suspected hyperparathyroidism, with the addition of PTHrP in some individuals with HHM. However, PTHrP is rarely informative if the PTH is not suppressed.92 In specific instances (e.g., lymphoma, sarcoidosis), measurement of 1,25(OH)2D may be useful. Very recent studies of four families with idiopathic hypercalcemia of infancy (see Box 52-2) have identified a genetic basis for this condition.270A Sequence analysis of CYP24A1, which encodes 25-hydroxyvitamin D 24-hydroxylase, the key enzyme for degrading the active metabolite of vitamin D3, revealed recessive mutations in the affected children. In addition, CYP24A1 mutations were identified in a group of infants in whom severe hypercalcemia developed after bolus prophylaxis with vitamin D. Functional characterization revealed a complete loss of function in all CYP24A1 mutations.270a The decreased ability to prevent vitamin D actions (see below) appears to explain the hypercalcemia seen in the patients. Free calcium is the biologically active fraction of blood calcium; it is tightly regulated by PTH and 1,25(OH)2D, and thus is the best indicator of calcium status. Methods for both total and free calcium are currently in use and have their own sources of error. Free calcium measurements have been recommended because of the consequences of delayed treatment and the cost of working-up patients with misleading total calcium results.30 Various methods have been described for total calcium measurement. At present, only photometric, ion selective electrode (ISE), and occasionally atomic absorption spectrophotometry methods are used in clinical laboratories for measuring serum and urine total calcium.272 Total calcium is most frequently measured by spectrophotometry using metallochromic indicators or dyes. Of the metallochromic indicators that change color on selectively binding calcium (Figure 52-5), o-cresolphthalein complexone (CPC) [3′,3″-bis({bis-[carboxymethyl]amino}-methyl)-5′,5″-dimethylphenolphthalein] and arsenazo III are most widely used. These methods, although less accurate and reproducible than atomic absorption spectrometry, have been readily automated on chemistry analyzers. o-Cresolphthalein Complexone Method: In alkaline solution, the metal-complexing dye CPC forms a red chromophore with calcium; the color is usually measured at a wavelength between 570 and 580 nm. The sample is diluted with acid to release protein-bound and complexed calcium. Organic base, most often diethylamine, 2-amino-2-methyl-1-propanol, or 2-ethylaminoethanol, is added to buffer the reaction and to produce an alkaline pH. Interference by magnesium is reduced: (1) by adding 8-hydroxyquinoline (see Figure 52-5); (2) by buffering the reaction mixture to near pH 12; and (3) by measuring the absorbance near 580 nm. Urea may be added to reduce the turbidity of lipemic specimens and to enhance complex formation. Blank absorbance may be reduced by adding ethanol or other organic solvents. Calcium forms both 1 : 1 and 2 : 1 complexes with CPC, with the former predominating at lower concentrations.60 Because the 1 : 1 complex has lower molar absorptivity, the calibration curves are nonlinear at low calcium concentrations. Multipoint calibration of CPC methods has been recommended. Linearity may be improved by adding sodium acetate.61 The temperature must be carefully controlled because the reaction is temperature sensitive. Arsenazo III Method: Arsenazo III [1,8-dihydroxynaphthalene-3,6-disulfonic acid-2,7-bis(azo-2)-phenylarsonic acid] (see Figure 52-5), at mildly acidic pH, has much higher affinity for calcium than magnesium and binds it to produce an intense, purple complex. A reaction pH of about 6 is commonly used; imidazole has been used to buffer the reaction. The solution must be thoroughly buffered because the spectral properties of arsenazo III are dependent on pH. Binding of calcium to arsenazo III can be influenced by buffer and sodium concentration. Interference from most biological pigments is reduced by measuring the calcium-dye complex near 650 nm. Citrate has been reported to cause negative interference, particularly with dry-slide techniques; in these, the only source of fluid is the sample, and the effective concentration of citrate is much higher than in wet-chemistry methods.16,106 Clinically significant interference may be noted in patients receiving citrated blood or blood products. Unlike CPC, which has limited stability when used as a single reagent, the arsenazo III reagent is stable.174 Many photometric methods should not be used within 24 hours after a magnetic resonance imaging (MRI) examination of the patient in which gadolinium has been used as contrast medium, particularly if the patient has impaired renal function.220 A predictive model has been described to calculate, in patients who have received gadodiamide, the minimum length of time to wait before blood collection to avoid pseudohypocalcemia when the Roche o-cresolphthalein method is used.156 The Clinical Laboratory and Standards Institute (CLSI) has approved a method by which atomic absorption spectrophotometry (AAS) is used as a reference method in measuring total serum calcium.44,333 This method has been compared with isotope dilution-mass spectrometry (ID-MS), the definitive method for total serum calcium developed by the National Institute of Standards and Technology. The reference method is reported to have an accuracy of 100 ± 2%, compared with 100 ± 0.2% for ID-MS.44 Although AAS provides better accuracy and precision for total serum calcium than the widely used photometric methods, it is used by only a few laboratories. It should continue to be used for validating new total calcium methods. For further information, see the previous edition of this book and the references.30,44,47,236,272,333 Wide variation in the concentrations of compounds that bind calcium in blood may be noted; this variation will affect the measured total calcium concentration without changing the free calcium fraction. Several types of calculation have been suggested to “adjust” the measured calcium concentration. The goal is to produce a corrected result that would have been found if the concentrations of all compounds that bind calcium had been within their respective reference intervals. In practice, only adjustments based on albumin are used. The term adjusted calcium is preferable to corrected calcium, because “corrected” may suggest that the result has been corrected because of an error.105,150,152 However, it has proved practically impossible to establish calculation methods that would be suitable for all pathologies and all laboratories. This is consistent with the fact that many factors affect the distribution of calcium among free, complexed, and protein-bound fractions (Box 52-3). The reliability of adjustment for serum albumin deteriorates in patients with very low serum albumin concentrations.105 Equations that are derived for a specific patient group, such as hemodialysis patients150 or patients with liver disease, may be better than no adjustment in that group of patients. Direct determination of free calcium by ISE is preferable to adjustments. Individual instruments and methods should be evaluated for their susceptibility to interference from magnesium, hemoglobin, bilirubin, turbidity, and other interferents. Care should be taken in handling specimens, calibrators, and solutions to prevent contamination with calcium. Any glassware or plastic ware that is reused should be washed with dilute hydrochloric acid (HCl), followed by distilled water, to eliminate calcium contamination. Corks should not be used because they can contaminate specimens with calcium. How the patient is prepared and how the specimen is obtained can have a significant effect on both free and total calcium measurements. For information on these preanalytical effects, see a later section in this chapter on the subject as well as Box 52-4. (See also Chapters 11 and 28.) Many blood gas analyzers, using ISEs, provide rapid whole blood determinations of plasma free calcium and electrolytes, as well as determinations of blood gases.30,40,71,272,277 The free calcium analyzer consists of a system of pumps under microprocessor control that transport calibration solutions, samples, and wash solutions through a measuring cell containing calcium ion-selective, reference, and pH electrodes. Sensitive potentiometers measure the voltage difference between the calcium or pH and reference electrodes for calibrating solutions or samples. A microprocessor calibrates the system and calculates calcium concentration and pH. Most instruments simultaneously measure the actual free calcium and pH at 37 °C. Calcium ISEs contain a calcium-selective membrane, which encloses an inner reference solution of calcium chloride often containing saturated silver chloride (AgCl) and physiologic concentrations of sodium chloride and potassium chloride (KCl) and an internal reference electrode.30,40,180,222,272 The reference electrode, usually of Ag/AgCl, is immersed in this inner reference solution. Modern calcium ISEs use liquid membranes containing the ion-selective calcium sensor dissolved in an organic liquid trapped in a polymeric matrix. The Ca2+ ionophores may be based on a polyvinyl chloride matrix and contain ETH1001 or ETH129 as a carrier. Instead of these two neutral carriers, ion exchangers, such as organophosphate sensors (Figure 52-6), have been used.30 Neutral carrier membranes contain an uncharged calcium-selective organic molecule, such as ETH1001, dissolved in a plasticizer and trapped in a polyvinyl chloride membrane. These molecules have a favorable steric and electrostatic pocket or site for selectively binding calcium. Ion exchangers or negatively charged carrier membranes are calcium salts, such as calcium bis(di-n-octylphenyl) phosphate, dissolved in di-n-octylphenyl phosphonate and trapped in a polyvinyl chloride membrane. Carryover has been minimized by various techniques, including (1) using flush solutions containing 5.0 mg/dL (1.25 mmol/L) of free calcium, (2) using the leading edge of the specimen to clean the fluid path, or (3) purging with air.40 Significant carryover is noted only at extremely low or high concentrations of free calcium. The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) has recommended a reference method for free calcium.41 Because ISEs measure ion activity, they are affected by the ionic strength of a specimen.71,272 Free calcium analyzers (and the associated calibrators) are optimized for specimens of serum, plasma, or whole blood. Because the ionic strength of these fluids is primarily a result of Na+ and Cl−, calibrators usually are prepared in buffer and NaCl with a final ionic strength of 160 mmol/kg.40,71,277 Although the range of Na+ and Cl+ concentrations usually observed in serum or plasma does not cause a clinically significant error in the measurement of free calcium, significant errors can occur with other specimens unless the matrices and the ionic strength of the calibrators and samples are matched closely. Modern electrodes have high selectivity for calcium over Na+, K+, Mg2+, H+, and Li+.40,71 At normal concentrations, these cations have little effect on the accuracy of free calcium measurements. Wide variations in the concentration of Na+ and high concentrations of Mg2+ and Li+ may influence the apparent concentration of free calcium. Electrodes are quite insensitive to H+, with insignificant interference noted between pH 5 and 9. Many physiologic anions, including protein, phosphate, citrate, lactate, sulfate, and oxalate, form complexes with calcium ions. Although these anions reduce the concentration of free calcium by complex formation, they do not directly interfere with measurement of the calcium that is free. Protein deposits on the electrode may act as a divalent cation exchanger, resulting in positive interference with high concentrations of Mg2+. Older electrodes were sensitive to the concentration of protein in the sample. Newer electrodes use a dialysis membrane or a neutral carrier to reduce or eliminate this protein effect,40,196,222,229 which typically is less than +0.02 mmol/L for 1 g/dL (10 g/L) of protein. Regular instrument maintenance and protein removal are reported to minimize this interference. The binding of calcium by protein and small anions is influenced by pH in vitro and in vivo.40,71 Albumin, with up to 30 binding sites for calcium,88,168 accounts for approximately 80% of the protein-bound calcium. Increasing the pH of a specimen in vitro increases the ionization and negative charge on albumin and other proteins, leading to an increase in protein-bound calcium and a decrease in free calcium. Decreasing pH in vitro decreases ionization and negative charge, thereby decreasing protein-bound calcium and increasing free calcium. Free calcium changes by about 5% for each 0.1 unit change in pH (Figure 52-7). Preanalytical considerations, including specimen collection and handling, are particularly important for free calcium. Specimens for free calcium must be collected and handled to minimize alterations in pH and free calcium caused by (1) loss of CO2, and (2) metabolism by blood cells. Free calcium may be measured in heparinized whole blood, heparinized plasma, or serum. For most laboratories in which specimens are analyzed rapidly (within minutes of sampling) by the use of blood gas analyzers, heparinized whole blood is preferable because it reduces processing time and the required specimen volume and avoids the alteration in pH associated with centrifugation. All syringes and evacuated tubes should be filled completely, kept tightly sealed, and handled anaerobically to prevent the loss of CO2 and the increase in pH that may occur when specimens are exposed to air. If other tests are ordered that are not available on the blood gas analyzer, it is best that the sample for free calcium is collected in a separate container to minimize the likelihood that the specimen may be analyzed aerobically. Specimens should also be handled to prevent the decrease in pH caused by the production of lactic acid by glycolysis, by erythrocytes, and by white blood cells. The IFCC has published recommendations on sampling, transport, and storage of the samples,25 and the Clinical and Laboratory Standards Institute (CLSI) has published guidelines (C-31A2) for free calcium specimen collection and handling.214 Ideally, whole blood specimens should be analyzed within 15 to 30 minutes of sampling,25 although free calcium is reported to be stable in whole blood specimens for at least 1 hour at room temperature and for 4 hours at 4 °C.25,40,307,313 A 2009 study reported that free calcium was stable for at least 7 hours at room temperature in an evacuated blood collection tube.129 If specimens cannot be analyzed promptly, they can be collected in an ice-water slurry to minimize metabolism. If measurements of plasma K+ are needed on the sample, cooling will not be appropriate; K+ concentrations will be increased because of the inhibition of ATPase activity at low temperature.87 For delayed analysis, serum may be the optimal sample type because of elimination of the anticoagulant and reduction in the occurrence of microclots. Serum specimens can be collected in evacuated gel tubes.173,313 These tubes should be filled completely and centrifuged to form an effective barrier between the serum and the clot with its cellular elements. Once centrifuged, specimens are stable for hours at 25 °C and for days at 4 °C, provided the tube remains sealed. Free calcium has been reported to be less stable in specimens from both acidotic and nonacidotic patients with uremia.217 The practice of using aerobic specimens for the measurement of free calcium with correction of the free calcium to pH 7.440,308 has been criticized40,263,306,330 and is not recommended. The free calcium value at pH 7.4 may be misleading in patients with respiratory and metabolic alkalosis or acidosis.217 Furthermore, aerobic handling of specimens may lead to irreversible precipitation of calcium-phosphate complexes and a decrease in free calcium in some specimens that have a high total calcium and phosphate content or a high pH (pH > 7.9). Because citrate, oxalate, and EDTA bind calcium and significantly reduce free calcium, heparin is the only acceptable anticoagulant for free calcium determinations. However, heparin, a polyanion, significantly lowers free calcium at the concentrations (≥30 to 100 U/mL) found in many conventional blood gas syringes (Table 52-3).42,71 In addition, the use of liquid heparin should be avoided; it can result in errors in free calcium caused by dilution, as well as high and variable concentrations of heparin. TABLE 52-3 Effects of Heparin on Free Calcium Modified from Toffaletti J. Ionized calcium. In: Pesce AJ, Kaplan LA, eds. Methods in clinical chemistry. St Louis: CV Mosby, 1987:1010-20. Several commercially available syringes containing lyophilized heparin are suitable for free calcium determinations: (1) electrolyte-balanced or calcium-titrated heparin syringes (final concentration of 40 to 50 U/mL); (2) very low heparin syringes with heparin in an inert filler, providing a final heparin concentration of 2 to 3 U/mL; and (3) lithium-zinc heparin syringes.314 With electrolyte-balanced or calcium-titrated heparin syringes, the heparin is titrated with calcium so that the free calcium is not significantly altered over most observed concentrations (3.6 to 6.4 mg/dL or 0.9 to 1.6 mmol/L); however, some bias may be apparent at very low and high free calcium concentrations. Electrolyte-balanced heparin may also produce a bias in specimens with pathologically low protein concentrations.189 In very low heparin syringes that contain 2 to 3 U/mL of heparin dispersed in a puff of inert proprietary material, the puff allows the heparin to be accurately dispensed during manufacturing and to rapidly dissolve with proper mixing, providing effective anticoagulation. A blend of lithium and zinc heparins has been reported to eliminate the heparin interference in free calcium measurements.171,300,316 In addition, lithium-zinc heparin did not alter total calcium, unlike electrolyte-balanced or calcium-titrated heparin. Three-milliliter syringes containing a total of 50 U of a 1 : 1 blend of lithium and zinc heparins did not alter results of any general chemistry tests except total magnesium, which was increased by 0.19 mg/dL or 0.08 mmol/L. In practice individuals obtaining the blood specimen should not place additional liquid heparin in heparinized syringes. Most evacuated collection tubes, when filled completely, contain concentrations of heparin (15 U/mL) that only slightly decrease free calcium.311 Specific brands of syringes, evacuated tubes, and heparin should be carefully evaluated. It is important that all syringes and tubes be filled completely to minimize dilution and/or heparin effects. Various calibration solutions are used by manufacturers for free calcium analyzers. The buffers in which these calibrators are prepared may have an effect on the liquid junction potential and on calcium binding; however, this is usually corrected for by the instrument software.40 Until reference solutions are available, it is best to use the calibrators provided by the instrument manufacturer. Aqueous quality control materials are commercially available for free calcium. Because simple aqueous controls may not reliably detect changes in performance with patient specimens, use of serum-based quality control materials has been recommended.315 Serum-based controls may be prepared by acidifying serum with 10 µL of 1 mol/L HCl and leaving it exposed in the refrigerator for 1 week to remove carbon dioxide. The pH is then adjusted to 7.4, and the serum is aliquoted and frozen. Alternatively, serum-based controls can be equilibrated with carbon dioxide before undergoing analysis. Patient preparation and the manner of specimen collection can significantly affect the results of total and free calcium determinations (see Box 52-4).40,115 A common and important source of preanalytical error in the measurement of calcium is the increase in total, but not free, calcium concentration associated with tourniquet use and venous occlusion during sampling.245 Errors of 0.5 to 1.0 mg/dL or 0.12 to 0.25 mmol/L in total calcium may result from the increase in protein-bound calcium caused by the efflux of water from the vascular compartment during stasis. Only small and clinically insignificant increases in free calcium have been reported with venous stasis. If a tourniquet is required, it should be applied just before sampling and released as soon as possible. Fist clenching or other forearm exercise should be avoided before phlebotomy245 because forearm exercise causes a decrease in pH (lactic acid production) and an increase in free calcium. The NCCLS and the IFCC have published recommendations on blood collection.42,214 Changes in posture cause fluid shifts within 10 minutes and thus alter the concentrations of cells and large molecules, including albumin and total calcium (as part of it is protein bound), in the vascular compartment. Standing decreases intravascular water and increases the total calcium concentration by 0.2 to 0.8 mg/dL or 0.05 to 0.20 mmol/L, whereas a much smaller effect has been reported for free calcium.40,115,245 One partial explanation (along with hypoalbuminemia) for the mild hypocalcemia observed in many hospital patients may be the hemodilution associated with recumbency. Prolonged immobilization and bed rest131,295 increase bone resorption, which increases total and free calcium. Hyperventilation and exercise decrease and increase the concentration of free calcium, respectively, because of changes in serum pH.40,245 Both serum free calcium concentration and calcium excretion are decreased during the night.45 Food ingestion has been reported to have various effects but usually causes a mild increase in serum calcium. Ingestion of calcium salts may increase serum calcium. Hemolysis can alter free calcium because of dilution and alterations in pH and binding (see previous discussion under “Interferences”). An analytical goal, or quality specification, for between-day imprecision, expressed as the coefficient of variation (CV), is 0.9% or less based on within-person biological variation.90 Current methods achieve between-day CVs of 1.5% or less within laboratories. For adults, the reference interval of free calcium has been reported as follows: Whole blood specimens develop a liquid junction potential different from that of serum or plasma because of the presence of erythrocytes.40,71,222 A positive bias that is directly proportional to the hematocrit has been reported. In addition, free calcium values have been reported to differ among capillary blood, venous blood, and serum samples because of differences in pH. Therefore, reference intervals should be determined by each laboratory using the local instrument, specimen type, and collection protocol, and reference subjects representative of the patient population served. Calcium has been reported to vary with age, gender, and season and during pregnancy.166,167,272 Total and free calcium have been reported to decline modestly and to remain unchanged in the elderly.281 In a healthy ambulatory group of men and women residing in the Southwest of the United States, no age-related decline or gender-related difference could be found in total or free calcium values.78 During pregnancy, total calcium declines in parallel with serum albumin, whereas free calcium is unchanged.166,167 The fetal circulation is relatively hypercalcemic,165,329 as evidenced by higher total and free calcium in cord blood than in maternal serum. Calcium concentrations decline after birth in healthy term neonates during the first few days, but soon rise to concentrations slightly greater than those observed in adults.166,329 Calcium status is more accurately determined by measuring free calcium, the tightly regulated, biologically active species.30,84 Interpretation of total serum calcium value is complicated by its association with protein and inorganic and organic ions. Interpretation of free calcium concentration is less complicated, provided the specimen has been properly obtained, handled, and analyzed. Disagreement between free and total calcium (abnormal total calcium and normal free calcium, or vice versa) values occurs in a high percentage of specimens. One study of 1213 patients suspected of having calcium disorders found disagreement between free calcium and total calcium or corrected calcium values in 18% and 31% of patients, respectively.309 Free calcium is reportedly more useful than total calcium determination in hospital patients, especially those undergoing major surgery (Figure 52-8) who have received citrated blood or platelets, heparin, bicarbonate, intravenous solutions, or calcium.40 Alterations in blood pH and temperature further reduce the usefulness of total calcium assay in these patients. Rapid measurement of free calcium, blood gases, and potassium permits maintenance of good cardiac function during liver transplant operations and other major operations such as those in which cardiopulmonary bypass is used. Free calcium is more useful than total calcium determination in patients in intensive care because of abnormal protein concentrations and putative circulating factors that alter calcium binding to albumin. Abnormally low free calcium is frequently found in critically ill patients.12,48,341 A study at one hospital where free calcium has replaced total calcium determinations found that 41% of free calcium concentrations were abnormal among inpatients.30 Of these cases, 31% were below and 10% were above the reference interval. The recovery room, surgical intensive care unit, renal transplantation ward, medical intensive care unit, and medical oncology unit had the highest percentages of abnormal values. Another study has questioned the value of frequent measurements of free calcium in hospital patients.11 In a large academic hospital setting, serial and daily testing was responsible for a large fraction of free calcium analyses. Half of all patients tested had free calcium concentrations below the reference interval. Free calcium was increased by intravenous (IV) administration of calcium, but the increase attributable to administered calcium was small compared with the increase that occurred in patients given no calcium. Retrospective analysis suggested that a total calcium below 8 mg/dL (2 mmol/L) identified most patients with low free calcium (<4 mg/dL, <1.0 mmol/L). Introduction of a reflexive strategy in which free calcium was measured only when total calcium was below that limit reduced free calcium testing by 72 to 76% and reduced IV calcium gluconate therapy by 45 to 81%. An outcomes study showed no evidence of an increase in adverse events. Free calcium may be useful in the diagnosis of hypercalcemia. Patients with subsequently surgically proven primary hyperparathyroidism more often have increases in free calcium than in total calcium (Figure 52-9). Free calcium is more sensitive than total calcium in detecting hypercalcemia associated with malignancy, as may be expected in patients who frequently have decreased serum albumin. Less commonly, paraproteins produced in myeloma may bind calcium, complicating the interpretation of total or adjusted calcium measurements. In neonates, assay of free calcium rather than total calcium is recommended because of its speed and the ability to use small skin-puncture specimens, and because of its greater validity in the presence of hyperphosphatemia, with alterations in pH, and with the persistence of α-fetoprotein after birth.329 The rate of urinary calcium excretion reflects calcium intake, intestinal absorption, skeletal resorption, and renal tubular filtration and reabsorption.249 Healthy men and women excrete up to 300 mg or ≈7.5 mmol of calcium per day on a diet with unrestricted calcium content, and up to 200 mg/d or ≈5 mmol/d on a calcium-restricted diet (500 mg or 12.5 mmol dietary calcium per day or less for several days). The reference data for urine calcium are not well established, and it may be preferable to use a value of 4 mg/kg body weight (or 0.1 mmol/kg body weight) as the upper reference limit in an individual patient. Calcium salts, such as calcium oxalate, precipitate in urine specimens during and after collection. Specimens may be collected in a container containing acid to prevent calcium salt precipitation. A commonly used acid is HCl, 6 mol/L, with 20 to 30 mL added to the container for a 24 hour collection (1 to 2 mL for a random specimen). The safety of the patient and the patient’s family in handling such a container may be a concern. The measured urinary calcium concentration must be corrected for dilution by the acid solution when the urinary volume is low. The specimen should be kept well mixed during collection. Specimens collected without acid should be acidified and allowed to stand for 1 hour before thorough remixing and aliquoting. Some have questioned the ability of postcollection acidification to redissolve all of the calcium salts with or without heating.115
Bone and Mineral Metabolism
Overview of Skeletal Metabolism
Cell Signaling in Bone
Bone Remodeling
Calcium

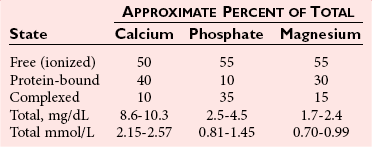
Biochemistry and Physiology
 of that in extracellular fluid.
of that in extracellular fluid.
Clinical Significance
Hypercalcemia
Measurement of Calcium
Measurement of Total Calcium
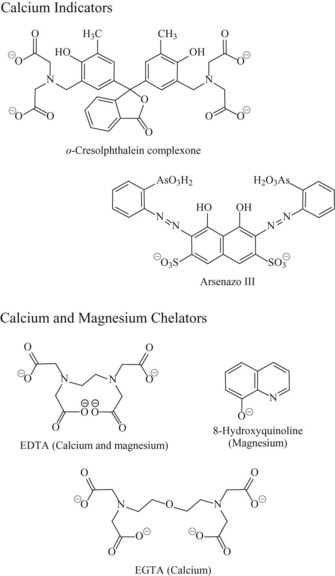
Photometric Methods
Atomic Absorption Spectrometry Methods
Adjusted or Corrected Total Calcium
Interferences
Measurement of Free (Ionized) Calcium
Interferences
Effect of pH
Specimen Requirements
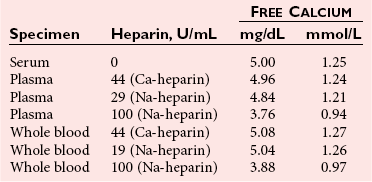
Effects of Anticoagulants
Calibrators and Quality Controls
Patient Preparation and Sources of Preanalytical Error for Total and Free Calcium Measurements
Reference Intervals for Total and Free Calcium in Serum and Plasma
Free Calcium
Physiologic Variation in Calcium
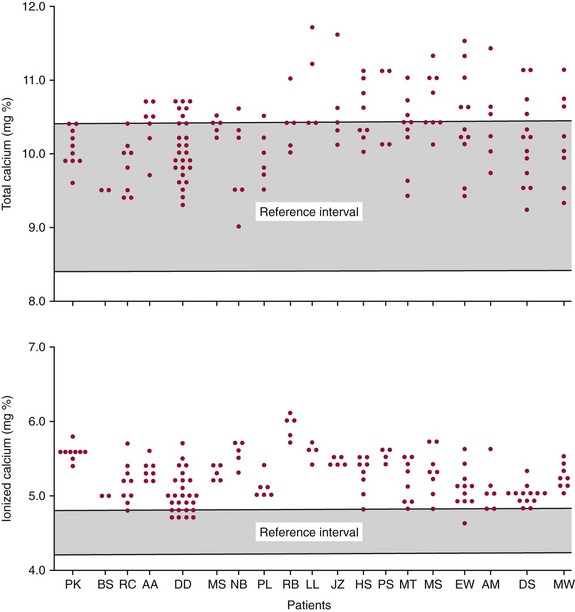
Interpretation of Total and Free Calcium Results
Urinary Calcium
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine

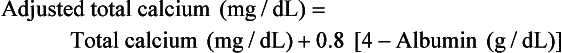
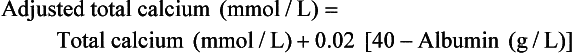
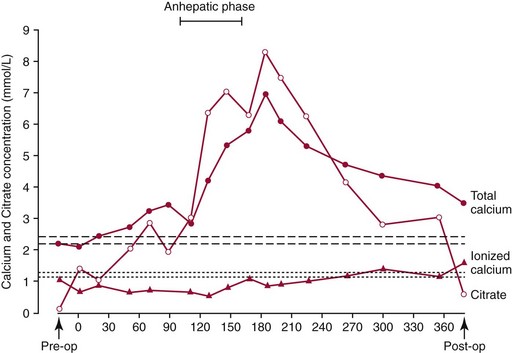
 ), total calcium (•), and citrate (
), total calcium (•), and citrate ( ) concentrations in a patient undergoing liver transplantation. Reference intervals are indicated by the upper (total calcium) and lower (free calcium) sets of dashed horizontal lines. The reference interval for citrate is 0.03 to 0.15 mmol/L.
) concentrations in a patient undergoing liver transplantation. Reference intervals are indicated by the upper (total calcium) and lower (free calcium) sets of dashed horizontal lines. The reference interval for citrate is 0.03 to 0.15 mmol/L.