Blood and Circulatory System Disorders
Learning Objectives
After studying this chapter, the student is expected to:
1. Define the terms describing abnormalities in the blood.
4. Describe hemophilia A: its pathophysiology, signs, and treatment.
6. Discuss the myelodysplastic syndrome and its relationship to other blood disorders.
Key Terms
achlorhydria
agglutination
autoregulation
bilirubin
cyanotic
demyelination
deoxyhemoglobin
diapedesis
dyscrasia
dyspnea
ecchymoses
erythrocytosis
erythropoietin
ferritin
gastrectomy
glossitis
hemarthrosis
hematocrit
hematopoiesis
hemolysis
hemoptysis
hemosiderin
hemostasis
hepatomegaly
hypochromic
interleukin
leukocytosis
leukopenia
leukopoiesis
macrocytes
macrophages
malabsorption
megaloblasts
microcytic
morphology
myelotoxins
myelodysplastic
neutropenia
oxyhemoglobin
pallor
pancytopenia
petechiae
phlebotomy
plasma
plethoric
reticulocyte
serum
splenomegaly
stomatitis
syncope
tachycardia
thrombocytopenia
Review of the Circulatory System and Blood
Anatomy, Structures, and Components
As any student of anatomy and physiology quickly discovers, although distinct in their specific structures and functions, all the systems of the human body are intricately interrelated and must interact constantly in order to maintain the proper functioning of the body. One component upon which all systems depend is blood that: transports essential oxygen to all tissues along with nutrients required for cellular metabolism, provides for the necessary removal of many cell wastes, plays a critical role in the body’s defenses/immune system and serves in maintaining body homeostasis. Blood and lymph, another essential body fluid, are transported throughout the body via a complex system of vessels and the pumping action of the heart. Due to the complexity and distinct features involved in the production and circulation of blood and lymph, this chapter examines blood itself along with a basic review of the vessels involved in the distribution of blood throughout the body and the associated blood disorders. Chapter 11 presents an examination of the lymphatic system and associated disorders. Chapter 12 presents a detailed examination of the cardiovascular system with specific emphasis on the heart and associated disorders along with disorders of the blood vessels themselves.
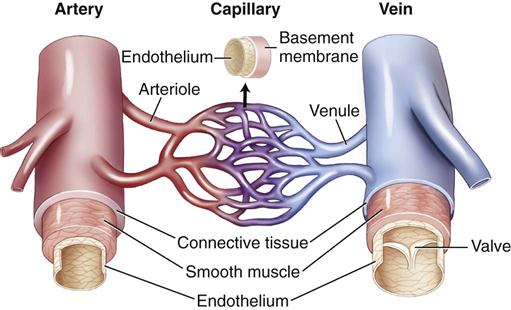
Blood Vessels
The arteries, capillaries, and veins constitute a closed system for the distribution of blood throughout the body. Major blood vessels, most of which are paired left and right, are shown in Figures 10-1 and 10-2.


To review the components of the circulatory system:
• Arteries transport blood away from the heart into the lungs or to body tissues.
• Small venules conduct blood from the capillary beds toward the heart.
The walls of arteries and veins are made up of three layers.
The vasa vasorum consists of tiny blood vessels that supply blood to the tissues of the wall itself. Normally the large arteries are highly elastic in order to adjust to the changes in blood volume that occur during the cardiac cycle. For example, the aorta must expand during systole to prevent systolic pressure from rising too high, and during diastole the walls must recoil to maintain adequate diastolic pressure. Veins have thinner walls than arteries and less smooth muscle (Fig. 10-3).
Localized vasodilation or vasoconstriction in arterioles is controlled by autoregulation, a reflex adjustment in a small area of a tissue or an organ, which varies depending on the needs of the cells in the area. For example, a decrease in pH, an increase in carbon dioxide, or a decrease in oxygen leads to local vasodilation. Release of chemical mediators such as histamine or an increase in temperature at a specific area can also cause vasodilation. These local changes do not affect the systemic blood pressure.
Norepinephrine and epinephrine increase systemic vasoconstriction by stimulating alpha1-adrenergic receptors in the arteriolar walls. Angiotensin is another powerful systemic vasoconstrictor. At all times, even at rest, vascular or vasomotor tone is maintained by constant input from the SNS that results in partial vasoconstriction throughout the body to ensure continued circulation of blood.
Capillary walls consist of a single endothelial layer to facilitate the exchange of fluid, oxygen, carbon dioxide, electrolytes, glucose and other nutrients, and wastes between the blood and the interstitial fluid. Capillary exchange and abnormal electrolyte shifts are discussed in Chapter 2.
Blood
Blood provides the major transport system of the body for essentials such as oxygen, glucose and other nutrients, hormones, electrolytes, and cell wastes. It serves as a critical part of the body’s defenses, carrying antibodies and white blood cells for the rapid removal of any foreign material. As a vehicle promoting homeostasis, blood provides a mechanism for controlling body temperature by distributing core heat throughout the peripheral tissues. Blood is the medium through which body fluid levels and blood pressure are measured and adjusted by various controls, such as hormones. Clotting factors in the circulating blood are readily available for hemostasis. Buffer systems in the blood maintain a stable pH of 7.35 to 7.45 (see discussion of acid-base balance in Chapter 2).
Composition of Blood
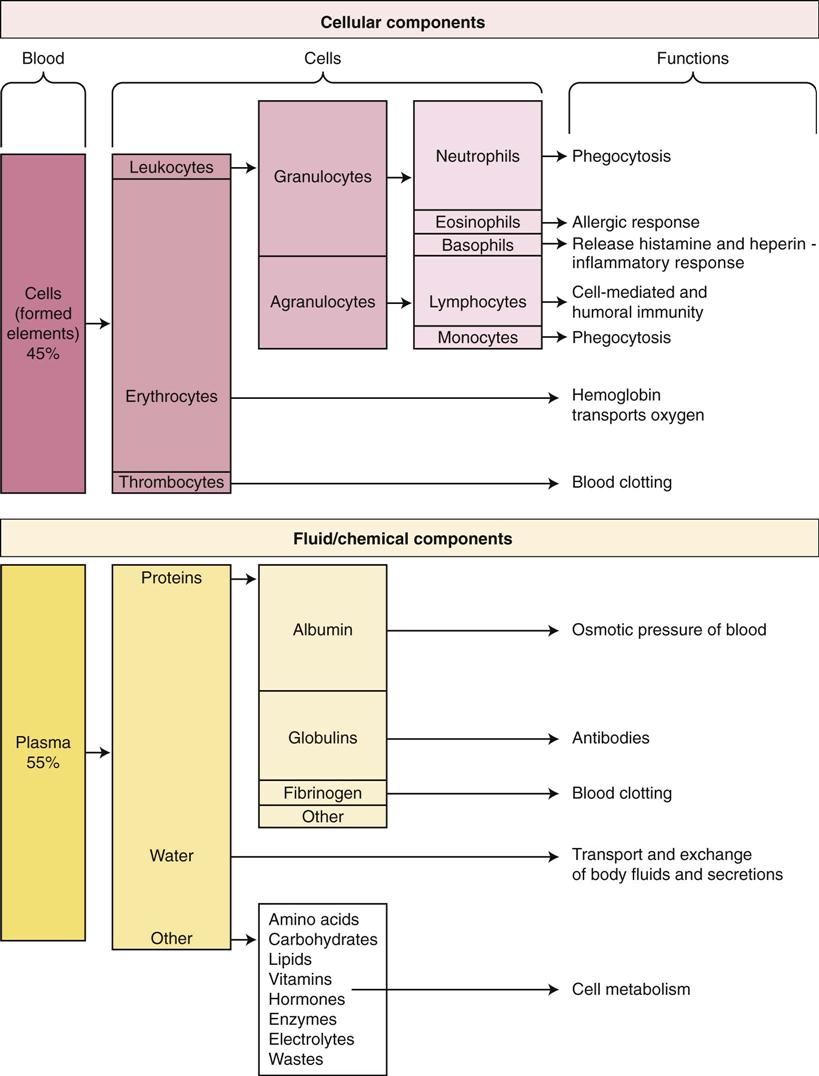
The adult body contains approximately 5 liters of blood. Blood consists of water and its dissolved solutes, which make up about 55% of the whole blood volume; the remaining 45% is composed of the cells or formed elements, the erythrocytes, along with leukocytes, and thrombocytes or platelets. Hematocrit refers to the proportion of cells (essentially the erythrocytes) in blood and indicates the viscosity of the blood. Males have a higher hematocrit, average 42% to 52%, than females, 37% to 47%. An elevated hematocrit could indicate dehydration (loss of fluid) or excess red blood cells. A low hematocrit might result from blood loss or anemia. Plasma is the clear yellowish fluid remaining after the cells have been removed, and serum refers to the fluid and solutes remaining after the cells and fibrinogen have been removed. The plasma proteins include albumin, which maintains osmotic pressure in the blood; globulins or antibodies; and fibrinogen, which is essential for the formation of blood clots.
The components of blood and their functions are summarized in Figure 10-4. Normal values for blood components are found inside the front cover of this book.
Blood Cells and Hematopoiesis
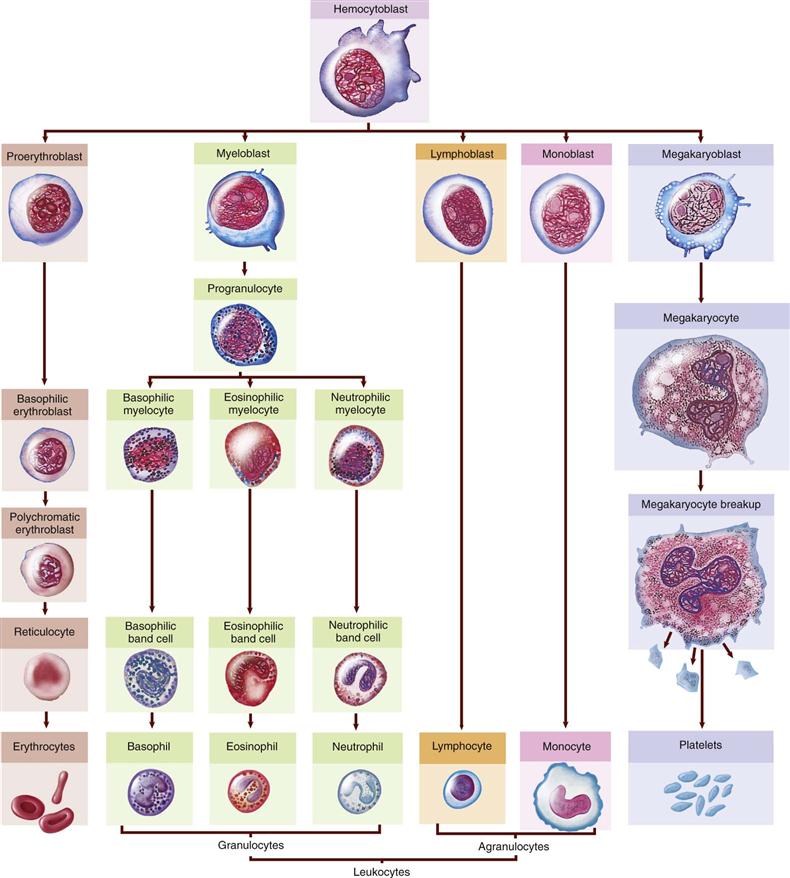
All blood cells originate from the red bone marrow. In the adult, red bone marrow is found in the flat and irregular bones, ribs, vertebrae, sternum, and pelvis. The iliac crest in the pelvic bone is a common site for a bone marrow aspiration for biopsy. The various blood cells develop from a single stem cell (pluripotential hematopoietic stem cell) during the process of hemopoiesis or hematopoiesis (Fig. 10-5). From this basic cell, the differentiation process forms committed stem cells for each type of blood cell. These cells then proliferate and mature, providing the specialized functional cells needed by the body. A pathological condition of the blood that usually refers to disorders involving the cellular components of blood is called dyscrasia. A number of specific blood dyscrasias are addressed later in the chapter.
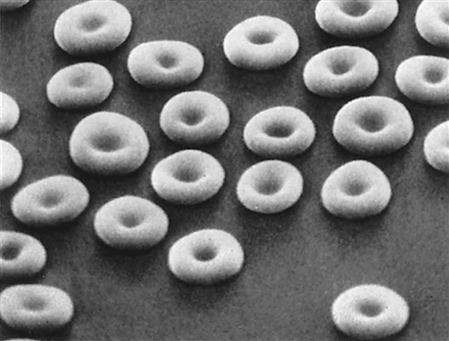
Erythrocytes or red blood cells (RBCs) are biconcave, flexible discs (like doughnuts but with thin centers rather than holes) that are non-nucleated when mature and contain hemoglobin (Fig. 10-6). The size and structure are essential for easy passage through small capillaries. The hormone erythropoietin, originating from the kidney, stimulates erythrocyte production in the red bone marrow in response to tissue hypoxia, or insufficient oxygen available to cells. Normally RBCs (4.2 to 6.2 million/mm3) constitute most of the cell volume in blood. Adequate RBC production and maturation depend on the availability of many raw materials, including amino acids, iron, vitamin B12, vitamin B6, and folic acid.
Hemoglobin consists of the globin portion, two pairs of amino acid chains, and four heme groups, each containing a ferrous iron atom, to which the oxygen molecule (O2) can attach (see Fig. 10-16A). Heme provides the red color associated with hemoglobin. Normally hemoglobin becomes fully saturated with oxygen in the lungs. Oxyhemoglobin is a bright red color, which distinguishes arterial blood from venous blood. As the blood circulates through the body, oxygen dissociates from hemoglobin, depending on local metabolism (see Fig. 13-6). Deoxygenated hemoglobin (deoxyhemoglobin or reduced hemoglobin) is dark or bluish-red in color and is found in venous blood.
Only a small proportion of the carbon dioxide (CO2) in blood is carried by hemoglobin (carbaminohemoglobin) attached to nitrogen in an amino acid group at a different site from that for oxygen. Most carbon dioxide is transported in blood as bicarbonate ion (in the buffer pair). Oxygen can easily be displaced from hemoglobin by carbon monoxide, which binds tightly to the iron, thus causing a fatal hypoxia (deficit of oxygen). Carbon monoxide poisoning can be recognized by the bright cherry-red color in the lips and face.
The lifespan of a normal RBC is approximately 120 days. As it ages, the cell becomes rigid and fragile and finally succumbs to phagocytosis in the spleen or liver and is broken down into globin and heme (Fig. 10-7). Globin is broken down into amino acids, which can be recycled in the amino acid pool, and the iron can be returned to the bone marrow and liver to be reused in the synthesis of more hemoglobin. Excess iron can be stored as ferritin or hemosiderin in the liver, blood, and other body tissues. A genetic disorder, hemochromatosis, otherwise known as iron overload, results in large amounts of hemosiderin accumulating in the liver, heart, and other organs, causing serious organ damage.
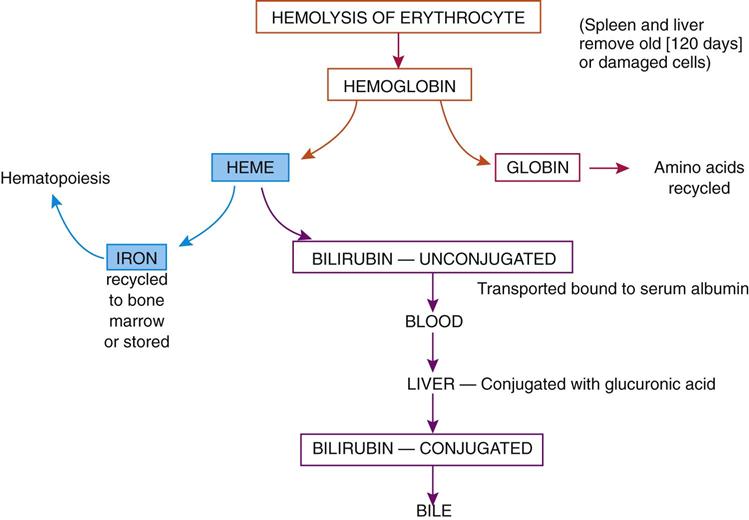
The balance of the heme component is converted to bilirubin and transported by the blood to the liver, where it is conjugated (or combined) with glucuronide to make it more soluble, and then excreted in the bile. Excessive hemolysis or destruction of RBCs may cause elevated serum bilirubin levels, which result in jaundice, the yellow color in the sclera of the eye and of the skin.
Hematopoiesis
Leukocytes, which number 5 to 10,000/mm3, make up only about 1% of blood volume. They are subdivided into three types of granulocytes and two types of agranulocytes. All types develop and differentiate from the original stem cell in bone marrow (see Fig. 10-5). Leukopoiesis, or production of white blood cells (WBCs), is stimulated by colony-stimulating factors (CSFs) produced by cells such as macrophages and T lymphocytes. For example, granulocyte CSF or multi-CSF (interleukin-3 [IL-3]) may be produced to increase certain types of WBCs during an inflammatory response (see Chapter 5). White blood cells may leave the capillaries and enter the tissues by diapedesis or ameboid action (movement through an intact capillary wall) when they are needed for defensive purposes.
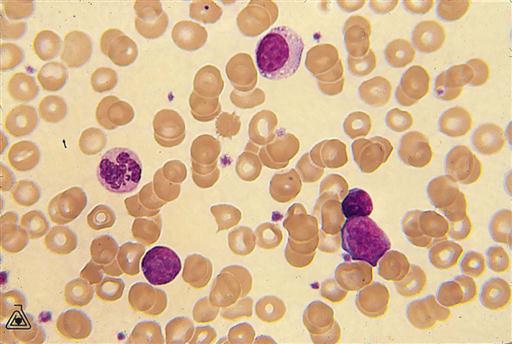
The five types of leukocytes vary in physical characteristics and functions (see Fig. 10-4). Some examples of WBCs are visible as large, nucleated cells (purple stain) in the blood smear in Figure 10-8.
• Lymphocytes make up 30% to 40% of the WBCs. The roles of B and T lymphocytes in the immune response are reviewed in Chapter 7. Some T cells are designated natural killer cells and are significant in immunity.
A differential count indicates the proportions of specific types of WBCs in the blood and frequently assists in making a diagnosis. For example, a bacterial infection or inflammatory condition stimulates an increase in neutrophils, whereas allergic reactions or parasitic infections increase the eosinophil count.
Thrombocytes, also called platelets, are an essential part of the blood-clotting process or hemostasis (Fig. 10-9). Thrombocytes are not cells; rather, they are very small, irregularly shaped, non-nucleated fragments from large megakaryocytes (see Fig. 10-8). Platelets stick to damaged tissue as well as to each other to form a platelet plug that seals small breaks in blood vessels, or they can adhere to rough surfaces and foreign material. The common drug ASA (aspirin) reduces this adhesion and can lead to an increased bleeding tendency. Thrombocytes can also initiate the coagulation process.
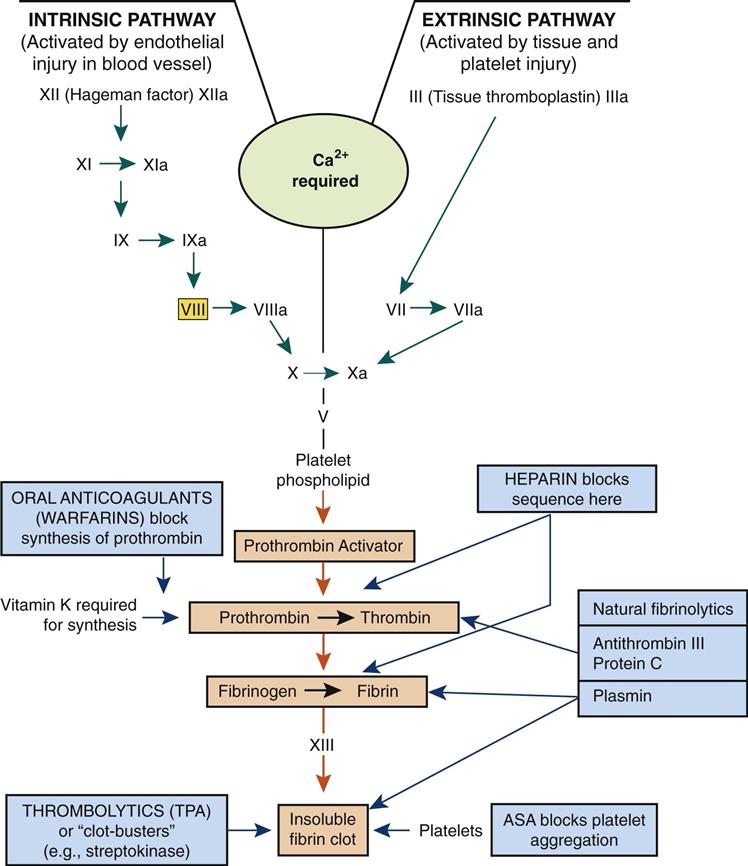
Blood Clotting
Hemostasis consists of three steps.
• The blood-clotting or coagulation mechanism is required in larger vessels, by which the clotting factors that are present in inactive forms in the circulating blood are activated through a sequence of reactions (see Fig. 10-9). Recent evidence indicates additional overlap in factor activity between the intrinsic and extrinsic pathways, but the cascade of reactions is the basis for coagulation.
Clot formation (coagulation) requires a sequence or cascade of events as summarized:
2. Prothrombin or factor II (inactive in the plasma) is converted into thrombin.
3. Fibrinogen (factor I) is converted into fibrin threads.
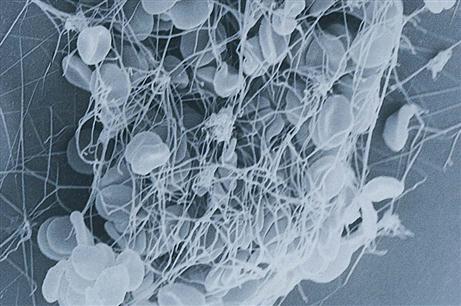
4. A fibrin mesh forms to trap cells, making up a solid clot, or thrombus, and stopping the flow of blood (Fig. 10-10).
The circulating clotting factors are produced primarily in the liver. Their numbers relate to the order of their discovery, not to the step in the clotting process. Vitamin K, a fat-soluble vitamin, is required for the synthesis of most clotting factors. Calcium ions are essential for many steps in the clotting process.
Other measures can be used by a person to facilitate this clotting process. For example, applying pressure and cold (a vasoconstrictor) to the site reduces blood flow in the area, or thrombin solution can be applied directly to speed up clotting.
Fibrinolysis
A delicate balance is always necessary between the tendency to clot to prevent blood loss and the tendency to form clots unnecessarily and cause infarctions. Some individuals tend to form clots very readily; others are predisposed to excessive bleeding. To prevent inappropriate thrombus formation, coagulation inhibitors such as antithrombin III circulate in the blood. Through thrombin, a prostaglandin is released to prevent platelets sticking to nearby undamaged tissue. Heparin, an anticoagulant, is released from basophils or mast cells in the tissues and exerts its major action by blocking thrombin. Heparin, as a drug, may be administered intravenously to patients at risk for thrombus formation. It does not dissolve clots, but will prevent further growth of the thrombus.
Also, there is a natural fibrinolytic process that can break down newly formed clots. Inactive plasminogen circulates in the blood. Following injury, it can be converted by tissue plasminogen activator (tPA) and streptokinase through a sequence of reactions, into plasmin. The product, plasmin, then breaks down fibrin and fibrinogen. This fibrinolysis is a localized event only, because plasmin is quickly inactivated by plasmin inhibitor. These numerous checks and balances are essential in the regulation of defense mechanisms. Application of this mechanism with “clot-buster” drugs such as streptokinase (Streptase) is proving very successful in minimizing the tissue damage resulting from blood clots causing strokes (cardiovascular accidents, CVAs) and heart attacks (myocardial infarctions, MIs). However, constant monitoring of blood-clotting times and careful administration technique are essential to prevent excessive bleeding or hematoma formation. New protocols for anticoagulant medications are under development in the United States to ensure greater safety for patients.
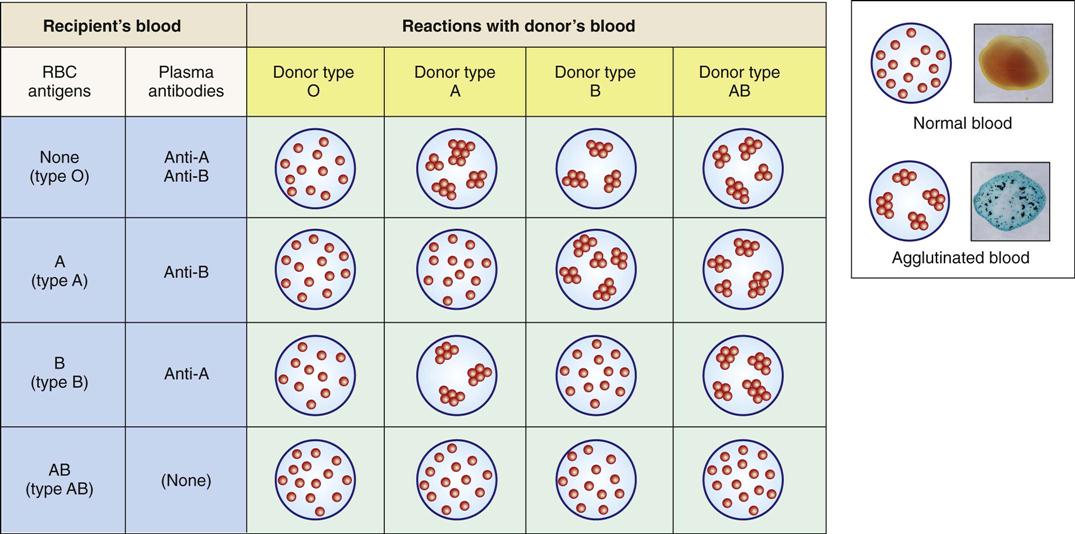
Antigenic Blood Types
An individual’s blood type (e.g., ABO and Rh groups) is determined by the presence of specific antigens on the cell membranes of that person’s erythrocytes. ABO groups are an inherited characteristic that depends on the presence of type A or B antigens or agglutinogens (Table 10-1). Shortly after birth, antibodies that can react with different antigens on another person’s RBCs form in the blood of the newborn infant. Such an antigen–antibody reaction would occur with, for example, an incompatible blood transfusion, resulting in agglutination (clumping) and hemolysis of the recipient’s RBCs (Fig. 10-11).
TABLE 10-1
ABO Blood Groups and Transfusion Compatibilities
| Blood Group | RBC Antigens | Antibodies in Plasma | For Transfusion, Can Receive Donor Blood Group |
| O | None | Anti-A and anti-B | O |
| A | A | Anti-B | O or A |
| B | B | Anti-A | O or B |
| AB | A and B | None | O, A, B, or AB |
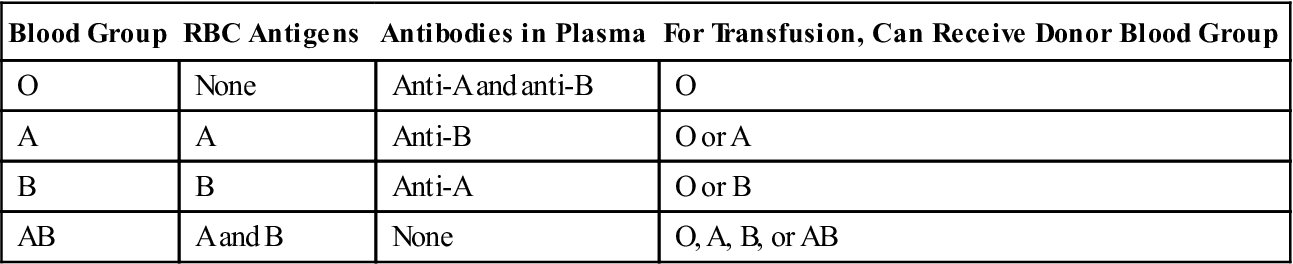
Blood types of both donor and recipient are carefully checked before transfusion. Persons with type O blood lack A and B antigens and therefore are considered universal donors. Persons with type AB blood are universal recipients. Signs of a transfusion reaction include a feeling of warmth in the involved vein, flushed face, headache, fever and chills, pain in the chest and abdomen, decreased blood pressure, and rapid pulse.
Another inherited factor in blood is the Rh factor, which may cause blood incompatibility if the mother is Rh-negative and the fetus is Rh positive (see Fig. 22-2). Rh blood incompatibility between maternal and fetal blood is reviewed in Chapter 22.
Plasma or colloidal volume-expanding solutions can be administered without risk of a reaction because they are free of antigens and antibodies.
Diagnostic Tests
The basic diagnostic test for blood is the complete blood count (CBC), which includes total RBCs, WBCs, platelet counts, and morphology (size and shape), a differential count for WBCs, hemoglobin, and hematocrit values (see normal values inside the front cover of this book). These tests are useful screening tools. For example, leukocytosis, an increase in WBCs in the circulation, is often associated with inflammation or infection. Leukopenia, a decrease in leukocytes, occurs with some viral infections as well as with radiation and chemotherapy. An increase in eosinophils is common with allergic responses. The characteristics of the individual cells observed in a blood smear, including size and shape, uniformity, maturity, and amount of hemoglobin, are very important. Different types of anemia are distinguished by the characteristic size and shape of the cell, and presence of a nucleus in the RBC. More specialized tests are available. A summary of the most common diagnostic tests is provided in Ready Reference 5.
The hematocrit shows the percentage of blood volume composed of RBCs and indicates fluid and cell content. It may be an indicator of anemia, a low RBC count. Hemoglobin is measured, and the amount of hemoglobin per cell is shown by the mean corpuscular volume (MCV). MCV indicates the oxygen-carrying capacity of the blood.
Bone marrow function can be assessed by the reticulocyte (immature non-nucleated RBC) count, plus a bone marrow aspiration and biopsy.
Chemical analysis of the blood can determine the serum levels of such components as iron, vitamin B12 and folic acid, cholesterol, urea, glucose, and bilirubin. The results can indicate metabolic disorders and disorders within various other body systems.
Blood-clotting disorders can be differentiated by tests such as bleeding time (measures platelet function—the time to plug a small puncture wound); prothrombin time or INR International Normalized Ratio (measures the extrinsic pathway); and partial thromboplastin time (PTT—intrinsic pathway), which measure the function of various factors in the coagulation process. They are also used to monitor anticoagulant therapy. The reference values for these tests are best established for individual patients based on their health history.
Blood Therapies
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree