http://evolve.elsevier.com/McCuistion/pharmacology/
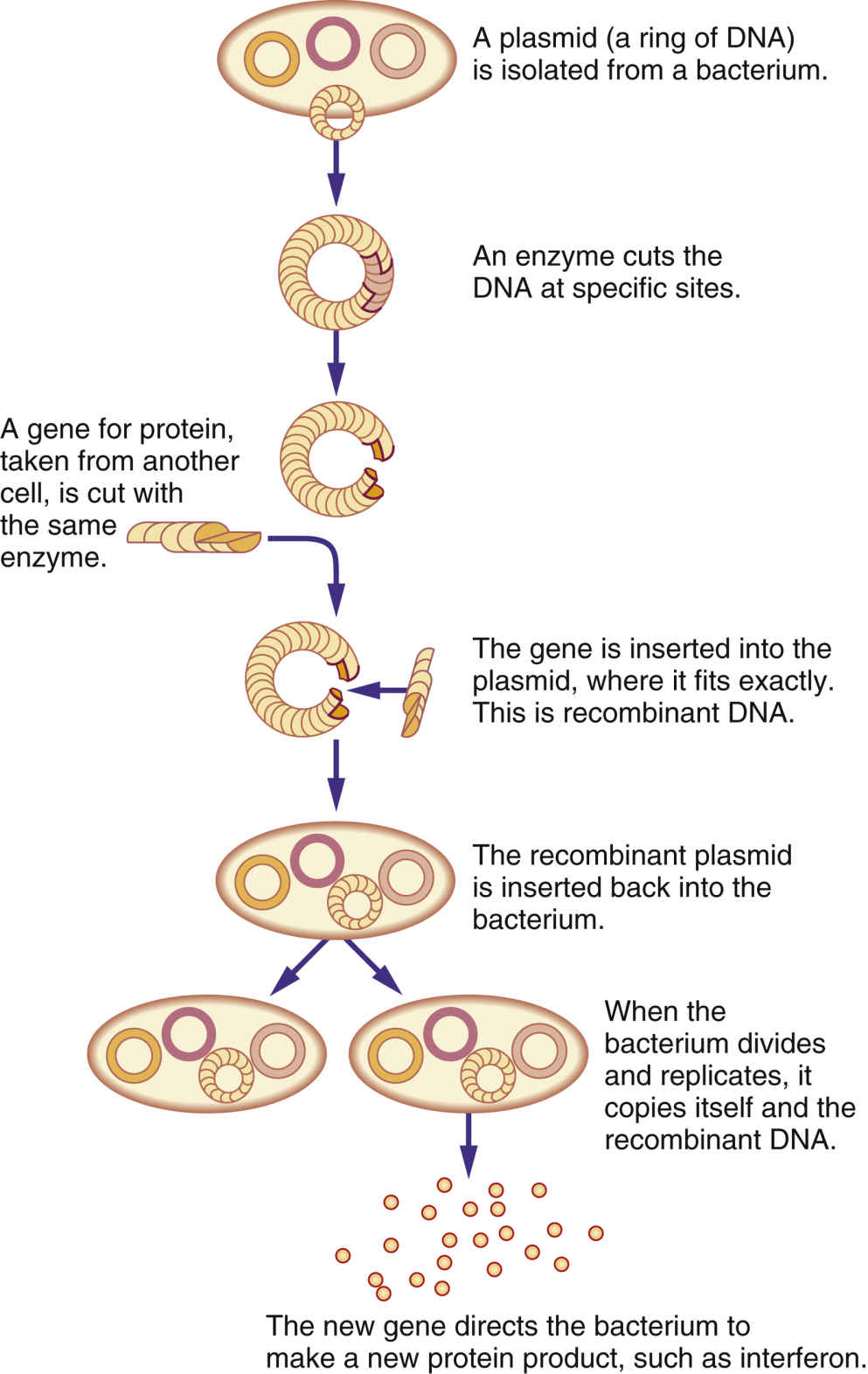
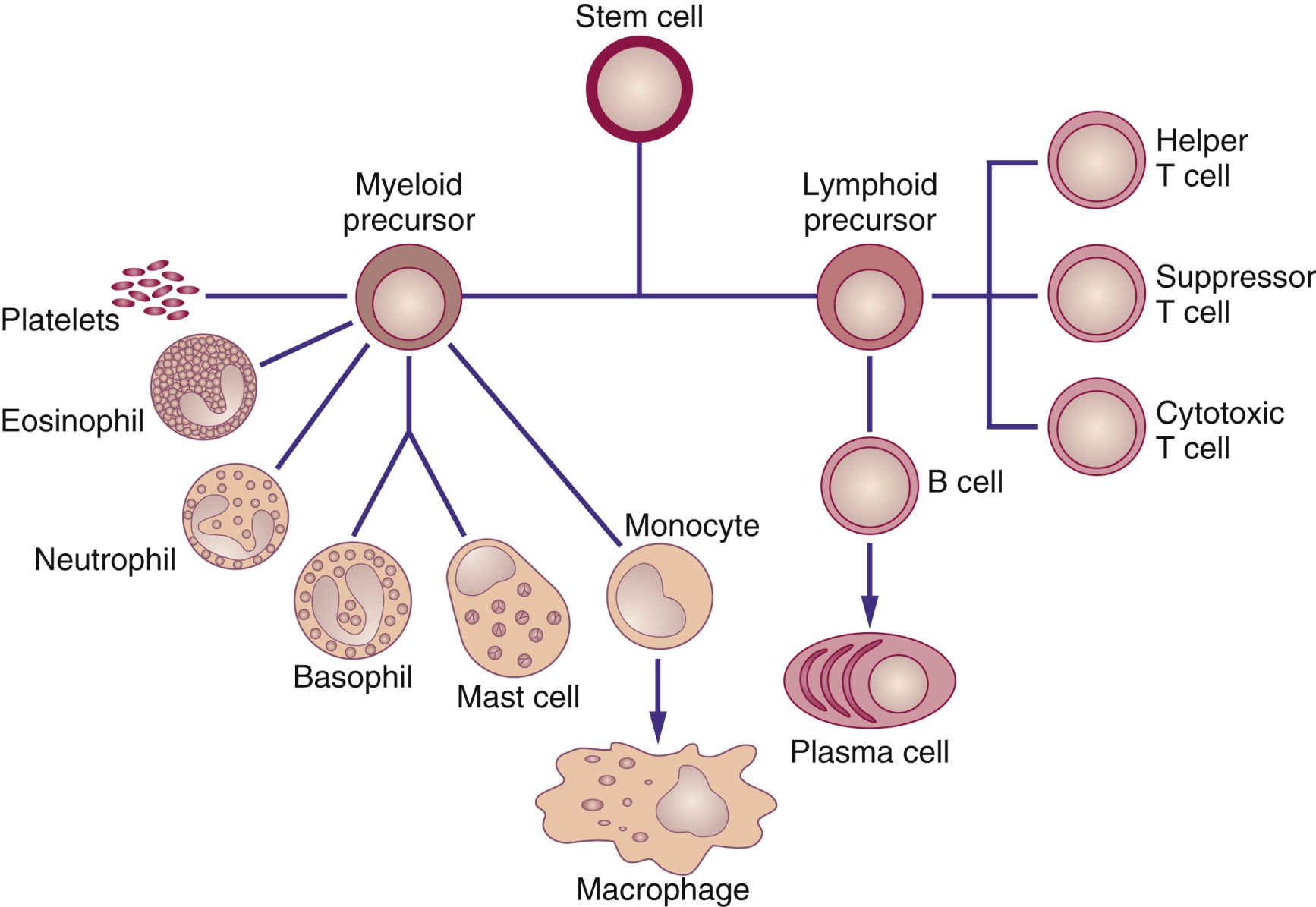
The immune system recognizes and protects the body from foreign invaders, such as bacteria or viruses; it also destroys damaged, diseased, or abnormal cells, including cancer cells. When the body detects an invader, an immune response is triggered, and substances such as white blood cells (WBCs) and natural killer cells (NKCs) provide a certain level of protection. Biologic response modifiers (BRMs), also called immunotherapies, are a class of pharmacologic drugs used to enhance, direct, or restore the body’s immune system. BRMs consist of substances naturally made in the body and those developed in the laboratory. They can kill cancer cells directly or indirectly. BRMs that target cancer cells directly are also called targeted therapy, and these are discussed in Chapter 33. Indirect therapies stimulate the body’s immune system and do not directly target cancer cells. Biologic therapies are used to treat the cancer or the side effects caused by other cancer treatments. Recombinant DNA, the genetic engineering process that combines two human DNA strands artificially, and hybridoma technology, the process that genetically makes monoclonal antibodies, are two advances that have led to commercial mass-production of BRMs (Fig. 34.1). Interferons (alfa, gamma, and beta), tumor necrosis factor (TNF), erythropoietin, vaccine therapy, colony-stimulating factors, interleukins, and monoclonal antibodies are some currently known BRMs. With the exception of monoclonal antibodies, BRMs are a complex set of proteins produced by the immune system (Fig. 34.2). Monoclonal antibodies are considered for targeted therapies and are further discussed in Chapter 33. Like chemotherapy, BRMs suppress the immune system; this immunocompromised state places patients at risk for complications such as infection. BRMs assist the immune system in several ways:
• They enhance the immune system’s ability to kill abnormal cells (immunomodulation).
• They change cancer cells to make them behave more like healthy cells.
• They inhibit normal cells from changing into cancer cells.
• They enhance the body’s ability to repair or replace damaged cells caused by other cancer treatments.
• They prevent cancer cells from metastasizing (spreading to other parts of the body).
New BRMs are always under development and investigation for clinical effectiveness. Several categories of these drugs have been approved by the U.S. Food and Drug Administration (FDA). Of the BRMs, interferons, colony-stimulating factors, and interleukin 2 are further discussed under those headings below.
Interferons
Interferons (IFNs) are a family of proteins that occur naturally in the body, and they are also produced in the laboratory. Various subtypes of IFNs have different mechanisms of action with some overlapping actions. IFNs work directly on cancer cells to slow their growth or cause cancer cells to behave more like normal cells. Some IFNs also stimulate certain types of WBCs to fight cancer, which includes NKCs, T cells, and macrophages. The two main types of IFNs are type I and type II. Type I interferons include IFN-alfa (leukocyte IFN) and IFN beta (fibroblast and epithelial cell IFN). Type II interferon includes IFN gamma produced by CD4+, CD8+, NKCs, and lymphokine-activated killer (LAK) cells. Table 34.1 provides information on IFNs with their dosages, uses, and considerations.
TABLE 34.1
Biologic Response Modifiers: Interferons
| Generic Drug Name | Route and Dosage | Uses and Considerations |
| Interferon alfa-2b | Hairy cell leukemia: A: IM/Subcut: 2 million units/m2 3 times/wk for up to 6 mo Kaposi sarcoma: A: IM/Subcut: Initially: 30 million units/m2 3 times/wk until severe intolerance or maximal response after 16 wk Malignant melanoma as adjuvant treatment: A: IV: Induction: 20 million units/m2 for 5 consecutive d/wk for 4 wk Subcut: Maint: 10 million units/m2 3 times/wk for 48 wk Follicular lymphoma (NHL): A: Subcut: 5 million units 3 times/wk for up to 18 mo | For hairy cell leukemia, adjuvant to surgical resection of malignant melanoma, follicular NHL, condyloma acuminata, AIDS-related Kaposi sarcoma, and chronic hepatitis B and non-A hepatitis. Monitor CBC, AST, ALT, ALP, LDH. Pregnancy category: C∗; PB: UK; t½: 2-3 h |
| Interferon gamma | Body surface area: >0.5 m2: A/C: Subcut: 50 mcg/m2 3 times/wk Body surface area: <0.5 m2: A/C >1 y: Subcut: 1.5 mcg/kg/dose 3 times/wk | For chronic granulomatous disease. Pregnancy category: C∗; PB: UK; t½: 0.5-6 h |
| Interferon beta-1b | Relapsing-remitting forms of MS: A: Subcut 250 mcg every other day. Incremental titration q2wk is recommended at 62.5 mcg every other day during wk 1 and 2, 125 mcg every other day during wk 3 and 4, and 187.5 mcg every other day during wk 5 and 6. | For MS. Pregnancy category: C∗; PB: UK; t½: 8 min-4.3 h |
Interferon Alpha
B-lymphocyte cells; non–B- and non–T-lymphocyte cells; and macrophages (mature monocytes) produce interferon alfa-2b (IFN-α-2b) endogenously in response to viral infection and other various exogenous inducers. Exogenous α-2b IFNs used as BRMs are considered second-generation IFNs. Interferon alfa-2a, a first-generation IFN, is currently not being marketed; however, both subtypes have similar actions. IFNs bind to cell receptors for biologic activities followed by activation of the tyrosine kinases (TKs). IFNs have been shown to have antiviral, antiproliferative, and immunomodulatory effects and they affect cellular differentiation, regulation of cell-surface major histocompatibility complex (MHC) antigen expression, and cytokine induction.
Interferon-α-2b is approved for hairy-cell leukemia, AIDS-related Kaposi sarcoma, malignant melanoma, and non-Hodgkin lymphoma (NHL); hepatitis B and C viruses; and human papillomavirus (HPV) infection. Additionally, it has been used in combination with antiviral or anticancer drugs in patients with Philadelphia-chromosome positive (Ph+) chronic myelogenous leukemia (CML), renal cell cancer (RCC), and T-cell leukemia/lymphoma.
Pharmacokinetics
Interferon alpha-2b is administered parenterally via intravenous (IV), intramuscular (IM), or subcutaneous routes. Mean serum concentrations after IM and subcutaneous administration were similar. IFN is catabolized by the renal system. Maximum concentration occurs in 3 to 12 hours, and elimination half-life is 2 to 3 hours. Following the IV route, the half-life is reached in about 2 hours and is undetectable in the serum in about 4 hours. IFN- α-2b is not cleared by hemodialysis.
Pharmacodynamics
IFN- α-2b has similar actions to native IFN-α. Endogenous IFNs are secreted by leukocytes in response to viral infection or various synthetic and biologic inducers. Once the IFN binds to cell surface receptors, tyrosine kinases are activated, which produce several IFN-stimulated enzymes that cause antiviral, antiproliferative, and immunomodulatory effects; cellular differentiation; regulation of cell-surface MHC antigen expression; and cytokine induction. Antiviral effects include inhibiting viral replication by inhibiting translation of viral proteins; inhibition of viral penetration and uncoating and/or viral assembly and release; and enhancement of the lytic (killing) effects of cytotoxic T lymphocytes. Viruses affected include hepatitis B, C, and D; herpes simplex virus types 1 and 2; human immunodeficiency virus (HIV); HPV; and rhinovirus, among others.
Antineoplastic effects may result from IFN’s ability to induce a host response to the tumor (e.g., immunomodulatory effects), cause a cytostatic effect on tumor cells, and slow the rate of cell proliferation by enhancing or inhibiting the synthesis of specific proteins, modifying cell-surface antigen expression, and/or modulating the immune system. IFNs prolong all phases of the cell cycle and promote cells to enter the G0 (resting) phase, which is thought to be important in treating hairy cell leukemia.
Side Effects and Adverse Reactions
Table 34.2 lists the common adverse effects for BRMs, including IFNs and any dosage adjustments or discontinuation of the drug for toxicity. The black-box warning includes the risk of fatal or life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Patients must be closely monitored with periodic clinical and laboratory evaluations. Table 34.3 compares the black-box warnings for IFN, interleukin 2 (IL-2), and erythropoietin (EPO). While taking IFN-α, close monitoring of complete blood count (CBC), chest radiography (CXR), electrocardiography (ECG), liver function tests (LFTs), triglycerides, and thyroid function tests (TFTs) should be conducted.
Interactions
It is unknown whether IFN-α-2b is metabolized by the liver; therefore caution is advised when IFN-α-2b is taken with other drugs metabolized through the hepatic cytochrome P450 enzyme system. The effect of IFN on the CYP450 system might increase enzyme degradation or inhibit CYP450 system. IFN-α-2b with concomitant use of theophylline may result in a 100% increase in theophylline concentrations. Caution is also warranted when taking drugs (e.g., antiretroviral nonnucleoside reverse transcriptase inhibitors [NNRTIs] and antiretroviral protease inhibitors) that can cause liver toxicity because IFNs also can cause liver damage. Hearing loss has been associated with IV eflornithine in combination with IFN-α-2b. Other drugs that may interact with IFN include barbiturates, colchicine, chemotherapy, and hydroxyurea. No information is available on drug-food or drug-herbal interactions.
Interferon Beta
Interferon beta (IFN-β) is a type I interferon produced by fibroblasts, macrophages, and epithelial cells. It has both antiviral and immune regulatory activities, especially against herpesvirus, HPV, hepatitis B and C, and HIV. IFN-β-1a is indicated for the treatment of multiple sclerosis (MS); IFN-β-1a inhibits the proinflammatory cytokines responsible for triggering the autoimmune reaction that leads to MS. IFN-β-1a also reduces T-cell migration across the blood-brain barrier and increases the production of nerve growth factor, which promotes axonal recovery; this may result in remyelination.
TABLE 34.2
Adverse Effects and Dosage Modifications for Biologic Response Modifiers
| Drug Class | Side Effects/Adverse Effects | Dose Adjustment or Discontinuation |
| Colony-stimulating factors: Erythropoietin (EPO) | Hypertension, flulike symptoms, rash, anaphylactoid reactions, antibody formation, arthralgia, myalgia, cephalgia, edema, red cell aplasia, thromboembolism, injection site reaction, urticaria, bronchospasm, cough, encephalopathy. | Stop treatment: Anaphylactoid reactions, red cell aplasia secondary to antibody formation Adjust dosage: Hgb rise >1 g/dL in 2 wk, CKD |
Colony-stimulating factors: Granulocyte colony–stimulating factor (G-CSF), granulocyte-macrophage colony–stimulating factor (GM-CSF) | Peripheral edema, flulike symptoms, chest pain, splenomegaly, anaphylactoid reactions, elevated hepatic enzymes, rash, cardiac arrhythmia, cephalgia, arthralgia, leukocytosis, asthenia, antibody formation, pulmonary disorders, capillary leak syndrome. | Stop treatment: Anaphylactoid reaction, capillary leak syndrome, ANC >10,000/mm3; ARDS, splenic involvement, sickle cell crisis, alveolar hemorrhage Adjust dosage: Vasculitis, renal disorders |
| Erythropoietin-stimulating agents (ESAs; interferons) | Flulike symptoms (fever, chills, tachycardia, malaise, myalgia, cephalgia), chest pain, fatigue, depression, drowsiness, dizziness, irritability, paresthesia, insomnia, alopecia, skin rash, amenorrhea, nausea, diarrhea, vomiting, xerostomia, abdominal pain, pancytopenia, dyspnea, cough, pharyngitis, infection. | Stop treatment: Severe depression, hypersensitivity reactions, hematologic toxicity (ANC <500/mm3 or platelets <25,000/mm3); severe hepatic decompensation Adjust dosages for other hematologic toxicity. |
| Interleukin (IL-2) | Hypotension, peripheral edema, tachycardia, SVT, flulike symptoms, rash, pruritus, electrolyte imbalances, diarrhea, vomiting, nausea, weight gain, anorexia, abdominal pain, pancytopenia, elevated liver function tests, weakness, renal impairment, respiratory disorders, antibody formation, infection. | See Table 34.5. |
TABLE 34.3
U.S. Food and Drug Administration Black-Box Warnings for Interferon, Interleukin 2, and Erythropoietin
| Interferon | Aldesleukin (IL-2) | Erythropoietin |
Autoimmune disorders Infectious disorders Ischemic disorders Neuropsychiatric disorders | Cardiac disease Coma Capillary leak syndrome Infection Pulmonary disease Treatment requires a specialized setting and experienced clinician. | Hgb concentration >11 g/dL Myocardial infarction Neoplastic disease Surgery Thromboembolic disease |
Interferon Gamma
Interferon gamma (IFN-γ) is a type II interferon produced endogenously by activating T lymphocytes and NKCs, and it is genetically produced from Escherichia coli. IFN-γ regulates the immune system and interacts with other interleukins, and it has direct and indirect antiviral activities by affecting attachment, penetration, uncoating, transcription, assembly, and maturation of viruses. It is also the primary factor for macrophage activation to kill parasites and cancer cells. IFN-γ enhances antigen processing and presentation by increasing the expression of MHC; it also increases humoral immunity and the expression of tumor suppressor genes; and it enhances recruitment of leukocytes to the sites of inflammation. IFN-γ is used for the treatment of chronic granulomatous disease and osteopetrosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree