Atypical Ductal Hyperplasia
Key Facts
Terminology
Atypical ductal hyperplasia (ADH) is an intraductal proliferation with some, but not all, features of low-grade DCIS
Etiology/Pathogenesis
Molecular studies support that ADH and low-grade DCIS are genetically related
ADH likely represents an early stage in a low-grade breast neoplasia pathway
Clinical Issues
ADH is considered a marker of increased risk of carcinoma and a nonobligate precursor of carcinoma
Increased relative risk is 4-5x or an actual lifetime risk of 13-17%
Both breasts are at increased risk
ADH on core needle biopsy should be excised due to risk of adjacent DCIS
Image Findings
ADH is most often detected associated with calcifications on mammographic screening
Microscopic Pathology
Diagnosis includes both qualitative and quantitative criteria
Differential diagnosis for ADH should include low-grade DCIS
Borderline lesions may be described as “ADH bordering on DCIS”
Top Differential Diagnoses
Usual ductal hyperplasia
Low-grade ductal carcinoma in situ
TERMINOLOGY
Abbreviations
Atypical ductal hyperplasia (ADH)
Synonyms
Ductal intraepithelial neoplasia (DIN) type 1b or 1c, depending on system used
Definitions
Clonal intraductal proliferation with architectural and cytologic features approaching those seen in low-grade ductal carcinoma in situ (LGDCIS)
ETIOLOGY/PATHOGENESIS
Molecular Pathology
DNA studies show genetic similarity between ADH and LGDCIS
Shared alterations include chromosomal gain of 1q and loss of 16q
Also seen in low-grade invasive ductal carcinoma (LGIDC)
Gene expression profiling data also show similarities shared by low-grade lesions
ADH, LGDCIS, and LGIDC have similar gene expression patterns
Differences are in quantitative levels of expression
No significant qualitative differences in expression patterns
Low-grade carcinomas express a unique set of genes rarely seen in high-grade carcinomas
ADH likely represents an early stage in a low-grade breast neoplasia pathway
However, majority of ADH does not progress to carcinoma
Factors responsible for progression from ADH to DCIS and IDC are poorly understood
CLINICAL ISSUES
Epidemiology
Incidence
Present in 15-20% of breast biopsies performed to evaluate mammographic microcalcifications
Age
Peak: Women in mid 40s
Presentation
Most commonly detected as clustered calcifications on screening mammography
Treatment
Risk reduction
Interventions to decrease risk of subsequent breast cancer must address both breasts
Surgical approach would require bilateral mastectomies
Chemoprevention with tamoxifen reduces risk of ER-positive cancers
Majority of women opt for careful surveillance
Prognosis
ADH is a marker of increased risk for developing invasive carcinoma and a nonobligate precursor of carcinoma
Associated with a 4-5x increased relative risk or a 13-17% lifetime risk of invasive carcinoma
Cancer risk approximately equal in both breasts
Some (but not all) studies show increased risk for women with positive family history
Core Needle Biopsy
ADH on core needle biopsy is an indication for excision
DCIS is found in adjacent tissue in ˜ 15-20% of cases
Likelihood of DCIS is less for larger bore core needle biopsies
Invasive carcinoma is present in < 5%
Extent or type of ADH on core does not predict with certainty which patients will or will not have DCIS on excision
Factors associated with increased likelihood of malignant diagnosis on surgical excision
Marked nuclear atypia
Multiple foci of ADH
Micropapillary architecture
IMAGE FINDINGS
Mammographic Findings
Amorphous calcifications most common finding
Clustered distribution
Other calcification morphologies (punctate, pleomorphic, fine linear) favor DCIS
ADH may be an incidental microscopic finding associated with other benign lesions
Papilloma, fibroadenoma, apocrine cysts, radial sclerosing lesion, gynecomastia
MACROSCOPIC FEATURES
Gross Findings
ADH does not form grossly apparent lesions
MICROSCOPIC PATHOLOGY
Histologic Features
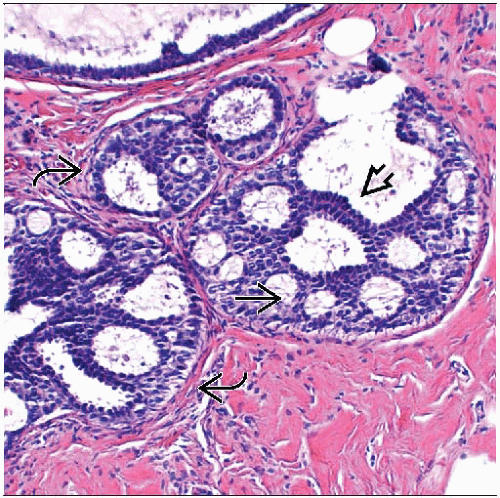
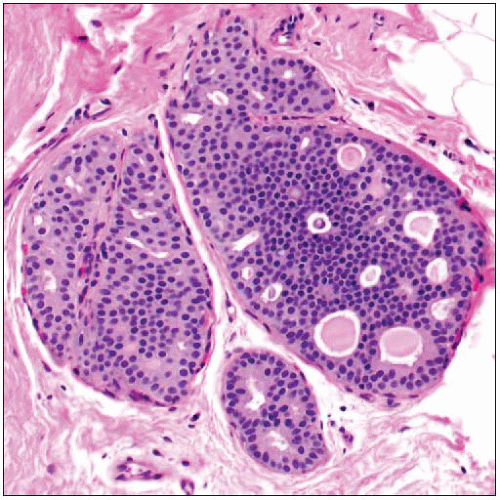
ADH is a proliferation of luminal-type cells
May involve terminal ductal lobular units or interlobular ducts
Diagnosis is based on both qualitative and quantitative assessment
Main differential diagnosis is between ADH and LGDCIS
LGDCIS requires cytologic, architectural, and size criteria to be met
ADH demonstrates some but not all features of LGDCIS
Qualitative assessment in diagnosis of ADH
Architectural features
Most common are cribriform spaces or arched bridges; spaces often not as uniform as those seen in DCIS
Bridges across cribriform spaces may be thin and show streaming of cells
Micropapillae may be present, but generally not extensive
Often associated with columnar cell change
Focal necrosis is rarely present
Cytologic features
Cells are all luminal in type; lack expression of high molecular weight keratin 5/6
Cells appear to stand apart from one another with well-defined borders
Usually partially uniform in appearance
Cell populations of different morphologies may be present (cuboidal, spindle, columnar)
Involved spaces usually contain more than 1 cell population; monomorphic-appearing cells may merge with areas of usual type hyperplasia
High-grade nuclei should not be seen in ADH
Quantitative assessment in diagnosis of ADH
Size &/or extent of involvement is used by some authorities to distinguish ADH from LGDCIS when qualitative criteria for DCIS are met
Different definitions required for diagnosis of DCIS have been proposed
At least 2 duct spaces must be involved
Lesion must be > 0.2 cm in size
Size is not a universally accepted criterion for diagnosis of ADH
No minimum size requirement for high-grade DCIS
In general, it is better to classify borderline lesions as “ADH bordering on DCIS”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree