42
Arboviruses
CHAPTER CONTENTS
INTRODUCTION
Arbovirus is an acronym for arthropod-borne virus and highlights the fact that these viruses are transmitted by arthropods, primarily mosquitoes and ticks. It is a collective name for a large group of diverse viruses, more than 600 at last count. In general, they are named either for the diseases they cause (e.g., yellow fever virus) or for the place where they were first isolated (e.g., St. Louis encephalitis virus).
A new group of viruses called roboviruses has recently emerged. The term robo refers to the fact that these viruses are rodent-borne (i.e., they are transmitted directly from rodents to humans without an arthropod vector). Transmission occurs when dried rodent excrement is inhaled into the human lung, as when sweeping the floor of a cabin. Two roboviruses cause a respiratory distress syndrome that is often fatal: Sin Nombre virus (a hantavirus) and Whitewater Arroyo virus (an arenavirus). These viruses are described in Chapter 46.
Important Properties
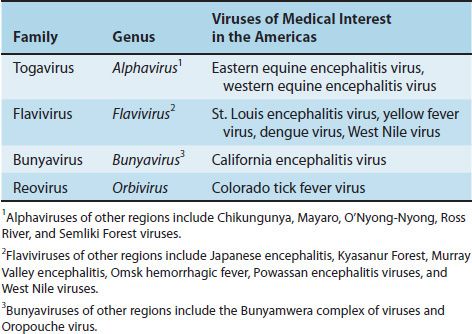
Most arboviruses are classified in three families,1 namely, togaviruses, flaviviruses, and bunyaviruses (Table 42–1).
(1) Togaviruses2 are characterized by an icosahedral nucleocapsid surrounded by an envelope and a single-stranded, positive-polarity RNA genome. They are 70 nm in diameter, in contrast to the flaviviruses, which are 40 to 50 nm in diameter (see later). Togaviruses are divided into two families, alphaviruses and rubiviruses. Only alphaviruses are considered here. The only rubivirus is rubella virus, which is discussed in Chapter 39.
(2) Flaviviruses3 are similar to togaviruses in that they also have an icosahedral nucleocapsid surrounded by an envelope and a single-stranded, positive-polarity RNA genome, but the flaviviruses are only 40 to 50 nm in diameter, whereas the togaviruses have a diameter of 70 nm.
(3) Bunyaviruses4 have a helical nucleocapsid surrounded by an envelope and a genome consisting of three segments of negative-polarity RNA that are hydrogen-bonded together.
Transmission
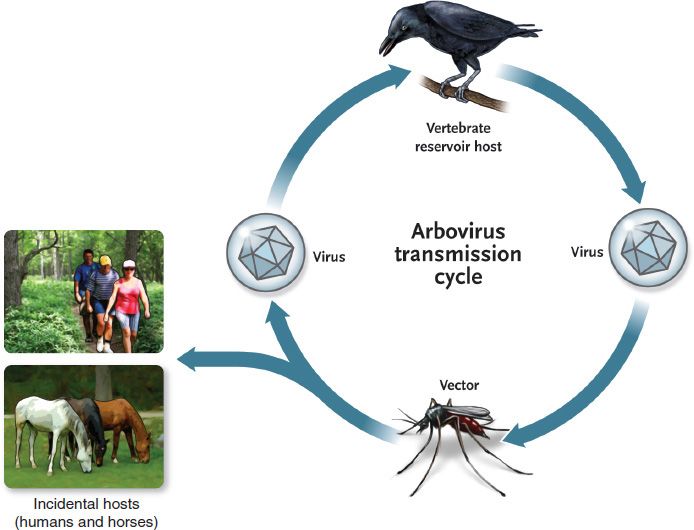
The life cycle of the arboviruses is based on the ability of these viruses to multiply in both the vertebrate host and the bloodsucking vector (Figure 42–1). For effective transmission to occur, the virus must be present in the bloodstream of the vertebrate host (viremia) in sufficiently high titer to be taken up in the small volume of blood ingested during an insect bite. After ingestion, the virus replicates in the gut of the arthropod and then spreads to other organs, including the salivary glands. Only the female of the species serves as the vector of the virus, because only she requires a blood meal in order for progeny to be produced. An obligatory length of time, called the extrinsic incubation period,5 must pass before the virus has replicated sufficiently for the saliva of the vector to contain enough virus to transmit an infectious dose. For most viruses, the extrinsic incubation period ranges from 7 to 14 days.
FIGURE 42–1 Arbovirus transmission cycle. Arboviruses typically cycle between the vertebrate reservoir host, often a bird, and the vector, often a mosquito. The infected vector can also bite other hosts, such as humans and horses, which are “dead-end” hosts because their viremia is too low to provide the vector with an infectious dose. (Modified from provider: Centers for Disease Control and Prevention.)
In addition to transmission through vertebrates, some arboviruses are transmitted by vertical “transovarian” passage from the mother tick to her offspring. Vertical transmission has important survival value for the virus if a vertebrate host is unavailable.
Humans are involved in the transmission cycle of arboviruses in two different ways. Usually, humans are dead-end hosts, because the concentration of virus in human blood is too low and the duration of viremia is too brief for the next bite to transmit the virus. However, in some diseases (e.g., yellow fever and dengue), humans have a high-level viremia and act as reservoirs of the virus.
Infection by arboviruses usually does not result in disease either in the arthropod vector or in the vertebrate animal that serves as the natural host. Disease occurs primarily when the virus infects dead-end hosts. For example, yellow fever virus cycles harmlessly among the jungle monkeys in South America, but when the virus infects a human, yellow fever can occur.
Clinical Findings & Epidemiology
The diseases caused by arboviruses range in severity from mild to rapidly fatal. The clinical picture usually fits one of three categories: (1) encephalitis; (2) hemorrhagic fever; or (3) fever with myalgias, arthralgias, and nonhemorrhagic rash. The pathogenesis of these diseases involves not only the cytocidal effect of the virus, but also, in some, a prominent immunopathologic component. After recovery from the disease, immunity is usually lifelong.
The arboviral diseases occur primarily in the tropics but are also found in temperate zones such as the United States and as far north as Alaska and Siberia. They have a tendency to cause sudden outbreaks of disease, generally at the interface between human communities and jungle or forest areas.
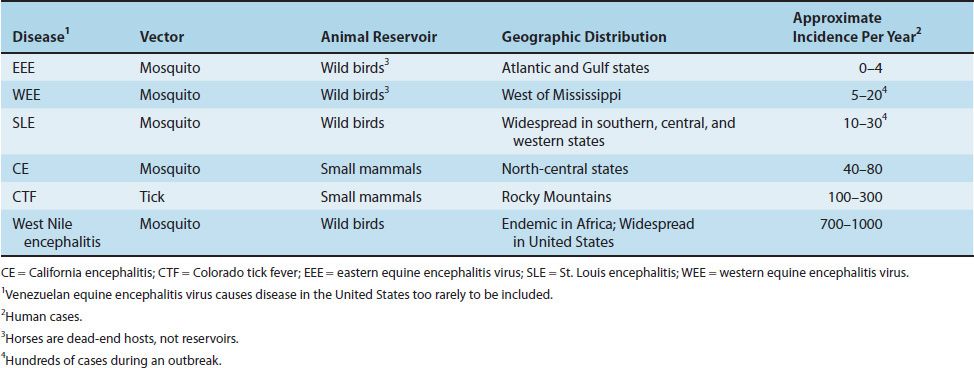
ARBOVIRUSES THAT CAUSE DISEASE IN THE UNITED STATES
Eastern Equine Encephalitis Virus
Of the four encephalitis viruses listed in Table 42–2, eastern equine encephalitis (EEE) virus causes the most severe disease and is associated with the highest fatality rate (approximately 50%). In its natural habitat, the virus is transmitted primarily by the swamp mosquito, Culiseta, among the small wild birds of the Atlantic and Gulf Coast states. Species of Aedes mosquitoes are suspected of carrying the virus from its wild bird reservoir to the principal dead-end hosts, horses and humans. The number of cases of human encephalitis caused by EEE virus in the United States usually ranges from zero to four per year, but outbreaks involving hundreds of cases also occur. Subclinical infections greatly exceed the number of overt cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree