88 | Stem Cell Biology |
Stem cell biology is a rapidly expanding field that explores the characteristics and possible clinical applications of a variety of stem cells that serve as the progenitors of more differentiated cell types. In addition to potential therapeutic applications (Chap. 90e), patient-derived stem cells can also be used as disease models and as a means of testing drug efficacy. Stem cells and their niche are a major focus of medical research because they play central roles in tissue and organ homeostasis and repair, which are important aspects of aging and disease.
IDENTIFICATION, ISOLATION, AND DERIVATION OF STEM CELLS
Resident Stem Cells The definition of stem cells remains elusive. Stem cells were originally postulated as unspecified or undifferentiated cells that provide a source of renewal of skin, intestine, and blood cells throughout life. These resident stem cells have been identified in a variety of organs (e.g., epithelia of the skin and digestive system, bone marrow, blood vessels, brain, skeletal muscle, liver, testis, and pancreas) based on their specific locations, morphology, and biochemical markers.
Isolated Stem Cells Unequivocal identification of stem cells requires their separation and purification, usually based on a combination of specific cell-surface markers. These isolated stem cells (e.g., hematopoietic stem [HS] cells) can be studied in detail and used in clinical applications, such as bone marrow transplantation (Chap. 89e). However, the lack of specific cell-surface markers for other types of stem cells has made it difficult to isolate them in large quantities. This challenge has been partially addressed in animal models by genetically marking different cell types with green-fluorescent protein driven by cell-specific promoters. Alternatively, putative stem cells have been isolated from a variety of tissues as side population (SP) cells using fluorescence-activated cell sorting after staining with the Hoechst 33342 dye.
Cultured Stem Cells It is desirable to culture and expand stem cells in vitro to obtain a sufficient quantity for analysis and potential therapeutic use. Although the derivation of stem cells in vitro has been a major obstacle in stem cell biology, the number and types of cultured stem cells have increased progressively (Table 88-1). Cultured stem cells derived from resident stem cells are often called adult stem cells or somatic stem cells to distinguish them from embryonic stem (ES) and embryonic germ (EG) cells. However, considering the existence of embryo-derived, tissue-specific stem cells (e.g., trophoblast stem [TS] cells) and the possible derivation of similar cells from an embryo/fetus (e.g., neural stem [NS] cells), it is more appropriate to use the term, tissue stem cells.
EXAMPLES OF CULTURED STEM CELLS |
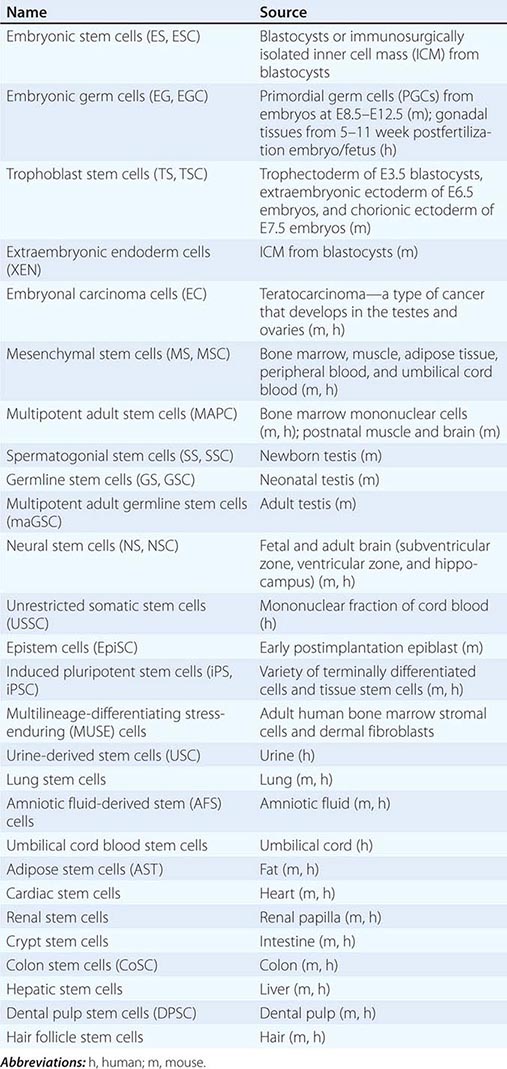
Successful derivation of cultured stem cells (both embryonic and tissue stem cells) often requires the identification of necessary growth factors and culture conditions, mimicking the microenvironment or niche of the resident stem cells. Recently, long-term maintenance of tissue stem cells in vitro is increasingly possible by growing them as three-dimensional (3D) organoids, which contain both stem cells and niche cells (Chap. 92e). For example, intestinal stem cells can now be cultured as “epithelial mini-guts” in the presence of R-spondin, epidermal growth factor (EGF), and noggin on Matrigel. Similarly, lung stem cells can be cultured as self-renewing “alveolospheres.” A growing list of cultured stem cells, although not comprehensive, is shown in Table 88-1. Please note that the establishment of cultured stem cells is often under dispute due to the difficulties in assessing the characteristics of these cells.
SELF-RENEWAL AND PROLIFERATION OF STEM CELLS
Symmetric and Asymmetric Cell Division The most widely accepted stem cell definition is a cell with a unique capacity to produce unaltered daughter cells (self-renewal) and to generate specialized cell types (potency). Self-renewal can be achieved in two ways. Asymmetric cell division produces one daughter cell that is identical to the parental cell and one daughter cell that is different from the parental cell and is a progenitor or differentiated cell. Asymmetric cell division does not increase the number of stem cells. Symmetric cell division produces two identical daughter cells. For stem cells to proliferate in vitro, they must divide symmetrically.
Unlimited Expansion In Vitro Resident stem cells are often quiescent and divide infrequently. However, once the stem cells are successfully cultured in vitro, they often acquire the capacity to divide continuously and the ability to proliferate beyond the normal passage limit typical of primary cultured cells (sometimes called immortality). These features are primarily seen in ES cells but have also been demonstrated for tissue stem cells, such as NS cells and mesenchymal stem (MS) cells, thereby enhancing the potential of these cells for therapeutic use (Table 88-1).
Stability of Genotype and Phenotype The capacity to actively proliferate is often associated with the accumulation of chromosomal abnormalities and mutations. Mouse ES cells appear to be an exception to this rule and tend to maintain their euploid karyotype and genome integrity. By contrast, human ES cells appear to be more susceptible to mutations after long-term culture. However, it is also important to note that even euploid mouse ES cells can form teratomas when injected into immunosuppressed animals, raising concerns about the possible formation of tumors after transplanting actively dividing stem cells.
POTENCY AND DIFFERENTIATION OF STEM CELLS
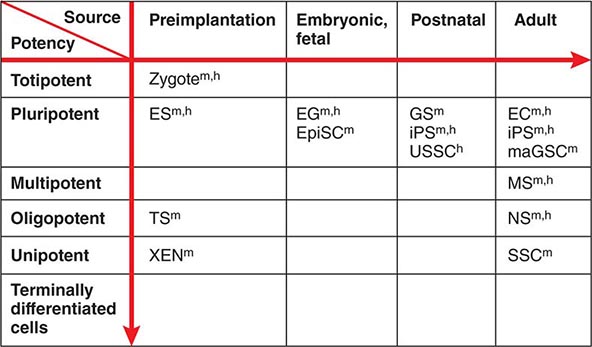
Developmental Potency The term potency is used to indicate a cell’s ability to differentiate into multiple specialized cell types. The current lack of knowledge about the molecular nature of potency requires the experimental manipulation of stem cells to demonstrate their potency. For example, in vivo testing can be done by injecting stem cells into mouse blastocysts or immunosuppressed adult mice and determining how many different cell types are formed from the injected cells. However, these in vivo assays are not applicable to human stem cells. In vitro testing can be performed by differentiating cells in various culture conditions to determine how many different cell types are formed from the cells. The formal test of self-renewal and potency is performed by demonstrating that a single cell possesses such abilities in vitro (clonality). Cultured stem cells are tentatively grouped according to their potency (Fig. 88-1). Only some examples are shown, because many cultured stem cells, especially human cells, lack definitive information about their developmental potency.
FIGURE 88-1 Potency and source developmental stage of cultured stem cells. For abbreviations of stem cells, see Table 88-1. Note that stem cells are often abbreviated with or without “cells,” e.g., ES cells or ESCs for embryonic stem cells. h, human; m, mouse.
From Totipotency to Unipotency Totipotent cells can form an entire organism autonomously. Only a fertilized egg (zygote) possesses this feature. Pluripotent cells (e.g., ES cells) can form almost all of the body’s cell lineages (endoderm, mesoderm, and ectoderm), including germ cells. Multipotent cells (e.g., HS cells) can form multiple cell lineages but cannot form all of the body’s cell lineages. Oligopotent cells (e.g., NS cells) can form more than one cell lineage but are more restricted than multipotent cells. Oligopotent cells are sometimes called progenitor cells or precursor cells; however, these terms are often more strictly used to define partially differentiated or lineage-committed cells (e.g., myeloid progenitor cells) that can divide into different cell types but lack self-renewing capacity. Unipotent cells or monopotent cells (e.g., spermatogonial stem [SS] cells) can form a single differentiated cell lineage.
Nuclear Reprogramming Development naturally progresses from totipotent fertilized eggs to pluripotent epiblast cells to multipotent cells and, finally, to terminally differentiated cells. According to Waddington’s epigenetic landscape, this is analogous to a ball moving down a slope. The reversal of the terminally differentiated cells to totipotent or pluripotent cells (called nuclear reprogramming) can thus be seen as an uphill gradient. Nuclear reprogramming has been achieved using nuclear transplantation, or nuclear transfer (NT), procedures (often called “cloning”), where the nucleus of a differentiated cell is transferred into an enucleated oocyte. Although this is an error-prone procedure with a very low success rate, live animals have been produced using adult somatic cells as donors in sheep, mice, and other mammals. In mice, it has been demonstrated that ES cells derived from blastocysts made by somatic cell NT are indistinguishable from normal ES cells. NT can potentially be used to produce patient-specific ES cells carrying a genome identical to that of the patient, although such strategies have not been pursued due to ethical issues and technical challenges. Recent success in generating human ES cells by NT has rekindled an interest in this area; however, the limited supply of human oocytes will still be a major problem for clinical applications of NT.
An alternative approach that has become a method of choice is the direct conversion of terminally differentiated cells into ES-like cells (called induced pluripotent stem [iPS] cells) by overexpressing a combination of key transcription factors (TFs). The original method was to infect mouse embryonic fibroblast cells with retrovirus vectors carrying four TFs [Pou5f1 (Oct4), Sox2, Klf4, and Myc] and to identify rare ES-like cells in culture. This approach was soon adapted to human cells, followed by a more refined procedure (e.g., the use of fewer TFs, different cell types, and different gene-delivery methods). Because a clinical trial using iPS cells is imminent, the safety of iPS-based therapy is a major concern and a variety of measures are being taken to ensure the safety. For example, it has now become a standard to use footprint-free methods such as an episomal vector, Sendai virus vector, and synthetic mRNAs to deliver reprogramming factors into cells, resulting in the production of patient-specific iPS cells with minimal alteration of their genetic makeup. In addition to cell replacement therapy, disease-specific iPS cells are expected to play a role in modeling human disease in vitro and in screening drugs for personalized medicine.
It has also become possible to convert one type of terminally differentiated cell (e.g., fibroblast cell) into another type of terminally differentiated cell (e.g., cardiac muscle, neuron, or hepatocyte) by overexpressing specific sets of TFs (called direct reprogramming). Direct reprogramming can bypass the step of making iPS cells, possibly providing the safer route to desired cell types for therapy; however, the technology is currently limited by its low efficiency.
Stem Cell Plasticity, Transdifferentiation, and Facultative Stem Cells The prevailing paradigm in developmental biology is that once cells are differentiated, their phenotypes are stable. However, more recent studies show that tissue stem cells, which have traditionally been thought to be lineage-committed multipotent cells, possess the capacity to differentiate into cell types outside their lineage restrictions (called transdifferentiation). For example, HS cells may be converted into neurons as well as germ cells. This feature may provide a means to use tissue stem cells derived directly from a patient for therapeutic purposes, thereby eliminating the need to use embryonic stem cells or elaborate procedures such as nuclear reprogramming of a patient’s somatic cells. However, more strict criteria and rigorous validation are required to establish tissue stem cell plasticity. For example, observations of transdifferentiation may reflect cell fusion, contamination with progenitor cells from other cell lineages, or persistence of pluripotent embryonic cells in adult organs. Therefore, the assignment of potency to each cultured stem cell in Fig. 88-1 should be considered with caution. Whether transdifferentiation exists and can be used for therapeutic purposes remains to be determined conclusively. A similar, but distinct, concept is the facultative stem cell, which is defined as a unipotent cell or a terminally differentiated cell that can function as a stem cell upon tissue injury. The presence of such cells has been proposed for some organs such as liver, intestine, pancreas, and testis, but is still debated.
Directed Differentiation of Stem Cells Pluripotent stem cells (e.g., ES and iPS cells) can differentiate into multiple cell types, but in culture, they normally differentiate into heterogeneous cell populations in a stochastic manner. However, for therapeutic uses, it is desirable to direct stem cells into specific cell types (e.g., insulin-secreting beta cells). This is an active area of stem cell research, and protocols are being developed to achieve this goal. In any of these directed cell differentiation systems, the cell phenotype must be evaluated critically. Alternatively, the heterogeneity of the cell population derived from pluripotent stem cells can be actively exploited, as different types of cells interact with each other in culture and further enhance their own differentiation. In some instances, e.g., optic cup, self-organizing tissue morphogenesis has been demonstrated in 3D culture.
MOLECULAR CHARACTERIZATION OF STEM CELLS
Genomics and Proteomics In addition to standard molecular biological approaches, high-throughput genomics and proteomics have been extensively applied to the analysis of stem cells. For example, DNA microarray analyses have revealed the expression levels of essentially all genes and identified specific markers for some stem cells. Chromatin immunoprecipitation coupled with next-generation sequencing technologies, capable of producing billions of sequence reads in a single run, has revealed chromatin modifications (“epigenetic marks”) relevant to stem cell properties. Similarly, the protein profiles of stem cells have been assessed by using mass spectrometry. These methods are beginning to provide a novel means to characterize and classify various stem cells and the molecular mechanisms that give them their unique characteristics.
ES Cell Regulation It is important to identify genes involved in the regulation of stem cell function and to examine the effects of altered gene expression on ES and other stem cells. For example, core networks of TFs such as Pou5f1 (Oct4), Nanog, and Sox2, govern key gene regulatory pathways/networks for the maintenance of self-renewal and pluripotency of mouse and human ES cells. These TF networks are modulated by specific external factors through signal transduction pathways, such as leukemia inhibitory factor (Lif)/Stat3, mitogen-activated protein kinase 1/3 (Mapk1/3), the transforming growth factor β (TGFβ) superfamily, and Wnt/glycogen synthase kinase 3 beta (Gsk3b). Inhibitors of Mapk1/3 and Gsk3b signaling enhance the derivation of ES cells and help maintain ES cells in full pluripotency (“ground” or “naive state”). Recent data also indicate that 20–25 nucleotide RNAs, called microRNAs (miRNAs), play an important role in regulating stem cell function by repressing the translation of their target genes. For example, it has been shown that miR-21 regulates cell cycle progression in ES cells and miR-128 prevents the differentiation of hematopoietic progenitor cells. These types of analyses should provide molecular clues about the function of stem cells and lead to a more effective means to manipulate stem cells for future therapeutic use.
89e | Hematopoietic Stem Cells |
All of the cell types in the peripheral blood and some cells in every tissue of the body are derived from hematopoietic (hemo: blood; poiesis: creation) stem cells. If the hematopoietic stem cell is damaged and can no longer function (e.g., due to a nuclear accident), a person would survive 2–4 weeks in the absence of extraordinary support measures. With the clinical use of hematopoietic stem cells, tens of thousands of lives are saved each year (Chap. 139e). Stem cells produce hundreds of billions of blood cells daily from a stem cell pool that is estimated to be only in the tens of thousands. How stem cells do this, how they persist for many decades despite the production demands, and how they may be better used in clinical care are important issues in medicine.
The study of blood cell production has become a paradigm for how other tissues may be organized and regulated. Basic research in hematopoiesis includes defining stepwise molecular changes accompanying functional changes in maturing cells, aggregating cells into functional subgroups, and demonstrating hematopoietic stem cell regulation by a specialized microenvironment; these concepts are worked out in hematology, but they offer models for other tissues. Moreover, these concepts may not be restricted to normal tissue function but extend to malignancy. Stem cells are rare cells among a heterogeneous population of cell types, and their behavior is assessed mainly in experimental animal models involving reconstitution of hematopoiesis. Thus, much of what we know about stem cells is imprecise and based on inferences from genetically manipulated animals.
CARDINAL FUNCTIONS OF HEMATOPOIETIC STEM CELLS
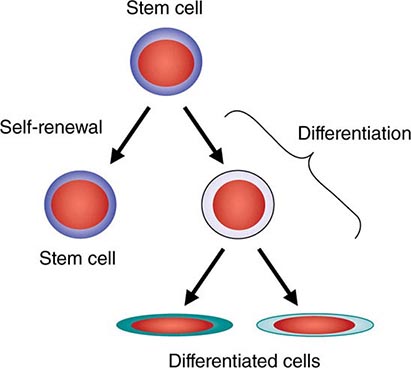
All stem cell types have two cardinal functions: self-renewal and differentiation (Fig. 89e-1). Stem cells exist to generate, maintain, and repair tissues. They function successfully if they can replace a wide variety of shorter-lived mature cells over prolonged periods. The process of self-renewal (see below) assures that a stem cell population can be sustained over time. Without self-renewal, the stem cell pool would become exhausted and tissue maintenance would not be possible. The process of differentiation leads to production of the effectors of tissue function: mature cells. Without proper differentiation, the integrity of tissue function would be compromised and organ failure or neoplasia would ensue.
FIGURE 89e-1 Signature characteristics of the stem cell. Stem cells have two essential features: the capacity to differentiate into a variety of mature cell types and the capacity for self-renewal. Intrinsic factors associated with self-renewal include expression of Bmi-1, Gfi-1, PTEN, STAT5, Tel/Atv6, p21, p18, MCL-1, Mel-18, RAE28, and HoxB4. Extrinsic signals for self-renewal include Notch, Wnt, SHH, and Tie2/Ang-1. Based mainly on murine studies, hematopoietic stem cells express the following cell surface molecules: CD34, Thy-1 (CD90), c-Kit receptor (CD117), CD133, CD164, and c-Mpl (CD110, also known as the thrombopoietin receptor).
In the blood, mature cells have variable average life spans, ranging from 7 h for mature neutrophils to a few months for red blood cells to many years for memory lymphocytes. However, the stem cell pool is the central, durable source of all blood and immune cells, maintaining a capacity to produce a broad range of cells from a single cell source, yet keeping itself vigorous over decades of life. As an individual stem cell divides, it has the capacity to accomplish one of three division outcomes: two stem cells, two cells destined for differentiation, or one stem cell and one differentiating cell. The former two outcomes are the result of symmetric cell division, whereas the latter indicates a different outcome for the two daughter cells—an event termed asymmetric cell division. The relative balance for these types of outcomes may change during development and under particular kinds of demands on the stem cell pool.
DEVELOPMENTAL BIOLOGY OF HEMATOPOIETIC STEM CELLS
During development, blood cells are produced at different sites. Initially, the yolk sac provides oxygen-carrying red blood cells, and then the placenta and several sites of intraembryonic blood cell production become involved. These intraembryonic sites engage in sequential order, moving from the genital ridge at a site where the aorta, gonadal tissue, and mesonephros are emerging to the fetal liver and then, in the second trimester, to the bone marrow and spleen. As the location of stem cells changes, the cells they produce also change. The yolk sac provides red cells expressing embryonic hemoglobins while intraembryonic sites of hematopoiesis generate red cells, platelets, and the cells of innate immunity. The production of the cells of adaptive immunity occurs when the bone marrow is colonized and the thymus forms. Stem cell proliferation remains high, even in the bone marrow, until shortly after birth, when it appears to dramatically decline. The cells in the bone marrow are thought to arrive by the bloodborne transit of cells from the fetal liver after calcification of the long bones has begun. The presence of stem cells in the circulation is not unique to a time window in development; however, hematopoietic stem cells appear to circulate throughout life. The time that cells spend freely circulating appears to be brief (measured in minutes in the mouse), but the cells that do circulate are functional and can be used for transplantation. The number of stem cells that circulate can be increased in a number of ways to facilitate harvest and transfer to the same or a different host.
MOBILITY OF HEMATOPOIETIC STEM CELLS
Cells entering and exiting the bone marrow do so through a series of molecular interactions. Circulating stem cells (through CD162 and CD44) engage the lectins (carbohydrate binding proteins) P- and E-selectin on the endothelial surface to slow the movement of the cells to a rolling phenotype. Stem cell integrins are then activated and accomplish firm adhesion between the stem cell and vessel wall, with a particularly important role for stem cell VCAM-1 engaging endothelial VLA-4. The chemokine CXCL12 (SDF1) interacting with stem cell CXCR4 receptors and ionic calcium interacting with the calcium sensing receptor appear to be important in the process of stem cells getting from the circulation to where they engraft in the bone marrow. This is particularly true in the developmental move from fetal liver to bone marrow.
However, the role for CXCR4 in adults appears to be more related to retention of stem cells in the bone marrow rather than the process of getting them there. Interrupting that retention process through either specific molecular blockers of the CXCR4/CXCL12 interaction, cleavage of CXCL12, or downregulation of the CXCR4 receptor can all result in the release of stem cells into the circulation. This process is an increasingly important aspect of recovering stem cells for therapeutic use as it has permitted the harvesting process to be done by leukapheresis rather than bone marrow punctures in the operating room. Granulocyte colony-stimulating factor and plerixafor, a macrocyclic compound that can block CXCR4, are both used clinically to mobilize marrow hematopoietic stem cells for transplant. Refining our knowledge of how stem cells get into and out of the bone marrow may improve our ability to obtain stem cells and make them more efficient at finding their way to the specific sites for blood cell production, the so-called stem cell niche.
HEMATOPOIETIC STEM CELL MICROENVIRONMENT
The concept of a specialized microenvironment, or stem cell niche, was first proposed to explain why cells derived from the bone marrow of one animal could be used in transplantation and again be found in the bone marrow of the recipient. This niche is more than just a housing site for stem cells, however. It is an anatomic location where regulatory signals are provided that allow the stem cells to thrive, to expand if needed, and to provide varying amounts of descendant daughter cells. In addition, unregulated growth of stem cells may be problematic based on their undifferentiated state and self-renewal capacity. Thus, the niche must also regulate the number of stem cells produced. In this manner, the niche has the dual function of serving as a site of nurture but imposing limits for stem cells: in effect, acting as both a nutritive and constraining home.
The niche for blood stem cells changes with each of the sites of blood production during development, but for most of human life it is located in the bone marrow. Within the bone marrow, the perivascular space particularly in regions of trabecular bone serves as a niche. The mesenchymal and endothelial cells of the marrow microvessels produce kit ligand and CXCL12, both known to be important for hematopoietic stem cells. Other cell types, such as sympathetic neurons, nonmyelinating Schwann cells, macrophages, osteoclasts, and osteoblasts, have been shown to regulate stem cells, but it is unclear whether their effects are direct or indirect. Extracellular matrix proteins like osteopontin also affect stem cell function. The endosteal region is particularly important for transplanted cells, suggesting that there may be distinctive features of that region that are yet to be defined that are important mediators of stem cell engraftment. The functioning of the niche as a supportive context for stem cells is of obvious importance for maintaining hematopoiesis and in transplantation. An active area of study involves determining whether the niche is altered in disease and whether drugs can modify niche function to improve transplantation or normal stem cell function in hematologic disease.
EXCESS CAPACITY OF HEMATOPOIETIC STEM CELLS
In the absence of disease, one never runs out of hematopoietic stem cells. Indeed, serial transplantation studies in mice suggest that sufficient stem cells are present to reconstitute several animals in succession, with each animal having normal blood cell production. The fact that allogeneic stem cell transplant recipients also never run out of blood cells in their life span, which can extend for decades, argues that even the limiting numbers of stem cells provided to them are sufficient. How stem cells respond to different conditions to increase or decrease their mature cell production remains poorly understood. Clearly, negative feedback mechanisms affect the level of production of most of the cells, leading to the normal tightly regulated blood cell counts. However, many of the regulatory mechanisms that govern production of more mature progenitor cells do not apply or apply differently to stem cells. Similarly, most of the molecules shown to be able to change the size of the stem cell pool have little effect on more mature blood cells. For example, the growth factor erythropoietin, which stimulates red blood cell production from more mature precursor cells, has no effect on stem cells. Similarly, granulocyte colony-stimulating factor drives the rapid proliferation of granulocyte precursors but has little or no effect on the cell cycling of stem cells. Rather, it changes the location of stem cells by indirect means, altering molecules such as CXCL12 that tether stem cells to their niche. Molecules shown to be important for altering the proliferation, self-renewal, or survival of stem cells, such as cyclin-dependent kinase inhibitors, transcription factors like Bmi-1, or microRNA-processing enzymes like Dicer, have little or different effects on progenitor cells. Hematopoietic stem cells have governing mechanisms that are distinct from the cells they generate.
HEMATOPOIETIC STEM CELL DIFFERENTIATION
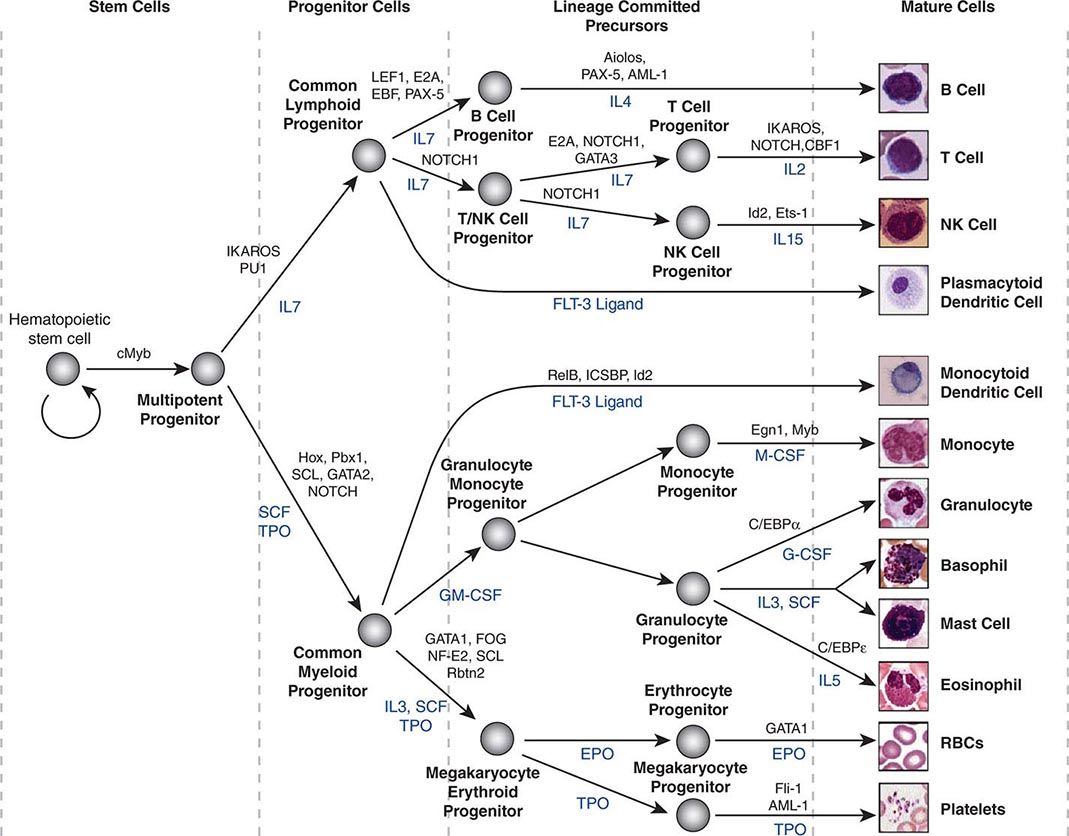
Hematopoietic stem cells sit at the base of a branching hierarchy of cells culminating in the many mature cell types that compose the blood and immune system (Fig. 89e-2). The maturation steps leading to terminally differentiated and functional blood cells take place both as a consequence of intrinsic changes in gene expression and niche-directed and cytokine-directed changes in the cells. Our knowledge of the details remains incomplete. As stem cells mature to progenitors, precursors, and, finally, mature effector cells, they undergo a series of functional changes. These include the obvious acquisition of functions defining mature blood cells, such as phagocytic capacity or hemoglobin synthesis. They also include the progressive loss of plasticity (i.e., the ability to become other cell types). For example, the myeloid progenitor can make all cells in the myeloid series but none in the lymphoid series. As common myeloid progenitors mature, they become precursors for either monocytes and granulocytes or erythrocytes and megakaryocytes, but not both. Some amount of reversibility of this process may exist early in the differentiation cascade, but that is lost beyond a distinct stage in normal physiologic conditions. With genetic interventions, however, blood cells, like other somatic cells, can be reprogrammed to become a variety of cell types.
FIGURE 89e-2 Hierarchy of hematopoietic differentiation. Stem cells are multipotent cells that are the source of all descendant cells and have the capacity to provide either long-term (measured in years) or short-term (measured in months) cell production. Progenitor cells have a more limited spectrum of cells they can produce and are generally a short-lived, highly proliferative population also known as transient amplifying cells. Precursor cells are cells committed to a single blood cell lineage but with a continued ability to proliferate; they do not have all the features of a fully mature cell. Mature cells are the terminally differentiated product of the differentiation process and are the effector cells of specific activities of the blood and immune system. Progress through the pathways is mediated by alterations in gene expression. The regulation of the differentiation by soluble factors and cell-cell communications within the bone marrow niche are still being defined. The transcription factors that characterize particular cell transitions are illustrated on the arrows; the soluble factors that contribute to the differentiation process are in blue. This picture is a simplification of the process. Active research is revealing multiple discrete cell types in the maturation of B cells and T cells and has identified cells that are biased toward one lineage or another (rather than uncommitted) in their differentiation. EPO, erythropoietin; RBC, red blood cell; SCF, stem cell factor; TPO, thrombopoietin.
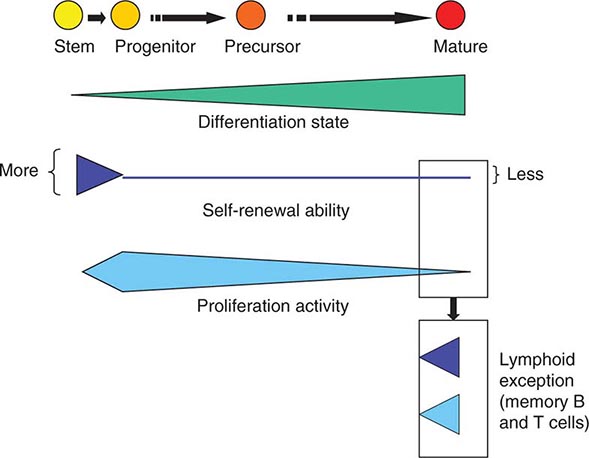
As cells differentiate, they may also lose proliferative capacity (Fig. 89e-3). Mature granulocytes are incapable of proliferation and only increase in number by increased production from precursors. The exceptions to the rule are some resident macrophages, which appear capable of proliferation, and lymphoid cells. Lymphoid cells retain the capacity to proliferate but have linked their proliferation to the recognition of particular proteins or peptides by specific antigen receptors on their surface. Like many tissues with short-lived mature cells such as the skin and intestine, blood cell proliferation is largely accomplished by a more immature progenitor population. In general, cells within the highly proliferative progenitor cell compartment are also relatively short-lived, making their way through the differentiation process in a defined molecular program involving the sequential activation of particular sets of genes. For any particular cell type, the differentiation program is difficult to speed up. The time it takes for hematopoietic progenitors to become mature cells is ~10–14 days in humans, evident clinically by the interval between cytotoxic chemotherapy and blood count recovery in patients.
FIGURE 89e-3 Relative function of cells in the hematopoietic hierarchy. The boxes represent distinct functional features of cells in the myeloid (upper box) versus lymphoid (lower box) lineages.
Although hematopoietic stem cells are generally thought to have the capacity to form all cells of the blood, it is becoming clear that individual stem cells may not be equal in their differentiation potential. That is, some stem cells are “biased” to become mature cells of a particular type. In addition, the general concept of cells having a binary choice of lymphoid or myeloid differentiation is not entirely accurate. A cell population with limited myeloid (monocyte and granulocyte) and lymphoid potential is now added to the commitment steps stem cells may undergo.
SELF-RENEWAL
The hematopoietic stem cell must balance its three potential fates: apoptosis, self-renewal, and differentiation. The proliferation of cells is generally not associated with the ability to undergo a self-renewing division except among memory T and B cells and among stem cells. Self-renewal capacity gives way to differentiation as the only option after cell division when cells leave the stem cell compartment, until they have the opportunity to become memory lymphocytes. In addition to this self-renewing capacity, stem cells have an additional feature characterizing their proliferation machinery. Stem cells in many mature adult tissues may be heterogeneous with some being deeply quiescent, serving as a deep reserve, whereas others are more proliferative and replenish the short-lived progenitor population. In the hematopoietic system, stem cells are generally cytokine-resistant, remaining dormant even when cytokines drive bone marrow progenitors to proliferation rates measured in hours. Stem cells, in contrast, are thought to divide at far longer intervals, measured in months to years, for the most quiescent cells. This quiescence is difficult to overcome in vitro, limiting the ability to effectively expand human hematopoietic stem cells. The process may be controlled by particularly high levels of cyclin-dependent kinase inhibitors like p57 or CDKN1c that restrict entry of stem cells into the cell cycle, blocking the G1-S transition. Exogenous signals from the niche also appear to enforce quiescence, including the activation of the tyrosine kinase receptor Tie2 on stem cells by angiopoietin 1 on niche cells.
The regulation of stem cell proliferation also appears to change with age. In mice, the cyclin-dependent kinase inhibitor p16INK4a accumulates in stem cells in older animals and is associated with a change in five different stem cell functions, including cell cycling. Lowering expression of p16INK4a in older animals improves stem cell cycling and capacity to reconstitute hematopoiesis in adoptive hosts, making them similar to younger animals. Mature cell numbers are unaffected. Therefore, molecular events governing the specific functions of stem cells are being gradually made clear and offer the potential of new approaches to changing stem cell function for therapy. One critical stem cell function that remains poorly defined is the molecular regulation of self-renewal.
For medicine, self-renewal is perhaps the most important function of stem cells because it is critical in regulating the number of stem cells. Stem cell number is a key limiting parameter for both autologous and allogeneic stem cell transplantation. Were we to have the ability to use fewer stem cells or expand limited numbers of stem cells ex vivo, it might be possible to reduce the morbidity and expense of stem cell harvests and enable use of other stem cell sources. Specifically, umbilical cord blood is a rich source of stem cells. However, the volume of cord blood units is extremely small, and therefore, the total number of hematopoietic stem cells that can be obtained in any single cord blood unit is generally only sufficient to transplant an individual of <40 kg. This limitation restricts what would otherwise be an extremely promising source of stem cells. Two features of cord blood stem cells are particularly important. (1) They are derived from a diversity of individuals that far exceeds the adult donor pool and therefore can overcome the majority of immunologic cross-matching obstacles. (2) Cord blood stem cells have a large number of T cells associated with them, but (paradoxically) they appear to be associated with a lower incidence of graft-versus-host disease when compared with similarly mismatched stem cells from other sources. If stem cell expansion by self-renewal could be achieved, the number of cells available might be sufficient for use in larger adults. An alternative approach to this problem is to improve the efficiency of engraftment of donor stem cells. Graft engineering is exploring methods of adding cell components that may enhance engraftment. Furthermore, at least some data suggest that depletion of host NK (natural killer) cells may lower the number of stem cells necessary to reconstitute hematopoiesis.
Some limited understanding of self-renewal exists and, intriguingly, implicates gene products that are associated with the chromatin state, a high-order organization of chromosomal DNA that influences transcription. These include members of the polycomb family, a group of zinc finger–containing transcriptional regulators that interact with the chromatin structure, contributing to the accessibility of groups of genes for transcription. One member, Bmi-1, is important in enabling hematopoietic stem cell self-renewal through modification of cell cycle regulators such as the cyclin-dependent kinase inhibitors. In the absence of Bmi-1 or of the transcriptional regulator, Gfi-1, hematopoietic stem cells decline in number and function. In contrast, dysregulation of Bmi-1 has been associated with leukemia; it may promote leukemic stem cell self-renewal when it is overexpressed. Other transcription regulators have also been associated with self-renewal, particularly homeobox, or “hox,” genes. These transcription factors are named for their ability to govern large numbers of genes, including those determining body patterning in invertebrates. HoxB4 is capable of inducing extensive self-renewal of stem cells through its DNA-binding motif. Other members of the hox family of genes have been noted to affect normal stem cells, but they are also associated with leukemia. External signals that may influence the relative self-renewal versus differentiation outcomes of stem cell cycling include specific Wnt ligands. Intracellular signal transducing intermediates are also implicated in regulating self-renewal. They include PTEN, an inhibitor of the AKT pathway, and STAT5, both of which are downstream of activated growth factor receptors and necessary for normal stem cell functions including self-renewal, at least in mouse models. The connections between these molecules remain to be defined, and their role in physiologic regulation of stem cell self-renewal is still poorly understood.
CANCER IS SIMILAR TO AN ORGAN WITH SELF-RENEWING CAPACITY
The relationship of stem cells to cancer is an important evolving dimension of adult stem cell biology. Cancer may share principles of organization with normal tissues. Cancer cells are heterogeneous even within a given patient and may have a hierarchical organization of cells with a base of stem-like cells capable of the signature stem cell features: self-renewal and differentiation. These stem-like cells might be the basis for perpetuation of the tumor and represent a slowly dividing, rare population with distinct regulatory mechanisms, including a relationship with a specialized microenvironment. A subpopulation of self-renewing cells has been defined for some, but not all, cancers. A more sophisticated understanding of the stem cell organization of cancers may lead to improved strategies for developing new therapies for the many common and difficult-to-treat types of malignancies that have been relatively refractory to interventions aimed at dividing cells.
Does the concept of cancer stem cells provide insight into the cellular origin of cancer? The fact that some cells within a cancer have stem cell–like properties does not necessarily mean that the cancer arose in the stem cell itself. Rather, more mature cells could have acquired the self-renewal characteristics of stem cells. Any single genetic event is unlikely to be sufficient to enable full transformation of a normal cell to a frankly malignant one. Rather, cancer is a multistep process, and for the multiple steps to accumulate, the cell of origin must be able to persist for prolonged periods. It must also be able to generate large numbers of daughter cells. The normal stem cell has these properties and, by virtue of its having intrinsic self-renewal capability, may be more readily converted to a malignant phenotype. This hypothesis has been tested experimentally in the hematopoietic system. Taking advantage of the cell-surface markers that distinguish hematopoietic cells of varying maturity, stem cells, progenitors, precursors, and mature cells can be isolated. Powerful transforming gene constructs were placed in these cells, and it was found that the cell with the greatest potential to produce a malignancy was dependent on the transforming gene. In some cases, it was the stem cell, but in others, the progenitor cell functioned to initiate and perpetuate the cancer. This shows that cells can acquire stem cell–like properties in malignancy.
WHAT ELSE CAN HEMATOPOIETIC STEM CELLS DO?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree