The first widespread use of serogroup C meningococcal conjugate vaccine (MenC) came in 1999 in the United Kingdom after a rise in serogroup C disease. A mass vaccination campaign involving all individuals <19 years of age was undertaken, and the number of laboratory-confirmed serogroup C cases fell from 955 in 1998–1999 to just 29 in 2011–2012. The effectiveness of the immunization program was attributed both to direct protection of immunized persons and to reduced transmission of the organism in the population as a result of decreased rates of colonization among the immunized (herd immunity). Data on immunogenicity and effectiveness have shown that the duration of protection is short when the vaccine is administered in early childhood; thus booster doses are needed to maintain population immunity. In contrast, immunity after a dose of vaccine given in adolescence appears to be prolonged.
The first quadrivalent conjugate meningococcal vaccine containing A, C, Y, and W polysaccharides conjugated to diphtheria toxoid was initially recommended for all children >11 years of age in the United States in 2005. In 2007 the license was extended to high-risk children 2–10 years of age. In the same year, the vaccine was licensed in Canada for persons 2–55 years of age. Uptake was slow, but current U.S. data suggest an efficacy rate of 82% in the first year after vaccination, with waning to 59% at 3–6 years after vaccination. Limited data from the U.S. Vaccine Adverse Events Reporting System indicated that there might be a short-term increase in the risk of Guillain-Barré syndrome after immunization with the diphtheria conjugate vaccine; however, further investigation has not confirmed this finding. Quadrivalent conjugate vaccines with tetanus or CRM197 as carrier protein are now available in many countries.
A monovalent serogroup A vaccine, manufactured in India, was licensed in 2010 and rolled out to countries in the sub-Saharan African meningitis belt. There is strong evidence that this vaccine has been highly effective in controlling epidemic meningococcal disease in the region, with some evidence of a >90% reduction in disease in vaccinated populations. However, disease caused by serogroup × and W persists.
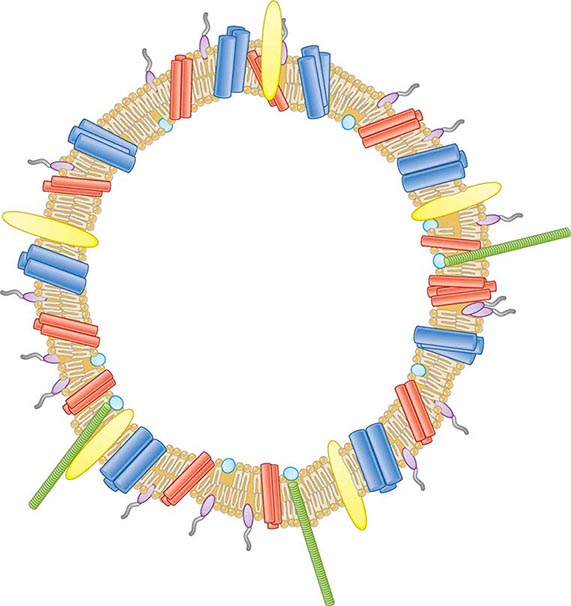
![]() Vaccines Based on Subcapsular Antigens The lack of immunogenicity of the serogroup B capsule has led to the development of vaccines based on subcapsular antigens. Various surface components have been studied in early-phase clinical trials. Outer-membrane vesicles (OMVs) containing outer-membrane proteins, phospholipid, and LPS can be extracted from cultures of N. meningitidis by detergent treatment (Fig. 180-7). OMVs prepared in this way were used in efficacy trials with a Norwegian outbreak strain and reduced the incidence of group B disease among 14- to 16-year-old schoolchildren by 53%. Similarly, OMV vaccines constructed from local outbreak strains in Cuba and New Zealand have had reported efficacy rates of >70%. These OMV vaccines appear to produce strain-specific immune responses, with only limited cross-protection, and are therefore best suited to clonal outbreaks (e.g., those in Cuba and New Zealand as well as others in Norway and the province of Normandy in France).
Vaccines Based on Subcapsular Antigens The lack of immunogenicity of the serogroup B capsule has led to the development of vaccines based on subcapsular antigens. Various surface components have been studied in early-phase clinical trials. Outer-membrane vesicles (OMVs) containing outer-membrane proteins, phospholipid, and LPS can be extracted from cultures of N. meningitidis by detergent treatment (Fig. 180-7). OMVs prepared in this way were used in efficacy trials with a Norwegian outbreak strain and reduced the incidence of group B disease among 14- to 16-year-old schoolchildren by 53%. Similarly, OMV vaccines constructed from local outbreak strains in Cuba and New Zealand have had reported efficacy rates of >70%. These OMV vaccines appear to produce strain-specific immune responses, with only limited cross-protection, and are therefore best suited to clonal outbreaks (e.g., those in Cuba and New Zealand as well as others in Norway and the province of Normandy in France).
FIGURE 180-7 Illustration of meningococcal outer-membrane vesicle containing outer-membrane structures.
Several purified surface proteins have been evaluated in phase 1 clinical trials but have not yet been developed further because of antigenic variability or poor immunogenicity (e.g., transferrin-binding proteins, neisserial surface protein A). Other vaccine candidates have been identified since sequencing of the meningococcal genome. A combination vaccine that includes the New Zealand OMV vaccine and three recombinant proteins (neisserial adhesin A, factor H–binding protein, and neisserial heparin-binding antigen) is immunogenic in infancy and has been licensed for use in Europe and Australia. Recommendations for its use are pending. Finally, a highly immunogenic vaccine based on two variants of the lipoprotein factor H–binding protein is undergoing clinical evaluation
MANAGEMENT OF CONTACTS
Close (household and kissing) contacts of individuals with meningococcal disease are at increased risk (up to 1000 times the rate for the general population) of developing secondary disease; a secondary case follows as many as 3% of sporadic cases. About one-fifth of secondary cases are actually co-primary cases—i.e., cases that occur soon after the primary case and in which transmission is presumed to have originated from the same third party. The rate of secondary cases is highest during the week after presentation of the index case. The risk falls rapidly but remains above baseline for up to 1 year after the index case; 30% of secondary cases occur in the first week, 20% in the second week, and most of the remainder over the next 6 weeks. In outbreaks of meningococcal disease, mass prophylaxis has been used; however, limited data support population intervention, and significant concerns have arisen about adverse events and the development of resistance. For these reasons, prophylaxis is usually restricted to (1) persons at greatest risk who are intimate and/or household contacts of the index case and (2) health care workers who have been directly exposed to respiratory secretions. In most cases, members of wider communities (e.g., at schools or colleges) are not offered prophylaxis.
![]() The aim of prophylaxis is to eradicate colonization of close contacts with the strain that has caused invasive disease in the index case. Prophylaxis should be given to all contacts at the same time to avoid recolonization by meningococci transmitted from untreated contacts and should also be used as soon as possible to treat early disease in secondary cases. If the index patient is treated with an antibiotic that does not reliably clear colonization (e.g., penicillin), he or she should be given a prophylactic agent at the end of treatment to prevent relapse or onward transmission. Although rifampin has been most widely used and studied, it is not the optimal agent because it fails to eradicate carriage in 15–20% of cases, rates of adverse events have been high, compliance is affected by the need for four doses, and emerging resistance has been reported. Ceftriaxone as a single IM or IV injection is highly (97%) effective in carriage eradication and can be used at all ages and in pregnancy. Reduced susceptibility of isolates to ceftriaxone has occasionally been reported. Ciprofloxacin or ofloxacin is preferred in some countries; these agents are highly effective and can be administered by mouth but are not recommended in pregnancy. Resistance to fluoroquinolones has been reported in some meningococci in North America, Europe, and Asia.
The aim of prophylaxis is to eradicate colonization of close contacts with the strain that has caused invasive disease in the index case. Prophylaxis should be given to all contacts at the same time to avoid recolonization by meningococci transmitted from untreated contacts and should also be used as soon as possible to treat early disease in secondary cases. If the index patient is treated with an antibiotic that does not reliably clear colonization (e.g., penicillin), he or she should be given a prophylactic agent at the end of treatment to prevent relapse or onward transmission. Although rifampin has been most widely used and studied, it is not the optimal agent because it fails to eradicate carriage in 15–20% of cases, rates of adverse events have been high, compliance is affected by the need for four doses, and emerging resistance has been reported. Ceftriaxone as a single IM or IV injection is highly (97%) effective in carriage eradication and can be used at all ages and in pregnancy. Reduced susceptibility of isolates to ceftriaxone has occasionally been reported. Ciprofloxacin or ofloxacin is preferred in some countries; these agents are highly effective and can be administered by mouth but are not recommended in pregnancy. Resistance to fluoroquinolones has been reported in some meningococci in North America, Europe, and Asia.
In documented serogroup A, C, Y, or W disease, contacts may be offered immunization (preferably with a conjugate vaccine) in addition to chemoprophylaxis to provide protection beyond the duration of antibiotic therapy. Mass vaccination has been used successfully to control disease during outbreaks in closed communities (educational and military establishments) as well as during epidemics in open communities.
181 | Gonococcal Infections |
DEFINITION
Gonorrhea is a sexually transmitted infection (STI) of epithelium and commonly manifests as cervicitis, urethritis, proctitis, and conjunctivitis. If untreated, infections at these sites can lead to local complications such as endometritis, salpingitis, tuboovarian abscess, bartholinitis, peritonitis, and perihepatitis in female patients; periurethritis and epididymitis in male patients; and ophthalmia neonatorum in newborns. Disseminated gonococcemia is an uncommon event whose manifestations include skin lesions, tenosynovitis, arthritis, and (in rare cases) endocarditis or meningitis.
MICROBIOLOGY
Neisseria gonorrhoeae is a gram-negative, nonmotile, non-spore-forming organism that grows singly and in pairs (i.e., as monococci and diplococci, respectively). Exclusively a human pathogen, the gonococcus contains, on average, three genome copies per coccal unit; this polyploidy permits a high level of antigenic variation and the survival of the organism in its host. Gonococci, like all other Neisseria species, are oxidase positive. They are distinguished from other neisseriae by their ability to grow on selective media and to use glucose but not maltose, sucrose, or lactose.
EPIDEMIOLOGY
![]() The incidence of gonorrhea has declined significantly in the United States, but there were still ~311,000 newly reported cases in 2012. Gonorrhea remains a major public health problem worldwide, is a significant cause of morbidity in developing countries, and may play a role in enhancing transmission of HIV.
The incidence of gonorrhea has declined significantly in the United States, but there were still ~311,000 newly reported cases in 2012. Gonorrhea remains a major public health problem worldwide, is a significant cause of morbidity in developing countries, and may play a role in enhancing transmission of HIV.
Gonorrhea predominantly affects young, nonwhite, unmarried, less educated members of urban populations. The number of reported cases probably represents half of the true number of cases—a discrepancy resulting from underreporting, self-treatment, and nonspecific treatment without a laboratory-proven diagnosis. The number of reported new cases of gonorrhea in the United States rose from ~250,000 in the early 1960s to a high of 1.01 million in 1978. The recorded incidence of gonorrhea in modern times peaked in 1975, with 468 reported new cases per 100,000 population in the United States. This peak was attributable to the interaction of several variables, including improved accuracy of diagnosis, changes in patterns of contraceptive use, and changes in sexual behavior. The incidence of the disease has since declined gradually and is currently estimated at 120 cases per 100,000, a figure that is still the highest among industrialized countries. A further decline in the overall incidence of gonorrhea in the United States over the past quarter-century may reflect increased condom use resulting from public health efforts to curtail HIV transmission. At present, the attack rate in the United States is highest among 15- to 19-year-old women and 20- to 24-year-old men; 60% of all reported cases occur in the preceding two groups together. From the standpoint of ethnicity, rates are highest among African Americans and lowest among persons of Asian or Pacific Island descent.
The incidence of gonorrhea is higher in developing countries than in industrialized nations. The exact incidence of any STI is difficult to ascertain in developing countries because of limited surveillance and variable diagnostic criteria. Studies in Africa have clearly demonstrated that nonulcerative STIs such as gonorrhea (in addition to ulcerative STIs) are an independent risk factor for the transmission of HIV (Chap. 226).
Gonorrhea is transmitted from males to females more efficiently than in the opposite direction. The rate of transmission to a woman during a single unprotected sexual encounter with an infected man is ~50–70%. Oropharyngeal gonorrhea occurs in ~20% of women who practice fellatio with infected partners. Transmission in either direction by cunnilingus is rare.
In any population, there exists a small minority of individuals who have high rates of new-partner acquisition. These “core-group members” or “high-frequency transmitters” are vital in sustaining STI transmission at the population level. Another instrumental factor in sustaining gonorrhea in the population is the large number of infected individuals who are asymptomatic or have minor symptoms that are ignored. These persons, unlike symptomatic individuals, may not cease sexual activity and therefore continue to transmit the infection. This situation underscores the importance of contact tracing and empirical treatment of the sex partners of index cases.
PATHOGENESIS, IMMUNOLOGY, AND ANTIMICROBIAL RESISTANCE
Outer-Membrane Proteins • PILI Fresh clinical isolates of N. gonorrhoeae initially form piliated (fimbriated) colonies distinguishable on translucent agar. Pilus expression is rapidly switched off with unselected subculture because of rearrangements in pilus genes. This change is a basis for antigenic variation of gonococci. Piliated strains adhere better to cells derived from human mucosal surfaces and are more virulent in organ culture models and human inoculation experiments than nonpiliated variants. In a fallopian tube explant model, pili mediate gonococcal attachment to nonciliated columnar epithelial cells. This event initiates gonococcal phagocytosis and transport through these cells to intercellular spaces near the basement membrane or directly into the subepithelial tissue. Pili are also essential for genetic competence and transformation of N. gonorrhoeae, which permit horizontal transfer of genetic material between different gonococcal lineages in vivo.
OPACITY-ASSOCIATED PROTEIN Another gonococcal surface protein that is important in adherence to epithelial cells is opacity-associated protein (Opa, formerly called protein II). Opa contributes to intergonococcal adhesion, which is responsible for the opaque nature of gonococcal colonies on translucent agar and the organism’s adherence to a variety of eukaryotic cells, including polymorphonuclear leukocytes (PMNs). Certain Opa variants promote invasion of epithelial cells, and this effect has been linked with the ability of Opa to bind vitronectin, glycosaminoglycans, and several members of the carcinoembryonic antigen–related cell adhesion molecule (CEACAM) receptor family. N. gonorrhoeae Opa proteins that bind CEACAM1, which is expressed by primary CD4+ T lymphocytes, suppress the activation and proliferation of these lymphocytes. This phenomenon may serve to explain the transient decrease in CD4+ T lymphocyte counts associated with gonococcal infection. Select Opa proteins can engage CEACAM3, which is expressed on neutrophils, with consequent nonopsonic phagocytosis (i.e., phagocytosis independent of antibody and complement) and killing of bacteria.
PORIN Porin (previously designated protein I) is the most abundant gonococcal surface protein, accounting for >50% of the organism’s total outer-membrane protein. Porin molecules exist as trimers that provide anion-transporting aqueous channels through the otherwise hydrophobic outer membrane. Porin exhibits stable interstrain antigenic variation and forms the basis for gonococcal serotyping. Two main serotypes have been identified: PorB.1A strains are often associated with disseminated gonococcal infection (DGI), whereas PorB.1B strains usually cause local genital infections only. DGI strains are generally resistant to the killing action of normal human serum and do not incite a significant local inflammatory response; therefore, they may not cause symptoms at genital sites. These characteristics may be related to the ability of PorB.1A strains to bind to complement-inhibitory molecules, resulting in a diminished inflammatory response. Porin can translocate to the cytoplasmic membrane of host cells—a process that could initiate gonococcal endocytosis and invasion.
OTHER OUTER-MEMBRANE PROTEINS Other notable outer-membrane proteins include H.8, a lipoprotein that is present in high concentration on the surface of all gonococcal strains and is an excellent target for antibody-based diagnostic testing. Transferrin-binding proteins (Tbp1 and Tbp2) and lactoferrin-binding protein are required for scavenging iron from transferrin and lactoferrin in vivo. Transferrin and iron have been shown to enhance the attachment of iron-deprived N. gonorrhoeae to human endometrial cells. IgA1 protease is produced by N. gonorrhoeae and may protect the organism from the action of mucosal IgA.
Lipooligosaccharide Gonococcal lipooligosaccharide (LOS) consists of a lipid A and a core oligosaccharide that lacks the repeating O-carbohydrate antigenic side chain seen in other gram-negative bacteria (Chap. 145e). Gonococcal LOS possesses marked endotoxic activity and contributes to the local cytotoxic effect in a fallopian tube model. LOS core sugars undergo a high degree of phase variation under different conditions of growth; this variation reflects genetic regulation and expression of glycotransferase genes that dictate the carbohydrate structure of LOS. These phenotypic changes may affect interactions of N. gonorrhoeae with elements of the humoral immune system (antibodies and complement) and may also influence direct binding of organisms to both professional phagocytes and nonprofessional phagocytes (epithelial cells). For example, gonococci that are sialylated at their LOS sites bind complement factor H and inhibit the alternative pathway of complement. LOS sialylation may also decrease nonopsonic Opa-mediated association with neutrophils and inhibit the oxidative burst in PMNs. The binding of the unsialylated terminal lactosamine residue of LOS to an asialoglycoprotein receptor on male epithelial cells facilitates adherence and subsequent gonococcal invasion of these cells. Moreover, oligosaccharide structures in LOS can modulate host immune responses. For example, the terminal monosaccharide expressed by LOS determines the C-type lectin receptor on dendritic cells that is targeted by the bacteria. In turn, the specific C-type lectin receptor engaged influences whether a TH1- or TH2-type response is elicited; the latter response may be less favorable for clearance of gonococcal infection.
Host Factors In addition to gonococcal structures that interact with epithelial cells, host factors seem to be important in mediating entry of gonococci into nonphagocytic cells. Activation of phosphatidylcholine-specific phospholipase C and acidic sphingomyelinase by N. gonorrhoeae, which results in the release of diacylglycerol and ceramide, is a requirement for the entry of N. gonorrhoeae into epithelial cells. Ceramide accumulation within cells leads to apoptosis, which may disrupt epithelial integrity and facilitate entry of gonococci into subepithelial tissue. Release of chemotactic factors as a result of complement activation contributes to inflammation, as does the toxic effect of LOS in provoking the release of inflammatory cytokines.
The importance of humoral immunity in host defenses against neisserial infections is best illustrated by the predisposition of persons deficient in terminal complement components (C5 through C9) to recurrent bacteremic gonococcal infections and to recurrent meningococcal meningitis or meningococcemia. Gonococcal porin induces T cell–proliferative responses in persons with urogenital gonococcal disease. A significant increase in porin-specific interleukin (IL) 4–producing CD4+ as well as CD8+ T lymphocytes is seen in individuals with mucosal gonococcal disease. A portion of these lymphocytes that show a porin-specific TH2-type response could traffic to mucosal surfaces and play a role in immune protection against the disease. Few data clearly indicate that protective immunity is acquired from a previous gonococcal infection, although bactericidal and opsonophagocytic antibodies to porin and LOS may offer partial protection. On the other hand, women who are infected and acquire high levels of antibody to another outer-membrane protein, Rmp (reduction modifiable protein, formerly called protein III), may be especially likely to become reinfected with N. gonorrhoeae because Rmp antibodies block the effect of bactericidal antibodies to porin and LOS. Rmp shows little, if any, interstrain antigenic variation; therefore, Rmp antibodies potentially may block antibody-mediated killing of all gonococci. The mechanism of blocking has not been fully characterized, but Rmp antibodies may noncompetitively inhibit binding of porin and LOS antibodies because of the proximity of these structures in the gonococcal outer membrane. In male volunteers who have no history of gonorrhea, the net effect of these events may influence the outcome of experimental challenge with N. gonorrhoeae. Because Rmp bears extensive homology to enterobacterial OmpA and meningococcal class 4 proteins, it is possible that these blocking antibodies result from prior exposure to cross-reacting proteins from these species and also play a role in first-time infection with N. gonorrhoeae.
Gonococcal Resistance to Antimicrobial Agents It is no surprise that N. gonorrhoeae, with its remarkable capacity to alter its antigenic structure and adapt to changes in the microenvironment, has become resistant to numerous antibiotics. The first effective agents against gonorrhea were the sulfonamides, which were introduced in the 1930s and became ineffective within a decade. Penicillin was then used as the drug of choice for the treatment of gonorrhea. By 1965, 42% of gonococcal isolates had developed low-level resistance to penicillin G. Resistance due to the production of penicillinase arose later.
![]() Gonococci become fully resistant to antibiotics either by chromosomal mutations or by acquisition of R factors (plasmids). Two types of chromosomal mutations have been described. The first type, which is drug specific, is a single-step mutation leading to high-level resistance. The second type involves mutations at several chromosomal loci that combine to determine the level as well as the pattern of resistance. Strains with mutations in chromosomal genes were first observed in the late 1950s. As recently as 2007, chromosomal mutations accounted for resistance to penicillin, tetracycline, or both in ~16% of strains surveyed in the United States.
Gonococci become fully resistant to antibiotics either by chromosomal mutations or by acquisition of R factors (plasmids). Two types of chromosomal mutations have been described. The first type, which is drug specific, is a single-step mutation leading to high-level resistance. The second type involves mutations at several chromosomal loci that combine to determine the level as well as the pattern of resistance. Strains with mutations in chromosomal genes were first observed in the late 1950s. As recently as 2007, chromosomal mutations accounted for resistance to penicillin, tetracycline, or both in ~16% of strains surveyed in the United States.
β-Lactamase (penicillinase)–producing strains of N. gonorrhoeae (PPNG) carrying plasmids with the Pcr determinant had rapidly spread worldwide by the early 1980s. N. gonorrhoeae strains with plasmid-borne tetracycline resistance (TRNG) can mobilize some β-lactamase plasmids, and PPNG and TRNG occur together, sometimes along with strains exhibiting chromosomally mediated resistance (CMRNG). Penicillin, ampicillin, and tetracycline are no longer reliable for the treatment of gonorrhea and should not be used.
![]() Quinolone-containing regimens were also recommended for treatment of gonococcal infections; the fluoroquinolones offered the advantage of antichlamydial activity when administered for 7 days. However, quinolone-resistant N. gonorrhoeae (QRNG) appeared soon after these agents were first used to treat gonorrhea. QRNG is particularly common in the Pacific Islands (including Hawaii) and Asia, where, in certain areas, all gonococcal strains are now resistant to quinolones. At present, QRNG is also common in parts of Europe and the Middle East. In the United States, QRNG has been identified in midwestern and eastern areas as well as in states on the Pacific coast, where resistant strains were first seen. Alterations in DNA gyrase and topoisomerase IV have been implicated as mechanisms of fluoroquinolone resistance.
Quinolone-containing regimens were also recommended for treatment of gonococcal infections; the fluoroquinolones offered the advantage of antichlamydial activity when administered for 7 days. However, quinolone-resistant N. gonorrhoeae (QRNG) appeared soon after these agents were first used to treat gonorrhea. QRNG is particularly common in the Pacific Islands (including Hawaii) and Asia, where, in certain areas, all gonococcal strains are now resistant to quinolones. At present, QRNG is also common in parts of Europe and the Middle East. In the United States, QRNG has been identified in midwestern and eastern areas as well as in states on the Pacific coast, where resistant strains were first seen. Alterations in DNA gyrase and topoisomerase IV have been implicated as mechanisms of fluoroquinolone resistance.
Resistance to spectinomycin, which has been used in the past as an alternative agent, has been reported. Because this agent usually is not associated with resistance to other antibiotics, spectinomycin can be reserved for use against multidrug-resistant strains of N. gonorrhoeae. Nevertheless, outbreaks caused by strains resistant to spectinomycin have been documented in Korea and England when the drug has been used for primary treatment of gonorrhea.
Third-generation cephalosporins have remained highly effective as single-dose therapy for gonorrhea, but the recent isolation of strains highly resistant to ceftriaxone (minimal inhibitory concentrations [MICs], 2 μg/mL) in Japan and some European countries is cause for concern. Even though the MICs of ceftriaxone against certain strains may reach 0.015–0.125 μg/mL (higher than the MICs of 0.0001–0.008 μg/mL for fully susceptible strains), these levels are greatly exceeded in the blood, the urethra, and the cervix when the routinely recommended parenteral dose of ceftriaxone is administered. The rising MICs of oral cefixime (the previously recommended alternative oral third-generation cephalosporin) against N. gonorrhoeae, combined with this drug’s limited capacity to reach levels sufficiently higher than MICs in the blood, the urethra, the cervix, and especially the pharynx, have resulted in the removal of cefixime from the list of first-line agents for treatment of uncomplicated gonorrhea. All N. gonorrhoeae strains with reduced susceptibility to ceftriaxone and cefixime (i.e., cephalosporin-intermediate/resistant strains) contain (1) a mosaic penA allele, which is the principal resistance determinant and encodes a penicillin-binding protein (PBP2) whose sequence differs in 60 amino acids from that of wild-type PBP2, and (2) additional genetic resistance determinants that are also required for high-level penicillin resistance.
CLINICAL MANIFESTATIONS
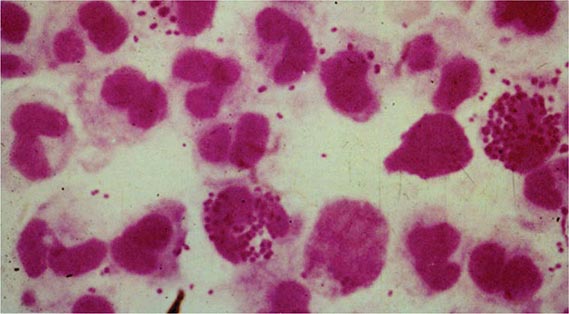
Gonococcal Infections in Men Acute urethritis is the most common clinical manifestation of gonorrhea in male patients. The usual incubation period after exposure is 2–7 days, although the interval can be longer and some men remain asymptomatic. Strains of the PorB.1A serotype tend to cause a greater proportion of cases of mild and asymptomatic urethritis than do PorB.1B strains. Urethral discharge and dysuria, usually without urinary frequency or urgency, are the major symptoms. The discharge initially is scant and mucoid but becomes profuse and purulent within a day or two. Gram’s staining of the urethral discharge may reveal PMNs and gram-negative intracellular monococci and diplococci (Fig. 181-1). The clinical manifestations of gonococcal urethritis are usually more severe and overt than those of nongonococcal urethritis, including urethritis caused by Chlamydia trachomatis (Chap. 213); however, exceptions are common, and it is often impossible to differentiate the causes of urethritis on clinical grounds alone. The majority of cases of urethritis seen in the United States today are not caused by N. gonorrhoeae and/or C. trachomatis. Although a number of other organisms may be responsible, many cases do not have a specific etiologic agent identified.
FIGURE 181-1 Gram’s stain of urethral discharge from a male patient with gonorrhea shows gram-negative intracellular monococci and diplococci. (From the Public Health Agency of Canada.)
Most symptomatic men with gonorrhea seek treatment and cease to be infectious. The remaining men, who are largely asymptomatic, accumulate in number over time and constitute about two-thirds of all infected men at any point in time; together with men incubating the organism (who shed the organism but are asymptomatic), they serve as the source of spread of infection. Before the antibiotic era, symptoms of urethritis persisted for ~8 weeks. Epididymitis is now an uncommon complication, and gonococcal prostatitis occurs rarely, if at all. Other unusual local complications of gonococcal urethritis include edema of the penis due to dorsal lymphangitis or thrombophlebitis, submucous inflammatory “soft” infiltration of the urethral wall, periurethral abscess or fistula, inflammation or abscess of Cowper’s gland, and seminal vesiculitis. Balanitis may develop in uncircumcised men.
Gonococcal Infections in Women • GONOCOCCAL CERVICITIS Mucopurulent cervicitis is a common STI diagnosis in American women and may be caused by N. gonorrhoeae, C. trachomatis, and other organisms, including Mycoplasma genitalium (Chap. 212). Cervicitis may coexist with candidal or trichomonal vaginitis. N. gonorrhoeae primarily infects the columnar epithelium of the cervical os. Bartholin’s glands occasionally become infected.
Women infected with N. gonorrhoeae usually develop symptoms. However, the women who either remain asymptomatic or have only minor symptoms may delay in seeking medical attention. These minor symptoms may include scant vaginal discharge issuing from the inflamed cervix (without vaginitis or vaginosis per se) and dysuria (often without urgency or frequency) that may be associated with gonococcal urethritis. Although the incubation period of gonorrhea is less well defined in women than in men, symptoms usually develop within 10 days of infection and are more acute and intense than those of chlamydial cervicitis.
The physical examination may reveal a mucopurulent discharge (mucopus) issuing from the cervical os. Because Gram’s stain is not sensitive for the diagnosis of gonorrhea in women, specimens should be submitted for culture or a nonculture assay (see “Laboratory Diagnosis,” below). Edematous and friable cervical ectopy and endocervical bleeding induced by gentle swabbing are more often seen in chlamydial infection. Gonococcal infection may extend deep enough to produce dyspareunia and lower abdominal or back pain. In such cases, it is imperative to consider a diagnosis of pelvic inflammatory disease (PID) and to administer treatment for that disease (Chaps. 163 and 213).
N. gonorrhoeae may also be recovered from the urethra and rectum of women with cervicitis, but these are rarely the only infected sites. Urethritis in women may produce symptoms of internal dysuria, which is often attributed to “cystitis.” Pyuria in the absence of bacteriuria seen on Gram’s stain of unspun urine, accompanied by urine cultures that fail to yield >102 colonies of bacteria usually associated with urinary tract infection, signifies the possibility of urethritis due to C. trachomatis. Urethral infection with N. gonorrhoeae may also occur in this context, but in this instance urethral cultures are usually positive.
GONOCOCCAL VAGINITIS The vaginal mucosa of healthy women is lined by stratified squamous epithelium and is rarely infected by N. gonorrhoeae. However, gonococcal vaginitis can occur in anestrogenic women (e.g., prepubertal girls and postmenopausal women), in whom the vaginal stratified squamous epithelium is often thinned down to the basilar layer, which can be infected by N. gonorrhoeae. The intense inflammation of the vagina makes the physical (speculum and bimanual) examination extremely painful. The vaginal mucosa is red and edematous, and an abundant purulent discharge is often present. Infection in the urethra and in Skene’s and Bartholin’s glands often accompanies gonococcal vaginitis. Inflamed cervical erosion or abscesses in nabothian cysts may also occur. Coexisting cervicitis may result in pus in the cervical os.
Anorectal Gonorrhea Because the female anatomy permits the spread of cervical exudate to the rectum, N. gonorrhoeae is sometimes recovered from the rectum of women with uncomplicated gonococcal cervicitis. The rectum is the sole site of infection in only 5% of women with gonorrhea. Such women are usually asymptomatic but occasionally have acute proctitis manifested by anorectal pain or pruritus, tenesmus, purulent rectal discharge, and rectal bleeding. Among men who have sex with men (MSM), the frequency of gonococcal infection, including rectal infection, fell by ≥90% throughout the United States in the early 1980s, but a resurgence of gonorrhea among MSM has been documented in several cities since the 1990s. Gonococcal isolates from the rectum of MSM tend to be more resistant to antimicrobial agents than are gonococcal isolates from other sites. Gonococcal isolates with a mutation in mtrR (multiple transferable resistance repressor) or in the promoter region of the gene that encodes for this transcriptional repressor develop increased resistance to antimicrobial hydrophobic agents such as bile acids and fatty acids in feces and thus are found with increased frequency in MSM. This situation may have been responsible for higher rates of failure of treatment for rectal gonorrhea with older regimens consisting of penicillin or tetracyclines.
Pharyngeal Gonorrhea Pharyngeal gonorrhea is usually mild or asymptomatic, although symptomatic pharyngitis does occasionally occur with cervical lymphadenitis. The mode of acquisition is oral-genital sexual exposure, with fellatio being a more efficient means of transmission than cunnilingus. In certain female adolescent populations in the United States, pharyngeal gonorrhea has become as common as genital gonorrhea. Most cases resolve spontaneously, and transmission from the pharynx to sexual contacts is rare. Pharyngeal infection almost always coexists with genital infection. Swabs from the pharynx should be plated directly onto gonococcal selective media. Pharyngeal colonization with Neisseria meningitidis needs to be differentiated from that with other Neisseria species.
Ocular Gonorrhea in Adults Ocular gonorrhea in an adult usually results from autoinoculation of N. gonorrhoeae from an infected genital site. As in genital infection, the manifestations range from severe to occasionally mild or asymptomatic disease. The variability in clinical manifestations may be attributable to differences in the ability of the infecting strain to elicit an inflammatory response. Infection may result in a markedly swollen eyelid, severe hyperemia and chemosis, and a profuse purulent discharge. The massively inflamed conjunctiva may be draped over the cornea and limbus. Lytic enzymes from the infiltrating PMNs occasionally cause corneal ulceration and rarely cause perforation.
Prompt recognition and treatment of this condition are of paramount importance. Gram’s stain and culture of the purulent discharge establish the diagnosis. Genital cultures should also be performed.
Gonorrhea in Pregnant Women, Neonates, and Children Gonorrhea in pregnancy can have serious consequences for both the mother and the infant. Recognition of gonorrhea early in pregnancy also identifies a population at risk for other STIs, particularly chlamydial infection, syphilis, and trichomoniasis. The risks of salpingitis and PID—conditions associated with a high rate of fetal loss—are highest during the first trimester. Pharyngeal infection, most often asymptomatic, may be more common during pregnancy because of altered sexual practices. Prolonged rupture of the membranes, premature delivery, chorioamnionitis, funisitis (infection of the umbilical cord stump), and sepsis in the infant (with N. gonorrhoeae detected in the newborn’s gastric aspirate during delivery) are common complications of maternal gonococcal infection at term. Other conditions and microorganisms, including Mycoplasma hominis, Ureaplasma urealyticum, C. trachomatis, and bacterial vaginosis (often accompanied by infection with Trichomonas vaginalis), have been associated with similar complications.
The most common form of gonorrhea in neonates is ophthalmia neonatorum, which results from exposure to infected cervical secretions during parturition. Ocular neonatal instillation of a prophylactic agent (e.g., 1% silver nitrate eye drops or ophthalmic preparations containing erythromycin or tetracycline) prevents ophthalmia neonatorum but is not effective for its treatment, which requires systemic antibiotics. The clinical manifestations are acute and usually begin 2–5 days after birth. An initial nonspecific conjunctivitis with a serosanguineous discharge is followed by tense edema of the eyelids, chemosis, and a profuse, thick, purulent discharge. Corneal ulcerations that result in nebulae or perforation may lead to anterior synechiae, anterior staphyloma, panophthalmitis, and blindness. Infections described at other mucosal sites in infants, including vaginitis, rhinitis, and anorectal infection, are likely to be asymptomatic. Pharyngeal colonization has been demonstrated in 35% of infants with gonococcal ophthalmia, and coughing is the most prominent symptom in these cases. Septic arthritis (see below) is the most common manifestation of systemic infection or DGI in the newborn. The onset usually comes at 3–21 days of age, and polyarticular involvement is common. Sepsis, meningitis, and pneumonia are seen in rare instances.
Any STI in children beyond the neonatal period raises the possibility of sexual abuse. Gonococcal vulvovaginitis is the most common manifestation of gonococcal infection in children beyond infancy. Anorectal and pharyngeal infections are common in these children and are frequently asymptomatic. The urethra, Bartholin’s and Skene’s glands, and the upper genital tract are rarely involved. All children with gonococcal infection should also be evaluated for chlamydial infection, syphilis, and possibly HIV infection.
Gonococcal Arthritis (DGI) DGI (gonococcal arthritis) results from gonococcal bacteremia. In the 1970s, DGI occurred in ~0.5–3% of persons with untreated gonococcal mucosal infection. The lower incidence of DGI at present is probably attributable to a decline in the prevalence of particular strains that are likely to disseminate. DGI strains resist the bactericidal action of human serum and generally do not incite inflammation at genital sites, probably because of limited generation of chemotactic factors. Strains recovered from DGI cases in the 1970s were often of the PorB.1A serotype, were highly susceptible to penicillin, and had special growth requirements—including arginine, hypoxanthine, and uracil—that made the organism more fastidious and more difficult to isolate.
Menstruation is a risk factor for dissemination, and approximately two-thirds of cases of DGI are in women. In about half of affected women, symptoms of DGI begin within 7 days of onset of menses. Complement deficiencies, especially of the components involved in the assembly of the membrane attack complex (C5 through C9), predispose to neisserial bacteremia, and persons with more than one episode of DGI should be screened with an assay for total hemolytic complement activity.
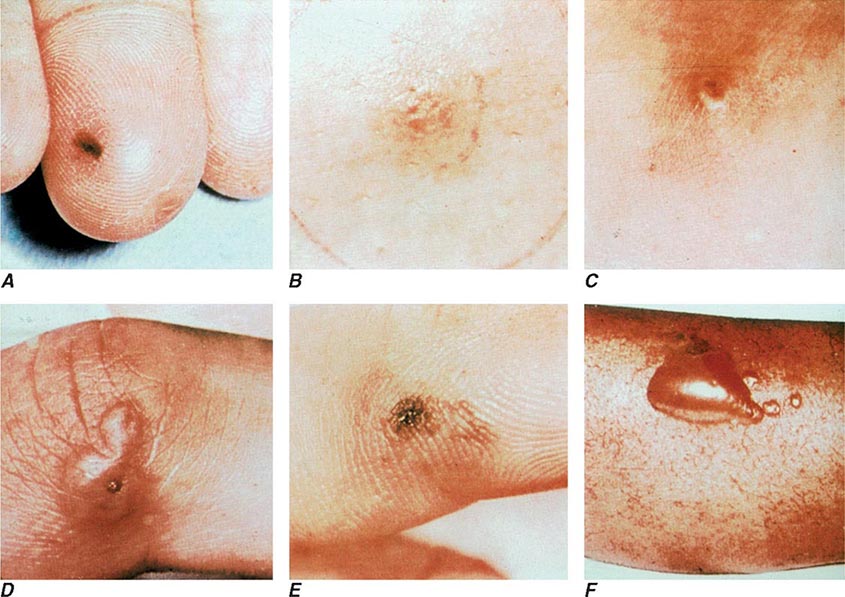
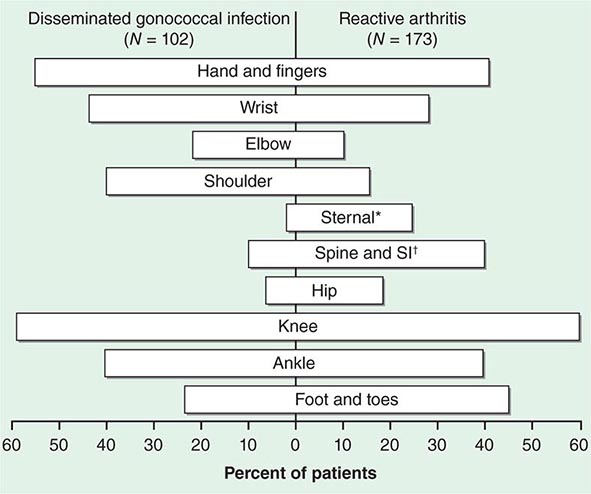
The clinical manifestations of DGI have sometimes been classified into two stages: a bacteremic stage, which is less common today, and a joint-localized stage with suppurative arthritis. A clear-cut progression usually is not evident. Patients in the bacteremic stage have higher temperatures, and chills more frequently accompany their fever. Painful joints are common and often occur together with tenosynovitis and skin lesions. Polyarthralgias usually include the knees, elbows, and more distal joints; the axial skeleton is generally spared. Skin lesions are seen in ~75% of patients and include papules and pustules, often with a hemorrhagic component (Fig. 181-2; see also Fig. 25e-44). Other manifestations of noninfectious dermatitis, such as nodular lesions, urticaria, and erythema multiforme, have been described. These lesions are usually on the extremities and number between 5 and 40. The differential diagnosis of the bacteremic stage of DGI includes reactive arthritis, acute rheumatoid arthritis, sarcoidosis, erythema nodosum, drug-induced arthritis, and viral infections (e.g., hepatitis B and acute HIV infection). The distribution of joint symptoms in reactive arthritis differs from that in DGI (Fig. 181-3), as do the skin and genital manifestations (Chap. 384).
FIGURE 181-2 Characteristic skin lesions in patients with proven gonococcal bacteremia. The lesions are in various stages of evolution. A. Very early petechia on finger. B. Early papular lesion, 7 mm in diameter, on lower leg. C. Pustule with central eschar resulting from early petechial lesion. D. Pustular lesion on finger. E. Mature lesion with central necrosis (black) on hemorrhagic base. F. Bullae on anterior tibial surface. (Reprinted with permission from KK Holmes et al: Disseminated gonococcal infection. Ann Intern Med 74:979, 1971.)
FIGURE 181-3 Distribution of joints with arthritis in 102 patients with disseminated gonococcal infection and 173 patients with reactive arthritis. *Includes the sternoclavicular joints. †SI, sacroiliac joint.
Suppurative arthritis involves one or two joints, most often the knees, wrists, ankles, and elbows (in decreasing order of frequency); other joints occasionally are involved. Most patients who develop gonococcal septic arthritis do so without prior polyarthralgias or skin lesions; in the absence of symptomatic genital infection, this disease cannot be distinguished from septic arthritis caused by other pathogens. The differential diagnosis of acute arthritis in young adults is discussed in Chap. 157. Rarely, osteomyelitis complicates septic arthritis involving small joints of the hand.
Gonococcal endocarditis, although rare today, was a relatively common complication of DGI in the preantibiotic era, accounting for about one-quarter of reported cases of endocarditis. Another unusual complication of DGI is meningitis.
Gonococcal Infections in HIV-Infected Persons The association between gonorrhea and the acquisition of HIV has been demonstrated in several well-controlled studies, mainly in Kenya and Zaire. The nonulcerative STIs enhance the transmission of HIV by three- to fivefold; transmission of HIV-infected immune cells and increased viral shedding by persons with urethritis or cervicitis may contribute (Chap. 226). HIV has been detected by polymerase chain reaction (PCR) more commonly in ejaculates from HIV-positive men with gonococcal urethritis than in those from HIV-positive men with nongonococcal urethritis. PCR positivity diminishes by twofold after appropriate therapy for urethritis. Not only does gonorrhea enhance the transmission of HIV, but it may also increase the individual’s risk for acquisition of HIV. A proposed mechanism is the significantly greater number of CD4+ T lymphocytes and dendritic cells that can be infected by HIV in endocervical secretions from women with nonulcerative STIs than in those from women with ulcerative STIs.
LABORATORY DIAGNOSIS
A rapid diagnosis of gonococcal infection in men may be obtained by Gram’s staining of urethral exudates (Fig. 181-1). The detection of gram-negative intracellular monococci and diplococci is usually highly specific and sensitive in diagnosing gonococcal urethritis in symptomatic males but is only ~50% sensitive in diagnosing gonococcal cervicitis. Samples should be collected with Dacron or rayon swabs. Part of the sample should be inoculated onto a plate of modified Thayer-Martin or other gonococcal selective medium for culture. It is important to process all samples immediately because gonococci do not tolerate drying. If plates cannot be incubated immediately, they can be held safely for several hours at room temperature in candle extinction jars prior to incubation. If processing is to occur within 6 h, transport of specimens may be facilitated by the use of nonnutritive swab transport systems such as Stuart or Amies medium. For longer holding periods (e.g., when specimens for culture are to be mailed), culture media with self-contained CO2-generating systems (such as the JEMBEC or Gono-Pak systems) may be used. Specimens should also be obtained for the diagnosis of chlamydial infection (Chap. 213).
PMNs are often seen in the endocervix on a Gram’s stain, and an abnormally increased number (≥30 PMNs per field in five 1000× oil-immersion microscopic fields) establishes the presence of an inflammatory discharge. Unfortunately, the presence or absence of gram-negative intracellular monococci or diplococci in cervical smears does not accurately predict which patients have gonorrhea, and the diagnosis in this setting should be made by culture or another suitable nonculture diagnostic method. The sensitivity of a single endocervical culture is ~80–90%. If a history of rectal sex is elicited, a rectal wall swab (uncontaminated with feces) should be cultured. A presumptive diagnosis of gonorrhea cannot be made on the basis of gram-negative diplococci in smears from the pharynx, where other Neisseria species are components of the normal flora.
Increasingly, nucleic acid probe tests are being substituted for culture for the direct detection of N. gonorrhoeae in urogenital specimens. A common assay uses a nonisotopic chemiluminescent DNA probe that hybridizes specifically with gonococcal 16S ribosomal RNA; this assay is as sensitive as conventional culture techniques. A disadvantage of non-culture-based assays is that N. gonorrhoeae cannot be grown from the transport systems. Thus a culture-confirmatory test and formal antimicrobial susceptibility testing, if needed, cannot be performed. Nucleic acid amplification tests (NAATs), including the Roche Cobas® Amplicor, Gen-Probe APTIMA COMBO 2®, and BD ProbeTec™ ET, also detect C. trachomatis and are more sensitive than culture identification of either N. gonorrhoeae or C. trachomatis. The Gen-Probe and BD tests offer the advantage that urine samples can be tested with a sensitivity similar to or greater than that obtained when urethral or cervical swab samples are assessed by other non-NAATs or culture, respectively. Several amplification tests are now available on semiautomated or fully automated platforms.
Because of the legal implications, the preferred method for the diagnosis of gonococcal infection in children is a standardized culture. Two positive NAATs, each targeting a different nucleic acid sequence, may be substituted for culture of the cervix or the urethra as legal evidence of infection in children. Although nonculture tests for gonococcal infection have not been approved by the U.S. Food and Drug Administration for use with specimens obtained from the pharynx and rectum of infected children, NAATs from these sites are preferred for diagnostic evaluation in adult victims of suspected sexual abuse, especially if the NAATs have been evaluated by the local laboratory and found to be superior. Cultures should be obtained from the pharynx and anus of both girls and boys, the urethra of boys, and the vagina of girls; cervical specimens are not recommended for prepubertal girls. For boys with a urethral discharge, a meatal specimen of the discharge is adequate for culture. Presumptive colonies of N. gonorrhoeae should be identified definitively by at least two independent methods.
Blood should be cultured in suspected cases of DGI. The use of Isolator blood culture tubes may enhance the yield. The probability of positive blood cultures decreases after 48 h of illness. Synovial fluid should be inoculated into blood culture broth medium and plated onto chocolate agar rather than selective medium because this fluid is not likely to be contaminated with commensal bacteria. Gonococci are infrequently recovered from early joint effusions containing <20,000 leukocytes/μL but may be recovered from effusions containing >80,000 leukocytes/μL. The organisms are seldom recovered from blood and synovial fluid of the same patient.
TREATMENT | GONOCOCCAL INFECTIONS |
Treatment failure can lead to continued transmission and the emergence of antibiotic resistance. The importance of adequate treatment with a regimen that the patient will adhere to cannot be overemphasized. Thus highly effective single-dose regimens have been developed for uncomplicated gonococcal infections. The modified 2010 treatment guidelines for gonococcal infections from the Centers for Disease Control and Prevention (CDC) are summarized in Table 181-1. Rising MICs of cefixime worldwide have led the CDC to discontinue its recommendation of this agent as first-line treatment for uncomplicated gonorrhea. The recommendations for uncomplicated gonorrhea apply to HIV-infected as well as HIV-uninfected patients.
RECOMMENDED TREATMENT FOR GONOCOCCAL INFECTIONS: ADAPTED FROM THE 2010 GUIDELINES OF THE CENTERS FOR DISEASE CONTROL AND PREVENTION |
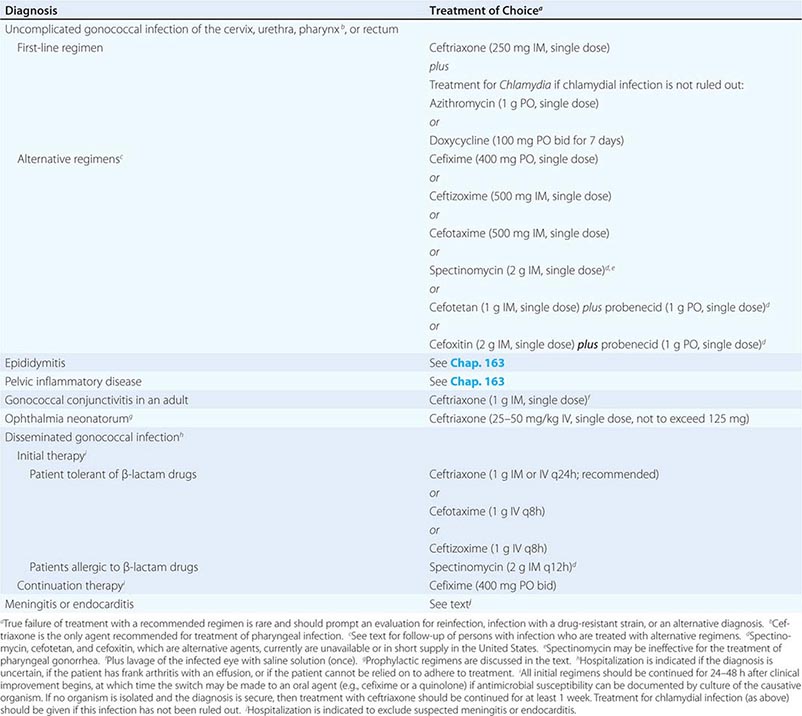
Currently, a single IM dose of the third-generation cephalosporin ceftriaxone is the mainstay of therapy for uncomplicated gonococcal infection of the urethra, cervix, rectum, or pharynx and almost always results in an effective cure. Quinolone-containing regimens are no longer recommended in the United States as first-line treatment because of widespread resistance. A recent multicenter trial of treatment for uncomplicated gonorrhea in the United States showed ≥99.5% efficacy of two combination regimens: (1) gemifloxacin (320 mg, single oral dose) plus azithromycin (2 g, single oral dose) or (2) azithromycin (2 g, single oral dose) plus gentamicin (a single IM dose of 240 mg or, in individuals who weigh ≤45 kg, 5 mg/kg).
Because co-infection with C. trachomatis occurs frequently, initial treatment regimens must also incorporate an agent (e.g., azithromycin or doxycycline) that is effective against chlamydial infection. Pregnant women with gonorrhea, who should not take doxycycline, should receive concurrent treatment with a macrolide antibiotic for possible chlamydial infection. A single 1-g dose of azithromycin, which is effective therapy for uncomplicated chlamydial infections, results in an unacceptably low cure rate (93%) for gonococcal infections and should not be used alone. A single 2-g dose of azithromycin, particularly in the extended-release microsphere formulation, delivers azithromycin to the lower gastrointestinal tract, thereby improving tolerability. Azithromycin is effective against sensitive strains, but this drug is expensive, causes gastrointestinal distress, and is not recommended for routine or first-line treatment of gonorrhea. Spectinomycin has been used as an alternative agent for the treatment of uncomplicated gonococcal infections in penicillin-allergic persons outside the United States but is not currently available in this country. Of note, the limited effectiveness of spectinomycin for the treatment of pharyngeal infection reduces its utility in populations among whom such infection is common, such as MSM.
Persons with uncomplicated infections who receive ceftriaxone do not need a test of cure; however, cultures for N. gonorrhoeae should be performed if symptoms persist after therapy with an established regimen, and any gonococci isolated should be tested for antimicrobial susceptibility. Persons given an alternative regimen should return for a test of cure targeting the infected anatomic site. This test ideally should be a culture. If culture is not readily available and a NAAT is positive, every effort should be made to perform a confirmatory culture. All positive cultures for test of cure should undergo antimicrobial susceptibility testing. Because of high rates of reinfection with N. gonorrhoeae and C. trachomatis within 6 months, repeat testing is recommended 3 months after treatment.
Symptomatic gonococcal pharyngitis is more difficult to eradicate than genital infection. Persons who cannot tolerate ceftriaxone and those in whom quinolones are contraindicated may be treated with spectinomycin if it is available, but this agent results in a cure rate of ≤52%. Persons given spectinomycin should have a pharyngeal sample cultured 3–5 days after treatment as a test of cure. A single 2-g dose of azithromycin may be used in areas where rates of resistance to azithromycin are low.
Treatments for gonococcal epididymitis and PID are discussed in Chap. 163. Ocular gonococcal infections in older children and adults should be managed with a single dose of ceftriaxone combined with saline irrigation of the conjunctivae (both undertaken expeditiously), and patients should undergo a careful ophthalmologic evaluation that includes a slit-lamp examination.
DGI may require higher dosages and longer durations of therapy (Table 181-1). Hospitalization is indicated if the diagnosis is uncertain, if the patient has localized joint disease that requires aspiration, or if the patient cannot be relied on to comply with treatment. Open drainage is necessary only occasionally—e.g., for management of hip infections that may be difficult to drain percutaneously. Nonsteroidal anti-inflammatory agents may be indicated to alleviate pain and hasten clinical improvement of affected joints.
Gonococcal meningitis and endocarditis should be treated in the hospital with high-dose IV ceftriaxone (1–2 g every 12 h); therapy should continue for 10–14 days for meningitis and for at least 4 weeks for endocarditis. All persons who experience more than one episode of DGI should be evaluated for complement deficiency.
PREVENTION AND CONTROL
Condoms, if properly used, provide effective protection against the transmission and acquisition of gonorrhea as well as other infections that are transmitted to and from genital mucosal surfaces. Spermicidal preparations used with a diaphragm or cervical sponges impregnated with nonoxynol 9 offer some protection against gonorrhea and chlamydial infection. However, the frequent use of preparations that contain nonoxynol 9 is associated with mucosal disruption that paradoxically may enhance the risk of HIV infection in the event of exposure. All patients should be instructed to refer sex partners for evaluation and treatment. All sex partners of persons with gonorrhea should be evaluated and treated for N. gonorrhoeae and C. trachomatis infections if their last contact with the patient took place within 60 days before the onset of symptoms or the diagnosis of infection in the patient. If the patient’s last sexual encounter was >60 days before onset of symptoms or diagnosis, the patient’s most recent sex partner should be treated. Partner-delivered medications or prescriptions for medications to treat gonorrhea and chlamydial infection diminish the likelihood of reinfection (or relapse) in the infected patient. In states where it is legal, this approach is an option for partner management. Patients should be instructed to abstain from sexual intercourse until therapy is completed and until they and their sex partners no longer have symptoms. Greater emphasis must be placed on prevention by public health education, individual patient counseling, and behavior modification. Sexually active persons, especially adolescents, should be offered screening for STIs. For male patients, a NAAT on urine or a urethral swab may be used for screening. Preventing the spread of gonorrhea may help reduce the transmission of HIV. No effective vaccine for gonorrhea is yet available, but efforts to test several candidates are under way.
ACKNOWLEDGMENT
The authors acknowledge the contributions of Dr. King K. Holmes and Dr. Stephen A. Morse to the chapter on this subject in earlier editions.
182 | Haemophilus and Moraxella Infections |
HAEMOPHILUS INFLUENZAE
MICROBIOLOGY
Haemophilus influenzae was first recognized in 1892 by Pfeiffer, who erroneously concluded that the bacterium was the cause of influenza. H. influenzae is a small (1- × 0.3-μm) gram-negative organism of variable shape; thus, it is often described as a pleomorphic coccobacillus. In clinical specimens such as cerebrospinal fluid (CSF) and sputum, H. influenzae frequently stains only faintly with safranin and therefore can easily be overlooked.
H. influenzae grows both aerobically and anaerobically. Its aerobic growth requires two factors: hemin (X factor) and nicotinamide adenine dinucleotide (V factor). These requirements are used in the clinical laboratory to identify the bacterium. Caution must be used to distinguish H. influenzae from H. haemolyticus, a respiratory tract commensal that has identical growth requirements. H. haemolyticus has classically been distinguished from H. influenzae by the hemolysis of the former species on horse blood agar. However, a significant proportion of isolates of H. haemolyticus have now been recognized as nonhemolytic. Analysis of various genotypic and phenotypic markers, including16S ribosomal sequences, superoxide dismutase, outer-membrane protein P6, protein D, and fuculose kinase, can be used to distinguish these two species.
Six major serotypes of H. influenzae have been identified; designated a through f, they are based on antigenically distinct polysaccharide capsules. In addition, some strains lack a polysaccharide capsule and are referred to as nontypable strains. Type b and nontypable strains are the most relevant strains clinically (Table 182-1), although encapsulated strains other than type b can cause disease. H. influenzae was the first free-living organism to have its entire genome sequenced.
CHARACTERISTICS OF TYPE b AND NONTYPABLE STRAINS OF HAEMOPHILUS INFLUENZAE |
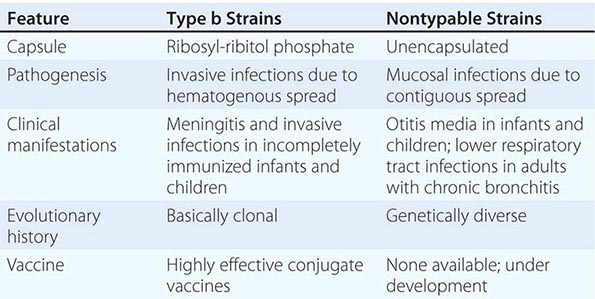
The antigenically distinct type b capsule is a linear polymer composed of ribosyl-ribitol phosphate. Strains of H. influenzae type b (Hib) cause disease primarily in infants and children <6 years of age. Nontypable strains are primarily mucosal pathogens but occasionally cause invasive disease.
EPIDEMIOLOGY AND TRANSMISSION
H. influenzae, an exclusively human pathogen, is spread by airborne droplets or by direct contact with secretions or fomites. Colonization with nontypable H. influenzae is a dynamic process; new strains are acquired and other strains are replaced periodically.
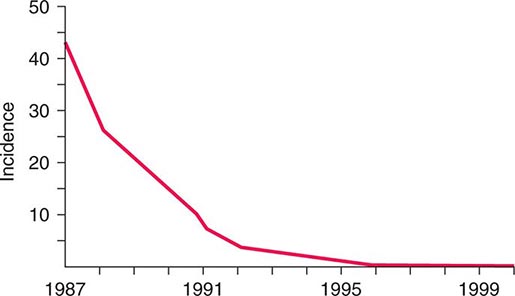
![]() The widespread use of Hib conjugate vaccines in many industrialized countries has resulted in striking decreases in the rate of nasopharyngeal colonization by Hib and in the incidence of Hib infection (Fig. 182-1). However, the majority of the world’s children remain unimmunized. Worldwide, invasive Hib disease occurs predominantly in unimmunized children and in those who have not completed the primary immunization series. Certain groups have a higher incidence of invasive Hib disease than the general population, including African-American children and Native American groups. Although this increased incidence has not yet been accounted for, several factors may be relevant, including age at exposure to the bacterium, socioeconomic conditions, and genetic differences.
The widespread use of Hib conjugate vaccines in many industrialized countries has resulted in striking decreases in the rate of nasopharyngeal colonization by Hib and in the incidence of Hib infection (Fig. 182-1). However, the majority of the world’s children remain unimmunized. Worldwide, invasive Hib disease occurs predominantly in unimmunized children and in those who have not completed the primary immunization series. Certain groups have a higher incidence of invasive Hib disease than the general population, including African-American children and Native American groups. Although this increased incidence has not yet been accounted for, several factors may be relevant, including age at exposure to the bacterium, socioeconomic conditions, and genetic differences.
FIGURE 182-1 Estimated incidence (rate per 100,000) of invasive disease due to Haemophilus influenzae type b among children <5 years of age: 1987–2000. Fewer than 40 cases per year have been reported since 2000. (Data from the Centers for Disease Control and Prevention.)
PATHOGENESIS
Hib strains cause systemic disease by invasion and hematogenous spread from the respiratory tract to distant sites such as the meninges, bones, and joints. The type b polysaccharide capsule is an important virulence factor affecting the bacterium’s ability to avoid opsonization and cause systemic disease.
Nontypable strains cause disease by local invasion of mucosal surfaces. Otitis media results when bacteria reach the middle ear by way of the eustachian tube. Adults with chronic bronchitis experience recurrent lower respiratory tract infection due to nontypable strains. In addition, persistent nontypable H. influenzae colonization of the lower airways of adults with chronic obstructive pulmonary disease (COPD) contributes to the airway inflammation that is a hallmark of the disease. Nontypable strains that cause infection in adults with COPD differ in pathogenic potential and genome content from strains that cause otitis media. In the middle ear, nontypable strains form biofilms. More resistant to host clearance mechanisms and to antibiotics than are planktonic bacteria, biofilms are associated with chronic and recurrent otitis media. The incidence of invasive disease caused by nontypable strains is low. Strains that cause invasive disease are genetically and phenotypically diverse.
IMMUNE RESPONSE
Antibody to the capsule is important in protection from infection by Hib strains. The level of (maternally acquired) serum antibody to the capsular polysaccharide, which is a polymer of polyribitol ribose phosphate (PRP), declines from birth to 6 months of age and, in the absence of vaccination, remains low until ~2 or 3 years of age. The age at the antibody nadir correlates with that of the peak incidence of type b disease. Antibody to PRP then appears partly as a result of exposure to Hib or cross-reacting antigens. Systemic Hib disease is unusual after the age of 6 years because of the presence of protective antibody. Vaccines in which PRP is conjugated to protein carrier molecules have been developed and are now used widely. These vaccines generate an antibody response to PRP in infants and effectively prevent invasive infections in infants and children.
Since nontypable strains lack a capsule, the immune response to infection is directed at noncapsular antigens. These antigens have generated considerable interest as immune targets and potential vaccine components. The human immune response to nontypable strains appears to be strain-specific, a characteristic that accounts in part for the propensity of these strains to cause recurrent otitis media and recurrent exacerbations of chronic bronchitis in immunocompetent hosts.
CLINICAL MANIFESTATIONS
Hib The most serious manifestation of infection with Hib is meningitis (Chap. 164), which primarily affects children <2 years of age. The clinical manifestations of Hib meningitis are similar to those of meningitis caused by other bacterial pathogens. Fever and altered central nervous system function are the most common features at presentation. Nuchal rigidity may or may not be evident. Subdural effusion, the most common complication, is suspected when, despite 2 or 3 days of appropriate antibiotic therapy, the infant has seizures, hemiparesis, or continued obtundation. The overall mortality rate from Hib meningitis is ~5%, and the morbidity rate is high. Of survivors, 6% have permanent sensorineural hearing loss, and about one-fourth have a significant handicap of some type. If more subtle handicaps are sought, up to half of survivors are found to have some neurologic sequelae, such as partial hearing loss and delayed language development.
Epiglottitis (Chap. 44) is a life-threatening Hib infection involving cellulitis of the epiglottis and supraglottic tissues. It can lead to acute upper airway obstruction. Its unique epidemiologic features are its occurrence in an older age group (2–7 years old) than other Hib infections and its absence among Navajo Indians and Alaskan Eskimos. Sore throat and fever rapidly progress to dysphagia, drooling, and airway obstruction. Epiglottitis also occurs in adults.
Cellulitis (Chap. 156) due to Hib occurs in young children. The most common location is on the head or neck, and the involved area sometimes takes on a characteristic bluish-red color. Most patients have bacteremia, and 10% have an additional focus of infection.
Hib causes pneumonia in infants. The infection is clinically indistinguishable from other types of bacterial pneumonia (e.g., pneumococcal pneumonia) except that Hib is more likely to involve the pleura. Several less common invasive conditions can be important clinical manifestations of Hib infection in children. These include osteomyelitis, septic arthritis, pericarditis, orbital cellulitis, endophthalmitis, urinary tract infection, abscesses, and bacteremia without an identifiable focus.
Non–type b encapsulated strains of H. influenzae (types a, c, d, e, and f) are unusual causes of invasive infection manifested predominantly by bacteremia and pneumonia. Most such infections occur in the setting of underlying conditions.
Nontypable H. influenzae Nontypable H. influenzae is the most common bacterial cause of exacerbations of COPD; these exacerbations are characterized by increased cough, sputum production, and shortness of breath. Fever is low-grade, and no infiltrates are evident on chest x-ray. Nontypable strains also cause community-acquired bacterial pneumonia in adults, especially among patients with COPD or AIDS. The clinical features of H. influenzae pneumonia are similar to those of other types of bacterial pneumonia, including pneumococcal pneumonia.
Nontypable H. influenzae is one of the three most common causes of childhood otitis media (the other two being Streptococcus pneumoniae and Moraxella catarrhalis) (Chap. 44). Infants are febrile and irritable, while older children report ear pain. Symptoms of viral upper respiratory infection often precede otitis media. The diagnosis is made by pneumatic otoscopy. An etiologic diagnosis, although not routinely sought, can be established by tympanocentesis and culture of middle-ear fluid. Clinical features associated with H. influenzae otitis media include a history of recurrent episodes, treatment failure, concomitant conjunctivitis, bilateral otitis media, and recent antimicrobial therapy. The increasing use of pneumococcal polysaccharide conjugate vaccines in infants is resulting in a relative increase in the proportion of otitis media cases that are caused by H. influenzae.
Nontypable H. influenzae also causes puerperal sepsis and is an important cause of neonatal bacteremia. These nontypable strains, which are closely related to H. haemolyticus, tend to be of biotype IV and cause invasive disease after colonizing the female genital tract.
Nontypable H. influenzae causes sinusitis (Chap. 44) in adults and children. In addition, the bacterium is a less common cause of various invasive infections. These infections include empyema, adult epiglottitis, pericarditis, cellulitis, septic arthritis, osteomyelitis, endocarditis, cholecystitis, intraabdominal infections, urinary tract infections, mastoiditis, aortic graft infection, and bacteremia without a detectable focus. While most H. influenzae invasive infections in countries where Hib vaccines are used widely are caused by nontypable strains, there is no convincing evidence of an increased incidence of infection by nontypable H. influenzae as a result of use of Hib vaccines. Continued monitoring will be important. Many patients with H. influenzae bacteremia have an underlying condition, such as HIV infection, cardiopulmonary disease, alcoholism, or cancer.
DIAGNOSIS
The most reliable method for establishing a diagnosis of Hib infection is recovery of the organism in culture. The presence of gram-negative coccobacilli in Gram-stained CSF is strong evidence for Hib meningitis. Recovery of the organism from CSF confirms the diagnosis. Cultures of other normally sterile body fluids, such as blood, joint fluid, pleural fluid, pericardial fluid, and subdural effusion, are confirmatory in other infections.
Detection of PRP is an important adjunct to culture in rapid diagnosis of Hib meningitis. Immunoelectrophoresis, latex agglutination, coagglutination, and enzyme-linked immunosorbent assay are effective in detecting PRP. These assays are particularly helpful when patients have received prior antimicrobial therapy and thus are especially likely to have negative cultures.
Because nontypable H. influenzae is primarily a mucosal pathogen, it is a component of a mixed flora; thus etiologic diagnosis is challenging. Nontypable H. influenzae infection is strongly suggested by the predominance of gram-negative coccobacilli among abundant polymorphonuclear leukocytes in a Gram-stained sputum specimen from a patient in whom pneumonia is suspected. Although bacteremia is detectable in a small proportion of patients with pneumonia due to nontypable H. influenzae, most such patients have negative blood cultures.
A diagnosis of otitis media is based on the detection by pneumatic otoscopy of fluid in the middle ear. An etiologic diagnosis requires tympanocentesis but is not routinely sought. An invasive procedure is also required to determine the etiology of sinusitis; thus, treatment is often empirical once the diagnosis is suspected in light of clinical symptoms and sinus radiographs.
TREATMENT | HAEMOPHILUS INFLUENZAE |
Initial therapy for meningitis due to Hib should consist of a cephalosporin such as ceftriaxone or cefotaxime. For children, the dosage of ceftriaxone is 75–100 mg/kg daily given in two doses 12 h apart. The pediatric dosage of cefotaxime is 200 mg/kg daily given in four doses 6 h apart. Adult dosages are 2 g every 12 h for ceftriaxone and 2 g every 4–6 h for cefotaxime. An alternative regimen for initial therapy is ampicillin (200–300 mg/kg daily in four divided doses) plus chloramphenicol (75–100 mg/kg daily in four divided doses). Therapy should continue for a total of 1–2 weeks.
Administration of glucocorticoids to patients with Hib meningitis reduces the incidence of neurologic sequelae. The presumed mechanism is reduction of the inflammation induced by bacterial cell-wall mediators of inflammation when cells are killed by antimicrobial agents. Dexamethasone (0.6 mg/kg per day intravenously in four divided doses for 2 days) is recommended for the treatment of Hib meningitis in children >2 months of age.
Invasive infections other than meningitis are treated with the same antimicrobial agents. For epiglottitis, the dosage of ceftriaxone is 50 mg/kg daily, and the dosage of cefotaxime is 150 mg/kg daily, given in three divided doses 8 h apart. Epiglottitis constitutes a medical emergency, and maintenance of an airway is critical. The duration of therapy is determined by the clinical response. A course of 1–2 weeks is usually appropriate.
Many infections caused by nontypable strains of H. influenzae, such as otitis media, sinusitis, and exacerbations of COPD, can be treated with oral antimicrobial agents. Approximately 20–35% of nontypable strains produce β-lactamase (with the exact proportion depending on geographic location), and these strains are resistant to ampicillin. Several agents have excellent activity against nontypable H. influenzae, including amoxicillin/clavulanic acid, various extended-spectrum cephalosporins, and the macrolides azithromycin and clarithromycin. Fluoroquinolones are highly active against H. influenzae and are useful in adults with exacerbations of COPD. However, fluoroquinolones are not currently recommended for the treatment of children or pregnant women because of possible effects on articular cartilage.
![]() In addition to β-lactamase production, alteration of penicillin-binding proteins—a second mechanism of ampicillin resistance—has been detected in isolates of H. influenzae. Although rare in the United States, these β-lactamase-negative ampicillin-resistant strains are common in Japan and are increasing in prevalence in Europe. Continued monitoring of the evolving antimicrobial susceptibility patterns of H. influenzae will be important.
In addition to β-lactamase production, alteration of penicillin-binding proteins—a second mechanism of ampicillin resistance—has been detected in isolates of H. influenzae. Although rare in the United States, these β-lactamase-negative ampicillin-resistant strains are common in Japan and are increasing in prevalence in Europe. Continued monitoring of the evolving antimicrobial susceptibility patterns of H. influenzae will be important.
PREVENTION
![]() Vaccination (See also Chap. 148) Two conjugate vaccines that prevent invasive infections with Hib in infants and children are licensed in the United States. In addition to eliciting protective antibody, these vaccines prevent disease by reducing rates of pharyngeal colonization with Hib. The widespread use of conjugate vaccines has dramatically reduced the incidence of Hib disease in developed countries. Even though the manufacture of Hib vaccines is costly, vaccination is cost-effective. The Global Alliance for Vaccines and Immunizations has recognized the underuse of Hib conjugate vaccines.
Vaccination (See also Chap. 148) Two conjugate vaccines that prevent invasive infections with Hib in infants and children are licensed in the United States. In addition to eliciting protective antibody, these vaccines prevent disease by reducing rates of pharyngeal colonization with Hib. The widespread use of conjugate vaccines has dramatically reduced the incidence of Hib disease in developed countries. Even though the manufacture of Hib vaccines is costly, vaccination is cost-effective. The Global Alliance for Vaccines and Immunizations has recognized the underuse of Hib conjugate vaccines.
The disease burden has been reduced in developing countries that have implemented routine vaccination (e.g., The Gambia, Chile). An important obstacle to more widespread vaccination is the lack of data on the epidemiology and burden of Hib disease in many developing countries.
All children should be immunized with an Hib conjugate vaccine, receiving the first dose at ~2 months of age, the rest of the primary series at 2–6 months of age, and a booster dose at 12–15 months of age. Specific recommendations vary for the different conjugate vaccines. The reader is referred to the recommendations of the American Academy of Pediatrics (Chap. 148 and www.cispimmunize.org).
Currently, no vaccines are available specifically for the prevention of disease caused by nontypable H. influenzae. However, a vaccine that contains protein D—a surface protein of H. influenzae—conjugated to pneumococcal polysaccharides is licensed in other countries and is used widely in Europe. The vaccine has shown partial efficacy in preventing H. influenzae otitis media in clinical trials. Additional progress in the development of vaccines against nontypable H. influenzae is anticipated.
Chemoprophylaxis The risk of secondary disease is greater than normal among household contacts of patients with Hib disease. Therefore, all children and adults (except pregnant women) in households with an index case and at least one incompletely immunized contact <4 years of age should receive prophylaxis with oral rifampin. When two or more cases of invasive Hib disease have occurred within 60 days at a child-care facility attended by incompletely vaccinated children, administration of rifampin to all attendees and personnel is indicated, as is recommended for household contacts. Chemoprophylaxis is not indicated in nursery and child-care contacts of a single index case. The reader is referred to the recommendations of the American Academy of Pediatrics.
HAEMOPHILUS DUCREYI
Haemophilus ducreyi is the etiologic agent of chancroid (Chap. 163), a sexually transmitted disease characterized by genital ulceration and inguinal adenitis. In addition to being a cause of morbidity in itself, chancroid is associated with HIV infection because of the role played by genital ulceration in HIV transmission. Chancroid increases the efficiency of transmission of and the degree of susceptibility to HIV infection.
MICROBIOLOGY
H. ducreyi is a highly fastidious coccobacillary gram-negative bacterium whose growth requires × factor (hemin). Although, in light of this requirement, the bacterium has been classified in the genus Haemophilus, DNA homology and chemotaxonomic studies have established substantial differences between H. ducreyi and other Haemophilus species. Taxonomic reclassification of the organism is likely in the future but awaits further study. Ulcers contain predominantly T cells. The fact that patients who have had chancroid may have repeated infections indicates that infection does not confer protection.
EPIDEMIOLOGY AND PREVALENCE
![]() The prevalence of chancroid has declined in the United States and worldwide. However, prevalence data must be interpreted with caution because of the difficulty of establishing a diagnosis. The infection appears to be more common in developing countries. Transmission is predominantly heterosexual, and cases in males have outnumbered those in females by ratios of 3:1 to 25:1 during outbreaks. Contact with commercial sex workers and illicit drug use are strongly associated with chancroid.
The prevalence of chancroid has declined in the United States and worldwide. However, prevalence data must be interpreted with caution because of the difficulty of establishing a diagnosis. The infection appears to be more common in developing countries. Transmission is predominantly heterosexual, and cases in males have outnumbered those in females by ratios of 3:1 to 25:1 during outbreaks. Contact with commercial sex workers and illicit drug use are strongly associated with chancroid.
CLINICAL MANIFESTATIONS AND DIFFERENTIAL DIAGNOSIS
Infection is acquired as the result of a break in the epithelium during sexual contact with an infected individual. After an incubation period of 4–7 days, the initial lesion—a papule with surrounding erythema—appears. In 2 or 3 days, the papule evolves into a pustule, which spontaneously ruptures and forms a sharply circumscribed ulcer that generally is not indurated (Fig. 182-2). The ulcers are painful and bleed easily; little or no inflammation of the surrounding skin is evident. Approximately half of patients develop enlarged, tender inguinal lymph nodes, which frequently become fluctuant and spontaneously rupture. Patients usually seek medical care after 1–3 weeks of painful symptoms.
FIGURE 182-2 Chancroid with characteristic penile ulcers and associated left inguinal adenitis (bubo).
The presentation of chancroid does not usually include all of the typical clinical features and is sometimes atypical. Multiple ulcers can coalesce to form giant ulcers. Ulcers can appear and then resolve, with inguinal adenitis (Fig. 182-2) and suppuration following 1–3 weeks later; this clinical picture can be confused with that of lymphogranuloma venereum (Chap. 213). Multiple small ulcers can resemble folliculitis. Other differential diagnostic considerations include the various infections causing genital ulceration, such as primary syphilis, secondary syphilis (condyloma latum), genital herpes, and donovanosis. In rare cases, chancroid lesions become secondarily infected with bacteria; the result is extensive inflammation.
DIAGNOSIS
Clinical diagnosis of chancroid is often inaccurate, and laboratory confirmation should be attempted in suspected cases. An accurate diagnosis of chancroid relies on culture of H. ducreyi from the lesion or from an aspirate of suppurative lymph nodes. Since the organism can be difficult to grow, the use of selective and supplemented media is necessary. No polymerase chain reaction assay for H. ducreyi is commercially available; such tests can be performed by Clinical Laboratory Improvement Amendment (CLIA)–certified clinical laboratories that have developed their own assays.
A probable diagnosis of chancroid can be made when the following criteria are met: (1) one or more painful genital ulcers; (2) no evidence of Treponema pallidum infection by dark-field examination of ulcer exudate or by a negative serologic test for syphilis performed at least 7 days after ulcer onset; (3) a typical clinical presentation for chancroid; and (4) a negative test for herpes simplex virus in the ulcer exudate.
TREATMENT | HAEMOPHILUS DUCREYI |
Treatment regimens recommended by the Centers for Disease Control and Prevention include (1) a single 1-g oral dose of azithromycin; (2) ceftriaxone (250 mg intramuscularly in a single dose); (3) ciprofloxacin (500 mg by mouth twice a day for 3 days); and (4) erythromycin base (500 mg by mouth three times a day for 7 days). Isolates from patients who do not respond promptly to treatment should be tested for antimicrobial resistance. In patients with HIV infection, healing may be slow and longer courses of treatment may be necessary. Clinical treatment failure in HIV-seropositive patients may reflect co-infection, especially with herpes simplex virus. Contacts of patients with chancroid should be identified and treated, whether or not symptoms are present, if they have had sexual contact with the patient during the 10 days preceding the patient’s onset of symptoms.
MORAXELLA CATARRHALIS
MICROBIOLOGY
M. catarrhalis is an unencapsulated gram-negative diplococcus whose ecologic niche is the human respiratory tract. The organism was initially designated Micrococcus catarrhalis. Its name was changed to Neisseria catarrhalis in 1970 because of phenotypic similarities to commensal Neisseria species. On the basis of more rigorous analysis of genetic relatedness, Moraxella catarrhalis is now the widely accepted name for this species.
EPIDEMIOLOGY
![]() Nasopharyngeal colonization by M. catarrhalis is common in infancy, with colonization rates ranging between 33% and 100% and depending on geographic location. Several factors probably account for this geographic variation, including living conditions, day-care attendance, hygiene, household smoking, and population genetics. The prevalence of colonization decreases steadily with age.
Nasopharyngeal colonization by M. catarrhalis is common in infancy, with colonization rates ranging between 33% and 100% and depending on geographic location. Several factors probably account for this geographic variation, including living conditions, day-care attendance, hygiene, household smoking, and population genetics. The prevalence of colonization decreases steadily with age.
The widespread use of pneumococcal conjugate vaccines in some countries has resulted in alterations in patterns of nasopharyngeal colonization in resident populations. A relative increase in colonization by nonvaccine pneumococcal serotypes, nontypable H. influenzae, and M. catarrhalis has occurred. These changes in colonization patterns may be altering the distribution of pathogens of both otitis media and sinusitis in children.
PATHOGENESIS
M. catarrhalis causes mucosal infections of the respiratory tract by contiguous spread from its colonizing site in the upper airway. A preceding viral upper respiratory tract infection is a common inciting event for otitis media. In exacerbations of COPD, the acquisition of new strains is critical for pathogenesis. Strains exhibit substantial genetic diversity and differences in virulence properties.
The expression of several adhesin molecules with differing specificities for various host cell receptors reflects the importance of adherence to the respiratory epithelial surface in the pathogenesis of infection. M. catarrhalis invades multiple cell types. Its intracellular residence in lymphoid tissue provides a potential reservoir for persistence in the human respiratory tract. Like many gram-negative bacteria, M. catarrhalis sheds vesicles into the surrounding environment. The vesicles are internalized by host cells and mediate several virulence mechanisms, including induction of inflammation and delivery of β-lactamase, that can promote the survival of co-pathogens.
CLINICAL MANIFESTATIONS
In children, M. catarrhalis causes predominantly mucosal infections when the bacterium migrates from the nasopharynx to the middle ear or the sinuses (Chap. 44). The inciting event for both otitis media and sinusitis is often a preceding viral infection. Overall, cultures of middle-ear fluid obtained by tympanocentesis indicate that M. catarrhalis causes 15–20% of cases of acute otitis media. Acute otitis media caused by M. catarrhalis or nontypable H. influenzae is clinically milder than otitis media caused by S. pneumoniae, with less fever and a lower prevalence of a red bulging tympanic membrane. However, substantial overlap makes it impossible to predict etiology in an individual child on the basis of clinical features.
A small proportion of viral upper respiratory tract infections are complicated by bacterial sinusitis. Cultures of sinus puncture aspirates show that M. catarrhalis accounts for ~20% of cases of acute bacterial sinusitis in children and for a smaller proportion in adults.
M. catarrhalis is a common cause of exacerbations in adults with COPD. The bacterium has been overlooked in this clinical setting because it has long been considered to be a commensal and because it is easily mistaken for commensal Neisseria species in cultures of respiratory secretions (see “Diagnosis,” below). Several independent lines of evidence have established M. catarrhalis as a pathogen in COPD. These include (1) the demonstration of M. catarrhalis in the lower airways during exacerbations, (2) the association of exacerbation with acquisition of new strains, (3) elevations of inflammatory markers in association with M. catarrhalis, and (4) the development of specific immune responses following infection. M. catarrhalis is the second most common bacterial cause of COPD exacerbations (after H. influenzae), as shown in a 10-year prospective study; the distribution of exacerbations associated with new-strain acquisitions is shown in Fig. 182-3. Not included are culture-negative cases or cases from which a pathogen had been previously isolated. With the application of rigorous clinical criteria for defining the etiology of exacerbations (both culture-positive and culture-negative), ~10% of all exacerbations in the same study were caused by M. catarrhalis. The clinical features of an exacerbation due to M. catarrhalis are similar to those of exacerbations due to other bacterial pathogens, including H. influenzae and S. pneumoniae. The cardinal symptoms are cough with increased sputum production, sputum purulence, and dyspnea in comparison with baseline symptoms.
FIGURE 182-3 Cumulative results of a prospective study (1994–2004) of bacterial infection in chronic obstructive pulmonary disease (COPD) showing etiology of exacerbations. The numbers of exacerbations shown indicate the acquisition of a new strain simultaneous with clinical symptoms of an exacerbation. NTHI, nontypable H. influenzae; M.cat, M. catarrhalis; S.pn, Streptococcus pneumoniae; PA, Pseudomonas aeruginosa. (Adapted from TF Murphy, GI Parameswaran: Clin Infect Dis 49:124, 2009, with permission. © 2009 Infectious Diseases Society of America.)
Pneumonia due to M. catarrhalis occurs in the elderly, particularly in the setting of underlying cardiopulmonary disease, but is infrequent. Invasive infections, such as bacteremia, endocarditis, neonatal meningitis, and septic arthritis, are rare.
DIAGNOSIS
Tympanocentesis is required for etiologic diagnosis of otitis media, but this procedure is not performed routinely. Therefore, treatment of otitis media is generally empirical. Similarly, an etiologic diagnosis of sinusitis requires an invasive procedure and thus is usually not available to the clinician. Isolation of M. catarrhalis from an expectorated sputum sample from an adult experiencing clinical symptoms of an exacerbation is suggestive, but not diagnostic, of M. catarrhalis as the cause.
Upon culture, colonies of M. catarrhalis resemble commensal neisseriae that are part of the normal upper airway flora. As mentioned above, the difficulty in distinguishing colonies of M. catarrhalis from neisserial colonies in cultures of respiratory secretions explains in part why M. catarrhalis has been overlooked as a pathogen. In contrast to these Neisseria species, M. catarrhalis colonies can be slid across the agar surface without disruption (the “hockey puck sign”). In addition, after 48 h of growth, M. catarrhalis colonies take on a pink color and tend to be larger than neisserial colonies. A variety of biochemical tests can distinguish M. catarrhalis from neisseriae. Kits that rely on these biochemical reactions are commercially available.
TREATMENT | MORAXELLA CATARRHALIS |
M. catarrhalis rapidly acquired β-lactamases during the 1970s and 1980s; antimicrobial susceptibility patterns have remained relatively stable since that time, with >90% of strains now producing β-lactamase and thus resistant to amoxicillin. Otitis media in children and exacerbations of COPD in adults are generally managed empirically with antimicrobial agents that are active against S. pneumoniae, H. influenzae, and M. catarrhalis. Most strains of M. catarrhalis are susceptible to amoxicillin/clavulanic acid, extended-spectrum cephalosporins, newer macrolides (azithromycin, clarithromycin), trimethoprim-sulfamethoxazole, and fluoroquinolones.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree