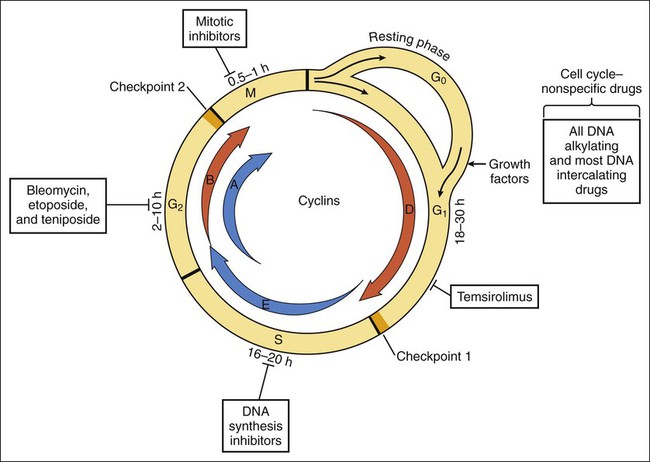
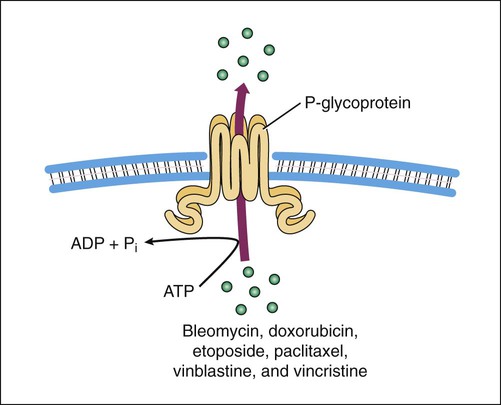
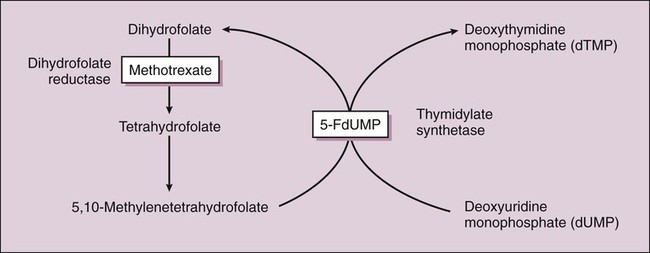
Cancer is a disease in which normal cells are transformed into neoplastic cells through alteration of their genetic material, leading to expression of oncogenes, inhibition of tumor suppressor genes, and uncontrolled growth. Oncogenes typically encode growth factors, many of which are kinases that activate cellular regulatory proteins that promote cell division. For example, the cyclins are proteins that activate cyclin-dependent kinases, leading to activation of enzymes that promote progress through the cell cycle of replication (Fig. 45-1). Some oncogenes express kinases that activate growth factor receptors, such as the epidermal growth factor receptor and the vascular endothelial growth factor receptor. In addition to their uncontrolled proliferation, neoplastic cells often invade previously unaffected organs through a process called metastasis. This process is dependent on angiogenesis, which is the formation of new blood vessels to support metastatic invasion and growth. Drugs that alter activity of the immune system are called immunomodulators. This group of drugs can be divided into immunosuppressants or immunostimulants agents, though a few drugs can both inhibit and stimulate the immune system through actions on different cells and receptors. A number of monoclonal antibody preparations are used as immunosuppressants to treat rheumatoid arthritis (see Chapter 30). The last major section of this chapter focuses on immunosuppressants that are used to treat autoimmune diseases and to prevent allograft rejection after organ or bone marrow transplantation. In the latter case, the drugs act by inhibiting the immune response to foreign antigens (alloantigens) contained in an allograft, which is a graft (transplant) of tissue between individuals of the same species but of disparate genotypes. The cytotoxic antineoplastic drugs can be classified as cell cycle–specific and cell cycle–nonspecific agents. As shown in Figure 45-1, the cycle of cell replication includes the G1, S, G2, and M phases. DNA is replicated during the S phase, and mitosis occurs during the M phase. Because early cytologists observed no activity between the S and M phases, they referred to the period before S as G1 (gap 1) and to the period before M as G2 (gap 2). It is now known that cells are actively preparing for DNA synthesis and mitosis during the G1 and G2 phases, respectively. Cyclins are growth factors that regulate the progression of cells through the cell cycle and are targets of new drug development. Examples of cyclins are shown in Figure 45-1. Drug resistance can occur through failure of the drug to reach its target because of drug efflux from tumor cells. Two important efflux pumps are the P-glycoprotein (Pgp) and the multidrug-resistance protein (MRP) as mentioned in Chapter 2 in the context of drug distribution. Pgp is the product of the multidrug resistance–1 gene and acts to transport many naturally occurring drugs out of neoplastic cells, including anthracyclines, taxanes, and vinca alkaloids (Fig. 45-2). Induction of Pgp by antineoplastic drugs can lead to multidrug resistance. The most common toxicities of traditional antineoplastic drugs (Table 45-1) result from nonspecific inhibition of cell replication in the bone marrow, gastrointestinal epithelium, and hair follicles. Many antineoplastic drugs also stimulate the chemoreceptor trigger zone in the medulla and thereby elicit nausea and vomiting. TABLE 45-1 Major Clinical Uses and Adverse Effects of Selected Antineoplastic Drugs The nausea and vomiting caused by antineoplastic drugs range from mild to severe. Among the antineoplastic drugs, the most emetic are cisplatin and carmustine. Their adverse effects can be prevented or substantially reduced, however, by pretreatment with a combination of antiemetic drugs, including serotonin antagonists (e.g., ondansetron) and corticosteroids (e.g., dexamethasone). The antiemetics are discussed in greater detail in Chapter 28. Most of the DNA synthesis inhibitors are analogues of purine or pyrimidine bases found in DNA or of folic acid. They act as antimetabolites to inhibit enzymes catalyzing various steps in DNA synthesis. The uses and adverse effects of selected inhibitors are listed in Table 45-1. Nearly 50 years ago, MTX was used successfully to induce remission in patients with acute childhood leukemia. Today it is the most widely used antimetabolite in cancer chemotherapy, and it is also used as an immunosuppressive drug in the treatment of rheumatoid arthritis, lupus erythematosus, and other conditions (see Chapter 30). MTX is actively transported into mammalian cells and inhibits dihydrofolate reductase, the enzyme that normally converts dietary folate to the tetrahydrofolate form required for thymidine and purine synthesis (Fig. 45-3).
Antineoplastic and Immunomodulating Drugs
Overview
Principles of Cancer Chemotherapy
Uses and Goals of Treatment
Cell Cycle Specificity
Limitations of Cancer Chemotherapy
Drug Resistance
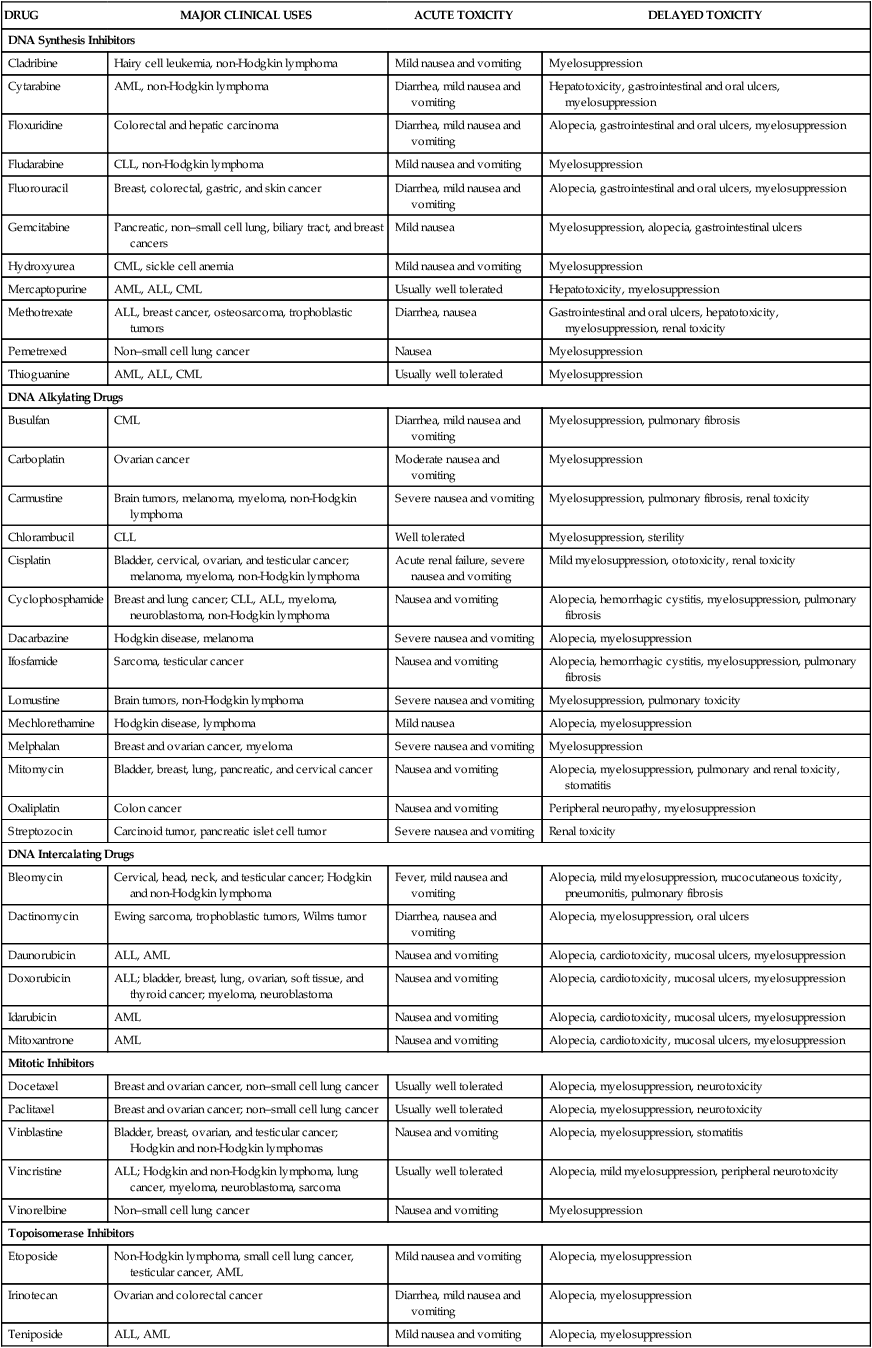
Drug Toxicity
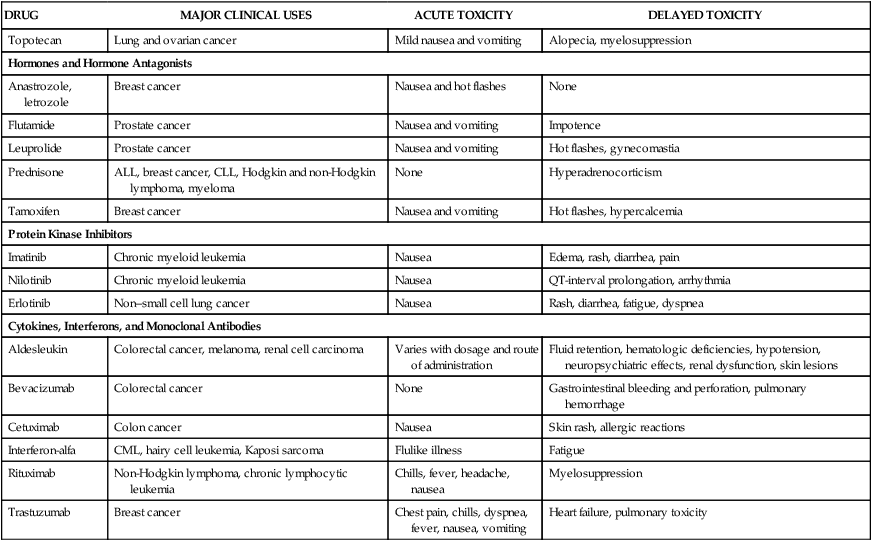
DRUG
MAJOR CLINICAL USES
ACUTE TOXICITY
DELAYED TOXICITY
DNA Synthesis Inhibitors
Cladribine
Hairy cell leukemia, non-Hodgkin lymphoma
Mild nausea and vomiting
Myelosuppression
Cytarabine
AML, non-Hodgkin lymphoma
Diarrhea, mild nausea and vomiting
Hepatotoxicity, gastrointestinal and oral ulcers, myelosuppression
Floxuridine
Colorectal and hepatic carcinoma
Diarrhea, mild nausea and vomiting
Alopecia, gastrointestinal and oral ulcers, myelosuppression
Fludarabine
CLL, non-Hodgkin lymphoma
Mild nausea and vomiting
Myelosuppression
Fluorouracil
Breast, colorectal, gastric, and skin cancer
Diarrhea, mild nausea and vomiting
Alopecia, gastrointestinal and oral ulcers, myelosuppression
Gemcitabine
Pancreatic, non–small cell lung, biliary tract, and breast cancers
Mild nausea
Myelosuppression, alopecia, gastrointestinal ulcers
Hydroxyurea
CML, sickle cell anemia
Mild nausea and vomiting
Myelosuppression
Mercaptopurine
AML, ALL, CML
Usually well tolerated
Hepatotoxicity, myelosuppression
Methotrexate
ALL, breast cancer, osteosarcoma, trophoblastic tumors
Diarrhea, nausea
Gastrointestinal and oral ulcers, hepatotoxicity, myelosuppression, renal toxicity
Pemetrexed
Non–small cell lung cancer
Nausea
Myelosuppression
Thioguanine
AML, ALL, CML
Usually well tolerated
Myelosuppression
DNA Alkylating Drugs
Busulfan
CML
Diarrhea, mild nausea and vomiting
Myelosuppression, pulmonary fibrosis
Carboplatin
Ovarian cancer
Moderate nausea and vomiting
Myelosuppression
Carmustine
Brain tumors, melanoma, myeloma, non-Hodgkin lymphoma
Severe nausea and vomiting
Myelosuppression, pulmonary fibrosis, renal toxicity
Chlorambucil
CLL
Well tolerated
Myelosuppression, sterility
Cisplatin
Bladder, cervical, ovarian, and testicular cancer; melanoma, myeloma, non-Hodgkin lymphoma
Acute renal failure, severe nausea and vomiting
Mild myelosuppression, ototoxicity, renal toxicity
Cyclophosphamide
Breast and lung cancer; CLL, ALL, myeloma, neuroblastoma, non-Hodgkin lymphoma
Nausea and vomiting
Alopecia, hemorrhagic cystitis, myelosuppression, pulmonary fibrosis
Dacarbazine
Hodgkin disease, melanoma
Severe nausea and vomiting
Alopecia, myelosuppression
Ifosfamide
Sarcoma, testicular cancer
Nausea and vomiting
Alopecia, hemorrhagic cystitis, myelosuppression, pulmonary fibrosis
Lomustine
Brain tumors, non-Hodgkin lymphoma
Severe nausea and vomiting
Myelosuppression, pulmonary toxicity
Mechlorethamine
Hodgkin disease, lymphoma
Mild nausea
Alopecia, myelosuppression
Melphalan
Breast and ovarian cancer, myeloma
Severe nausea and vomiting
Myelosuppression
Mitomycin
Bladder, breast, lung, pancreatic, and cervical cancer
Nausea and vomiting
Alopecia, myelosuppression, pulmonary and renal toxicity, stomatitis
Oxaliplatin
Colon cancer
Nausea and vomiting
Peripheral neuropathy, myelosuppression
Streptozocin
Carcinoid tumor, pancreatic islet cell tumor
Severe nausea and vomiting
Renal toxicity
DNA Intercalating Drugs
Bleomycin
Cervical, head, neck, and testicular cancer; Hodgkin and non-Hodgkin lymphoma
Fever, mild nausea and vomiting
Alopecia, mild myelosuppression, mucocutaneous toxicity, pneumonitis, pulmonary fibrosis
Dactinomycin
Ewing sarcoma, trophoblastic tumors, Wilms tumor
Diarrhea, nausea and vomiting
Alopecia, myelosuppression, oral ulcers
Daunorubicin
ALL, AML
Nausea and vomiting
Alopecia, cardiotoxicity, mucosal ulcers, myelosuppression
Doxorubicin
ALL; bladder, breast, lung, ovarian, soft tissue, and thyroid cancer; myeloma, neuroblastoma
Nausea and vomiting
Alopecia, cardiotoxicity, mucosal ulcers, myelosuppression
Idarubicin
AML
Nausea and vomiting
Alopecia, cardiotoxicity, mucosal ulcers, myelosuppression
Mitoxantrone
AML
Nausea and vomiting
Alopecia, cardiotoxicity, mucosal ulcers, myelosuppression
Mitotic Inhibitors
Docetaxel
Breast and ovarian cancer, non–small cell lung cancer
Usually well tolerated
Alopecia, myelosuppression, neurotoxicity
Paclitaxel
Breast and ovarian cancer; non–small cell lung cancer
Usually well tolerated
Alopecia, myelosuppression, neurotoxicity
Vinblastine
Bladder, breast, ovarian, and testicular cancer; Hodgkin and non-Hodgkin lymphomas
Nausea and vomiting
Alopecia, myelosuppression, stomatitis
Vincristine
ALL; Hodgkin and non-Hodgkin lymphoma, lung cancer, myeloma, neuroblastoma, sarcoma
Usually well tolerated
Alopecia, mild myelosuppression, peripheral neurotoxicity
Vinorelbine
Non–small cell lung cancer
Nausea and vomiting
Myelosuppression
Topoisomerase Inhibitors
Etoposide
Non-Hodgkin lymphoma, small cell lung cancer, testicular cancer, AML
Mild nausea and vomiting
Alopecia, myelosuppression
Irinotecan
Ovarian and colorectal cancer
Diarrhea, mild nausea and vomiting
Alopecia, myelosuppression
Teniposide
ALL, AML
Mild nausea and vomiting
Alopecia, myelosuppression
Topotecan
Lung and ovarian cancer
Mild nausea and vomiting
Alopecia, myelosuppression
Hormones and Hormone Antagonists
Anastrozole, letrozole
Breast cancer
Nausea and hot flashes
None
Flutamide
Prostate cancer
Nausea and vomiting
Impotence
Leuprolide
Prostate cancer
Nausea and vomiting
Hot flashes, gynecomastia
Prednisone
ALL, breast cancer, CLL, Hodgkin and non-Hodgkin lymphoma, myeloma
None
Hyperadrenocorticism
Tamoxifen
Breast cancer
Nausea and vomiting
Hot flashes, hypercalcemia
Protein Kinase Inhibitors
Imatinib
Chronic myeloid leukemia
Nausea
Edema, rash, diarrhea, pain
Nilotinib
Chronic myeloid leukemia
Nausea
QT-interval prolongation, arrhythmia
Erlotinib
Non–small cell lung cancer
Nausea
Rash, diarrhea, fatigue, dyspnea
Cytokines, Interferons, and Monoclonal Antibodies
Aldesleukin
Colorectal cancer, melanoma, renal cell carcinoma
Varies with dosage and route of administration
Fluid retention, hematologic deficiencies, hypotension, neuropsychiatric effects, renal dysfunction, skin lesions
Bevacizumab
Colorectal cancer
None
Gastrointestinal bleeding and perforation, pulmonary hemorrhage
Cetuximab
Colon cancer
Nausea
Skin rash, allergic reactions
Interferon-alfa
CML, hairy cell leukemia, Kaposi sarcoma
Flulike illness
Fatigue
Rituximab
Non-Hodgkin lymphoma, chronic lymphocytic leukemia
Chills, fever, headache, nausea
Myelosuppression
Trastuzumab
Breast cancer
Chest pain, chills, dyspnea, fever, nausea, vomiting
Heart failure, pulmonary toxicity
Cytotoxic Agents
DNA Synthesis Inhibitors
Folate Antagonists
Methotrexate.
Chemistry and Mechanisms.
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Antineoplastic and Immunomodulating Drugs
Only gold members can continue reading. Log In or Register to continue