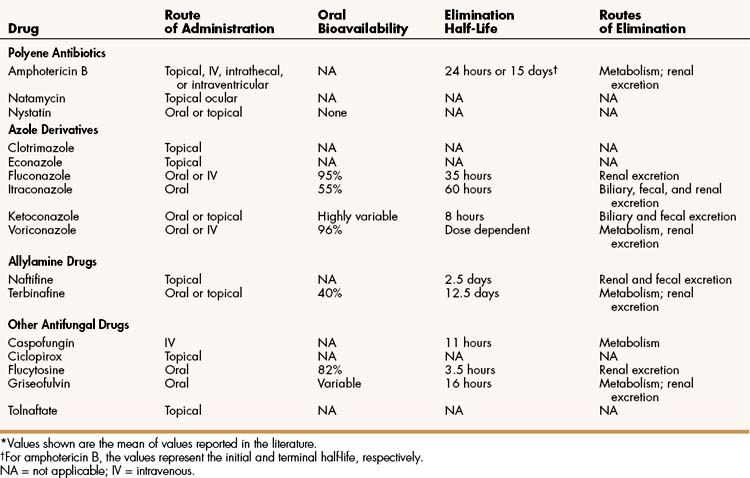
Many antifungal drugs act by impairing plasma membrane function in fungal cells. The selective toxicity of these drugs is caused by the difference in the sterols found in fungal and mammalian cell membranes. Fungal cell membranes contain ergosterol, whereas mammalian cell membranes contain cholesterol. Some antifungal drugs bind to ergosterol and thereby increase plasma membrane permeability, whereas other drugs inhibit the synthesis of ergosterol (Fig. 42–1 and Table 42–1).
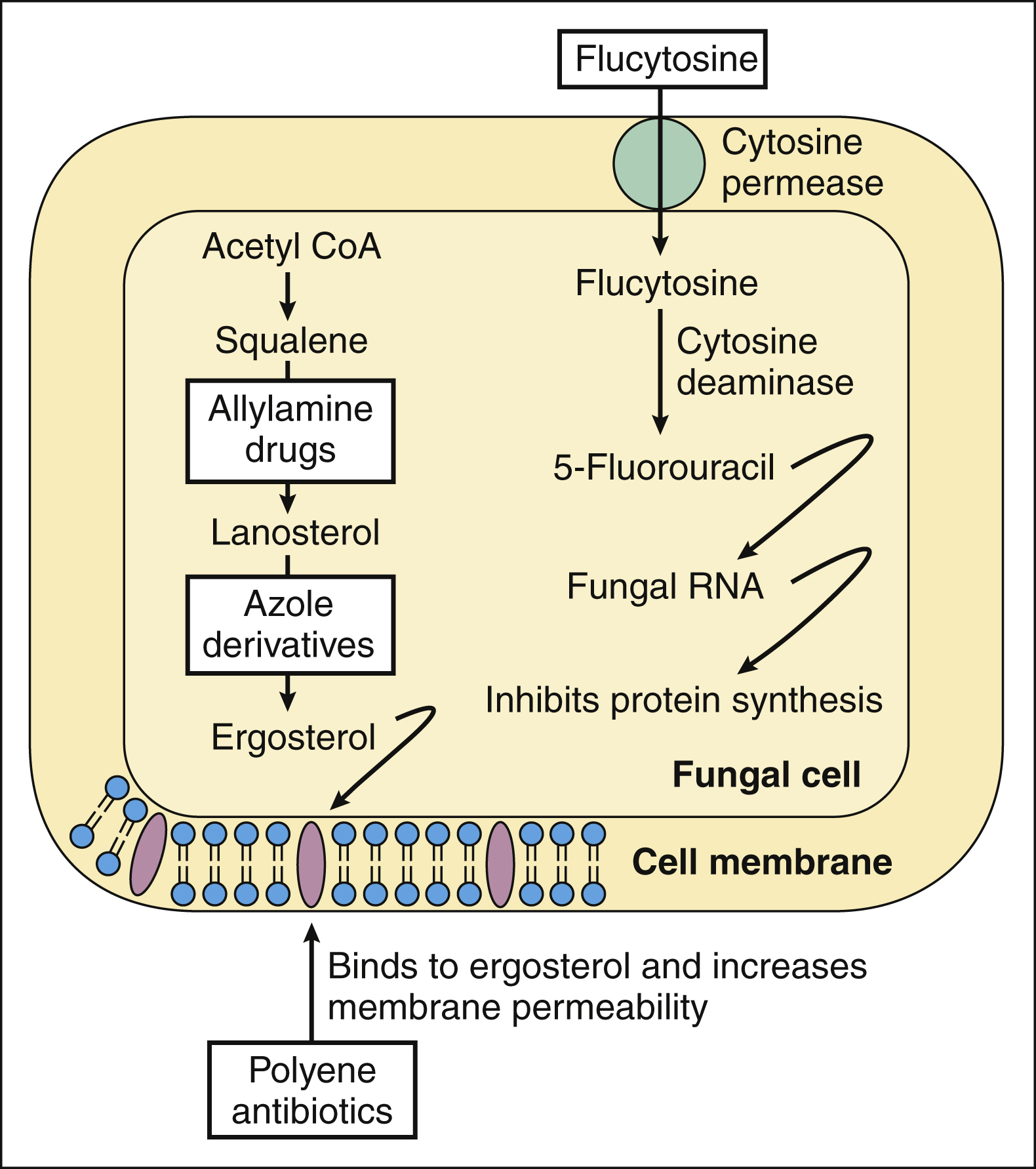
Figure 42–1 Mechanisms of action of antifungal drugs. The synthesis of ergosterol is inhibited by allylamine drugs and by azole derivatives. Amphotericin B and other polyene antibiotics bind to ergosterol in fungal cell membranes and increase membrane permeability. Ciclopirox increases membrane permeability by another mechanism (not shown). Flucytosine is accumulated by fungal cells and converted to 5-fluorouracil (5-FU). When 5-FU is incorporated into fungal RNA, protein synthesis is inhibited. Griseofulvin interferes with microtubule function and blocks mitosis (not shown). Caspofungin inhibits fungal cell wall synthesis (not shown).
Polyene antibiotics selectively bind to ergosterol in fungal membranes. This action increases fungal plasma membrane permeability and allows the cytoplasmic contents to escape from the cell. The polyene drugs can also bind to cholesterol in mammalian cells, and this may account for their ability to produce kidney damage. Ciclopirox increases fungal cell membrane permeability by a different mechanism. It prevents amino acid transport into fungal cells, thereby altering membrane structure and allowing the escape of intracellular material.
The allylamine drugs and the azole derivatives block distinct steps in ergosterol biosynthesis, but these groups of drugs have little effect on cholesterol biosynthesis in humans. The allylamine drugs inhibit squalene monooxygenase, which converts squalene to squalene-2,3-oxide, the immediate precursor of lanosterol. The azoles inhibit 14-α-demethylase, a cytochrome P450 enzyme that converts lanosterol to ergosterol.
The echinocandin drugs (e.g., caspofungin) represent a new class of antifungal agents that inhibit the synthesis of a fungal cell wall component, β-(1,3)-D-glucan. The fungal cell wall surrounds the plasma membrane and protects the cell from osmotic and mechanical stress.
Flucytosine, a pyrimidine antimetabolite, is the only antifungal drug that affects nucleic acid. Flucytosine is converted to 5-fluorouracil (5-FU) in fungal cells by cytosine deaminase, an enzyme not found in mammalian cells. 5-FU is then incorporated into fungal RNA, and this inhibits fungal protein synthesis.
Griseofulvin acts by binding to fungal microtubules and thereby inhibiting microtubule function and mitosis. The mechanism of action of tolnaftate is unknown.
DRUGS
Polyene Antibiotics
The polyene antibiotics are produced by various soil organisms of the family Streptomycetaceae. Examples are amphotericin A and B, natamycin, and nystatin. Each of these compounds consists of a macrolide (lactone) ring containing conjugated double bonds (polyene), with acidic and basic side groups. Because the acidic group and basic group are capable of donating or accepting a proton, respectively, the polyene drugs are amphoteric.
Amphotericin B has greater antifungal activity than does amphotericin A, which is not used clinically. Amphotericin B is the only polyene drug used to treat systemic and subcutaneous mycoses. The other polyene drugs are limited to topical application for the treatment of superficial mycoses.
Amphotericin B
PHARMACOKINETICS
The pharmacokinetic properties of amphotericin B are listed in Table 42–2. Amphotericin B is not absorbed from the gut and is available as a deoxycholate complex and as three lipid formulations for parenteral administration. The drug is also available in topical preparations for the treatment of superficial infections.
The dosage and route of parenteral treatment depend on the site and severity of the infection and on the immune status of the patient. Higher doses of amphotericin B are used to treat infections caused by more resistant fungi, especially Aspergillus species, and lower doses are generally used to treat esophageal and urinary tract infections. Concentrations of the drug in cerebrospinal fluid are only 2% to 3% of those in plasma, reflecting that amphotericin B does not penetrate the blood-brain barrier very well. Nevertheless, the drug is usually administered intravenously to treat fungal meningitis and other systemic mycoses. Intrathecal administration or intraventricular administration with an Ommaya reservoir is usually reserved for extremely ill patients and those who do not respond to intravenous therapy.
Amphotericin B is extensively metabolized in the liver, and the metabolites are slowly excreted in the urine. The biotransformation pathways are not well understood. Amphotericin B has a biphasic half-life, with an initial half-life of about 24 hours and a terminal half-life of about 15 days.
SPECTRUM AND INDICATIONS
Amphotericin B is active against a wide variety of fungi (Table 42–3), and it has been the standard for comparison of other drugs in the treatment of serious fungal infections.
TABLE 42–3 Causes and Management of Selected Systemic and Subcutaneous Mycoses
| Mycosis | Pathogens | Treatments |
|---|---|---|
| Aspergillosis | Aspergillus fumigatus | Voriconazole, caspofungin, or both; amphotericin B |
| Blastomycosis | Blastomyces dermatitidis | Itraconazole or amphotericin B |
| Candidiasis | Candida albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. krusei | Fluconazole, voriconazole, amphotericin B ± flucytosine, or caspofungin |
| Coccidioidomycosis∗ | Coccidioides immitis | Itraconazole or fluconazole (mild to moderate disease); amphotericin B (severe disease); fluconazole for meningitis |
| Cryptococcosis | Cryptococcus neoformans | Amphotericin B + flucytosine followed by fluconazole for meningitis; fluconazole or amphotericin B for nonmeningeal infections |
| Fusariosis | Fusaria species | Voriconazole |
| Histoplasmosis | Histoplasma capsulatum | Itraconazole for moderate disease; amphotericin B for severe disease and meningitis |
| Mucormycosis | Absidia, Rhizopus, and Rhizomucor species | Amphotericin B |
| Pseudallescheriasis | Pseudallescheria boydii | Voriconazole, itraconazole, or miconazole, with surgery |
| Sporotrichosis | Sporothrix schenckii | Itraconazole, fluconazole, or saturated potassium iodide solution |
∗ No treatment needed for uncomplicated pulmonary infection in normal host (valley fever).
FUNGAL RESISTANCE
Although polyene antibiotics have been used to treat fungal infections for nearly 50 years, few reports have been issued of fungal resistance to these drugs. Fungi that do become resistant to polyenes have a reduced content of ergosterol in their cell membranes.
ADVERSE EFFECTS
Amphotericin B has been called “amphoterrible” and is probably the most toxic antibiotic in use today. It causes some degree of renal toxicity in about 80% of patients who receive it. Renal toxicity reduces the glomerular filtration rate and contributes to the development of hypokalemia and hypomagnesemia. It also leads to accumulation of creatinine and urea in the blood (azotemia). Electrolytes (especially sodium, potassium, and magnesium) should be monitored weekly during treatment and replacements administered as needed.
Lipid formulations of amphotericin B cause less renal toxicity and should be used in persons with renal impairment and those who are intolerant of the traditional deoxycholate formulation. These preparations include amphotericin B cholesteryl complex, amphotericin B lipid complex, and amphotericin B liposomal complex. The lipid formulations have unique pharmacokinetic characteristics that reduce renal drug concentrations and toxicity. Following intravenous administration, the lipid formulations are sequestered by cells of the reticuloendothelial system in the liver and spleen, which slowly release amphotericin B into the circulation over several days, resulting in lower but more sustained plasma levels of the drug.
In addition to causing nephrotoxicity, amphotericin B can cause acute liver failure, cardiac arrhythmias, and hematopoietic disorders such as anemia, leukopenia, and thrombocytopenia. The drug frequently causes less severe but unpleasant effects, including chills, fever, headache, nausea, and vomiting. The severity of these minor adverse effects can be lessened by pretreatment with corticosteroids, antipyretic drugs (e.g., acetaminophen), and antihistamine drugs.
Nystatin and Natamycin
Nystatin, which is active against Candida species, is available in various formulations, including the following: creams, ointments, and powders for mucocutaneous candidiasis; lozenges (troches) for oral candidiasis; orally administered tablets and suspensions for intestinal candidiasis; and vaginal tablets for vaginal candidiasis.
Natamycin is active against Aspergillus, Candida, Fusarium, and Penicillium species and is available as an ophthalmic suspension for the treatment of fungal blepharitis, conjunctivitis, or keratitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree