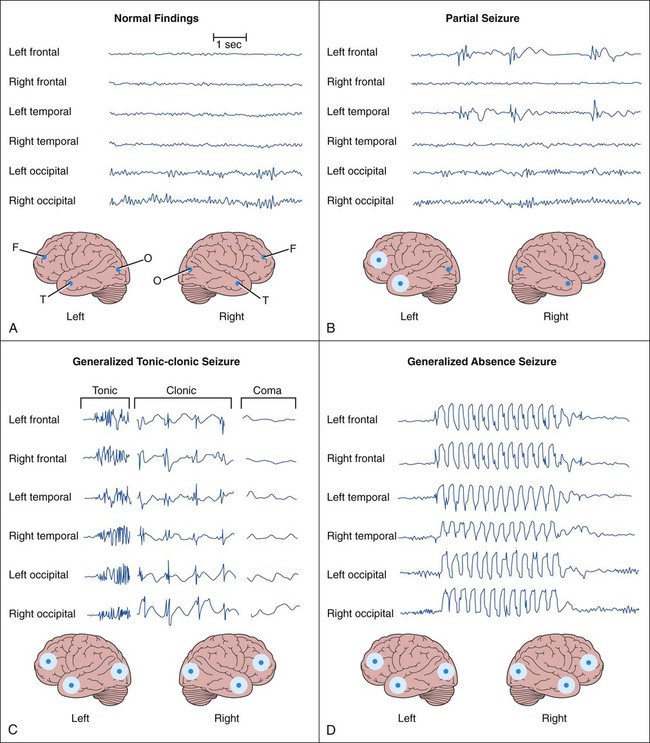
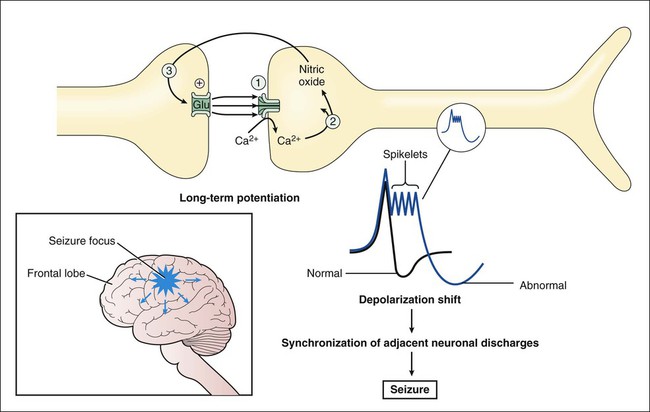
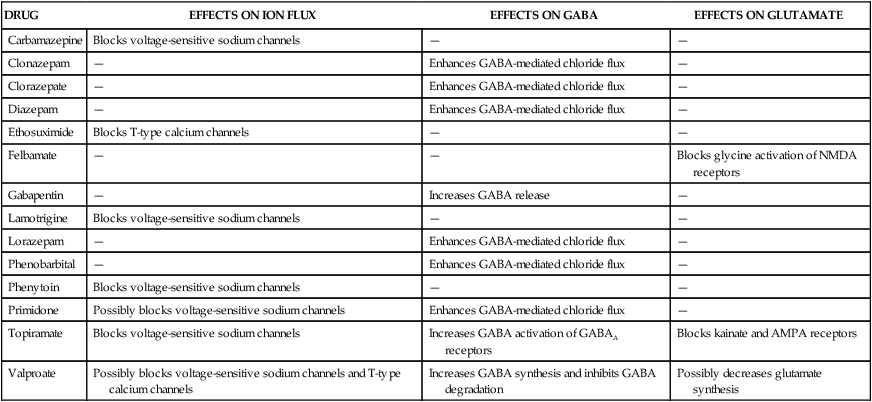
The two main categories of seizures are partial (focal) seizures and generalized seizures (Table 20-1). A partial seizure originates in one cerebral hemisphere, and the patient does not lose consciousness during the seizure. A generalized seizure arises in both cerebral hemispheres and involves loss of consciousness. Seizures are accompanied by characteristic changes in the electroencephalogram (EEG), as shown in Figure 20-1. Most seizures are self-limited and last from about 10 seconds to 5 minutes. Some seizures are preceded by an aura, which is a sensation or mood that may help identify the anatomic location of the seizure focus. TABLE 20-1 International Classification of Partial and Generalized Seizures Less-common types of generalized seizures include myoclonic seizures and atonic seizures (see Table 20-1). Epileptic seizures are caused by synchronous neuronal discharges within a particular group of neurons, or seizure focus, which is often located in the cerebral cortex but can be found in other areas of the brain. Once initiated, the abnormal discharges spread to other parts of the brain and produce abnormal movements, sensations, or thoughts. The neuronal mechanisms that initiate a seizure are not fully understood, but growing evidence indicates the involvement of excessive excitatory neurotransmission mediated by glutamate (Fig. 20-2). Investigators believe that excessive activation by glutamate of N-methyl-D-aspartate (NMDA) receptors displaces Mg2+ ions from the NMDA receptor–calcium ion channel and thereby facilitates calcium entry into neurons. Calcium contributes to the long-term potentiation of excitatory glutamate neurotransmission by activating the synthesis of nitric oxide. Antiepileptic drugs are believed to suppress the formation or spread of abnormal electrical discharges in the brain. As shown in Table 20-2, the currently available drugs accomplish these actions via three mechanisms: (1) inhibition of the sodium or calcium influx responsible for neuronal depolarization, (2) augmentation of inhibitory GABA neurotransmission, and (3) inhibition of excitatory glutamate neurotransmission. TABLE 20-2 Mechanisms of Selected Antiepileptic Drugs Antiepileptic drugs facilitate GABA neurotransmission by various means. Benzodiazepines, such as clonazepam, and barbiturates, such as phenobarbital, enhance GABA activation of the GABAA receptor–chloride ion channel (see Chapter 19). Topiramate also activates the GABAA receptor. Gabapentin increases GABA release, whereas valproate inhibits GABA degradation. Drugs that augment GABA may serve to counteract the excessive excitatory neurotransmission responsible for initiating and spreading abnormal electrical discharges. The pharmacologic properties of antiepileptic drugs are described in the following sections, whereas the mechanisms of action, adverse effects, contraindications, and drug interactions are given in Tables 20-2, 20-3, and 20-4. With regard to adverse effects, the results of recent U.S. Food and Drug Administration (FDA) studies showed an increased risk of suicidal thoughts and behavior with all antiepileptic agents. TABLE 20-3 Adverse Effects, Contraindications, and Pregnancy Risk Data for Select Antiepileptic Drugs
Antiepileptic Drugs
Overview
Classification of Seizures
CLASSIFICATION
CHARACTERIZATION
Partial (Focal) Seizures
Arise in one cerebral hemisphere
Simple partial seizure
No alteration of consciousness
Complex partial seizure
Altered consciousness, automatisms, and behavioral changes
Secondarily generalized seizure
Focal seizure becomes generalized and is accompanied by loss of consciousness
Generalized Seizures
Arise in both cerebral hemispheres and are accompanied by loss of consciousness
Tonic-clonic (grand mal) seizure
Increased muscle tone followed by spasms of muscle contraction and relaxation
Tonic seizure
Increased muscle tone
Clonic seizure
Spasms of muscle contraction and relaxation
Myoclonic seizure
Rhythmic, jerking spasms
Atonic seizure
Sudden loss of all muscle tone
Absence (petit mal) seizure
Brief loss of consciousness, with minor muscle twitches and eye blinking
Neurobiology of Seizures
Mechanisms of Antiepileptic Drugs
DRUG
EFFECTS ON ION FLUX
EFFECTS ON GABA
EFFECTS ON GLUTAMATE
Carbamazepine
Blocks voltage-sensitive sodium channels
—
—
Clonazepam
—
Enhances GABA-mediated chloride flux
—
Clorazepate
—
Enhances GABA-mediated chloride flux
—
Diazepam
—
Enhances GABA-mediated chloride flux
—
Ethosuximide
Blocks T-type calcium channels
—
—
Felbamate
—
—
Blocks glycine activation of NMDA receptors
Gabapentin
—
Increases GABA release
—
Lamotrigine
Blocks voltage-sensitive sodium channels
—
—
Lorazepam
—
Enhances GABA-mediated chloride flux
—
Phenobarbital
—
Enhances GABA-mediated chloride flux
—
Phenytoin
Blocks voltage-sensitive sodium channels
—
—
Primidone
Possibly blocks voltage-sensitive sodium channels
Enhances GABA-mediated chloride flux
—
Topiramate
Blocks voltage-sensitive sodium channels
Increases GABA activation of GABAA receptors
Blocks kainate and AMPA receptors
Valproate
Possibly blocks voltage-sensitive sodium channels and T-type calcium channels
Increases GABA synthesis and inhibits GABA degradation
Possibly decreases glutamate synthesis
Effects on GABAergic Systems
Treatment of Seizure Disorders
DRUG
MAJOR ADVERSE EFFECTS
CONTRAINDICATIONS
RISK CATEGORY AND EFFECTS OF USE DURING PREGNANCY*
Carbamazepine
Aplastic anemia (rare); ataxia, drowsiness, and other symptoms of central nervous system (CNS) depression; gastrointestinal reactions; and nausea
Hypersensitivity
Category C; increased risk of birth defects, abnormal facial features, neural tube defects such as spina bifida, reduced head size, and other anomalies
Clonazepam
Arrhythmia, CNS depression, drug dependence, hypotension, and mild respiratory depression
Acute angle-closure glaucoma, hypersensitivity, and severe liver disease
Category C; apnea in newborns, increased risk of birth defects
Clorazepate
Confusion, drowsiness, drug tolerance, and lethargy
Hypersensitivity
Category D; increased risk of birth defects
Diazepam
Same as clonazepam
Same as clonazepam
Category D; increased risk of birth defects
Ethosuximide
Dizziness, drowsiness, gastric distress, lethargy, and nausea
Hypersensitivity
Category C
Felbamate
Aplastic anemia, fatigue, gastrointestinal reactions, headache, hepatic toxicity, and insomnia
Bone marrow depression, hepatic disease, and hypersensitivity
Category C
Gabapentin
Ataxia, dizziness, drowsiness, nystagmus, and tremor
Hypersensitivity
Category C
Lamotrigine
Ataxia, diplopia, dizziness, drowsiness, headache, nausea, rash, and Stevens-Johnson syndrome
Risk of aseptic meningitis
Hypersensitivity: use cautiously in patients who are taking valproate or have hepatic or renal disease.
Category C; may reduce folate levels
Levetiracetam
Somnolence, asthenia (weakness), infection, and dizziness
Hypersensitivity
Category C; levetiracetam produced evidence of developmental toxicity at doses similar to or greater than human therapeutic doses
Lorazepam
Same as clonazepam
Same as clonazepam
Category D; increased risk of birth defects
Phenobarbital
Ataxia, cognitive impairment, dizziness, drowsiness, drug dependence, rash, and respiratory depression
Hypersensitivity, porphyria, respiratory depression, and severe liver disease
Category D; bleeding at birth, minor congenital defects
Phenytoin
Cerebellar symptoms, gastrointestinal disturbances, gingival hyperplasia, hirsutism, megaloblastic anemia and other blood cell deficiencies, osteomalacia, and psychiatric changes
Bradycardia, hypersensitivity, and severe atrioventricular block or sinoatrial dysfunction; Asians with polymorphism in human leukocyte antigen (HLA) allele at higher risk for skin reactions.
Category D; may reduce folate levels, two to three times increased risk of birth defects, fetal hydantoin syndrome
Primidone
Same as phenobarbital
Hypersensitivity
Category D
Tiagabine
Dizziness, somnolence, nausea, nervousness, abdominal pain, and difficulty with concentration or attention
Hypersensitivity
Category C; adverse effects on embryo-fetal development, including teratogenic effects at doses greater than the human therapeutic dose
Topiramate
Ataxia, dizziness, drowsiness, nystagmus, paresthesia, and psychomotor impairment
Hypersensitivity; use cautiously during pregnancy, during lactation, or in the presence of hepatic or renal disease.
Category D; recent data showed increase incidence of cleft palate.
Valproate
Drowsiness, gastrointestinal disturbances, hepatic toxicity (rare), pancreatitis (rare), nausea, and weight gain
Hepatic disease and hypersensitivity
Category D; may reduce folate levels; teratogenic during the first trimester; neural tube defects such as spina bifida; decreased cognitive development
Vigabatrin
Amnesia, blurred vision, blue-yellow color blindness, decreased vision or other vision changes, eye pains, increase in seizures (rare)
Hypersensitivity
Category C; studies in rabbits have shown that vigabatrin causes birth defects.
Zonisamide
Somnolence, anorexia, dizziness, headache, nausea, and agitation or irritability; metabolic acidosis in younger patients
Hypersensitivity
Category C; teratogenic in mice, rats, and dogs and embryolethal in monkeys when administered during the period of organogenesis at zonisamide dosage and maternal plasma levels similar to or lower than therapeutic levels in humans ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Antiepileptic Drugs
Only gold members can continue reading. Log In or Register to continue