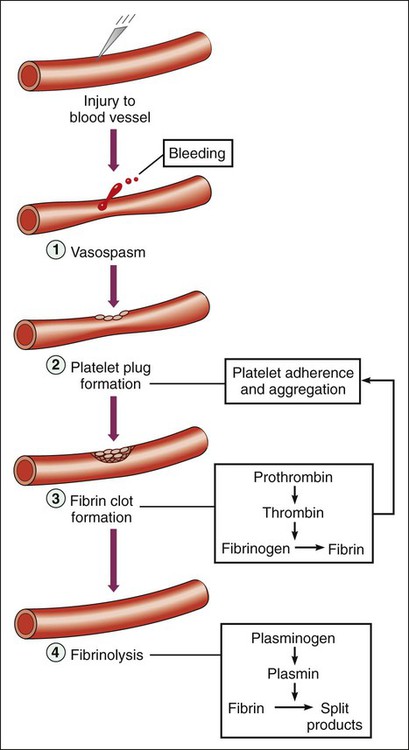
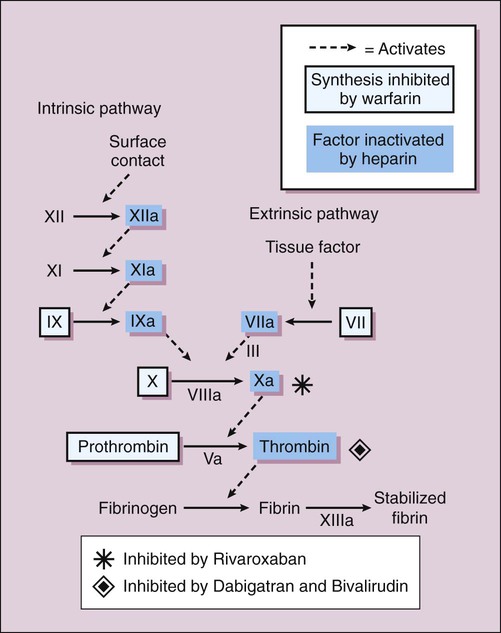
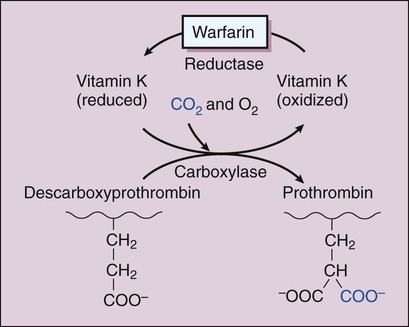
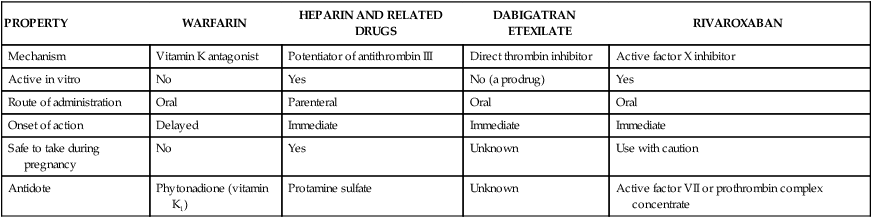
When a small blood vessel is injured, hemorrhage is prevented by vasospasm, the formation of a platelet plug and a fibrin clot (Fig. 16-1). After the vessel is repaired, the clot is removed via the process of fibrinolysis. Vasospasm reduces bleeding and blood flow and thereby facilitates platelet aggregation and blood coagulation. Exposure of the blood to extravascular collagen causes adherence of platelets to the injured vessel wall and initiates the sequential activation (cascade) of numerous coagulation factors (blood clotting factors), most of which are serine proteases. In this cascade, the inactive factors are converted to active enzymes by the previous coagulation factor or stimulus. The factors and their synonyms are listed in Table 16-1, and the coagulation pathways are illustrated in Figure 16-2. The intrinsic pathway may be activated by surface contact with a foreign body or extravascular tissue, whereas the extrinsic pathway is activated by a complex tissue factor called thromboplastin. The pathways converge with the activation of factor X, which is the major rate-limiting step in the coagulation cascade. The activation of factor X leads to the formation of thrombin, and thrombin, in turn, catalyzes the conversion of fibrinogen to fibrin. Thrombin is also a powerful stimulant of platelet aggregation. The fibrin meshwork traps erythrocytes and platelets to complete the formation of a hemostatic thrombus (clot). TABLE 16-1 *Factor VI is no longer considered to be a coagulation factor. †Proteins C and S, which are endogenous anticoagulants that inactivate factors Va and VIIIa and promote fibrinolysis, are also dependent on vitamin K. Anticoagulants are drugs that impede blood coagulation and prevent the occurrence or expansion of a thrombus. The anticoagulants are classified according to their mechanism of action and include vitamin K antagonists, drugs that potentiate antithrombin III, and drugs that directly inhibit thrombin or active factor X. Table 16-2 compares the properties of various anticoagulant drugs. TABLE 16-2 Comparison of the Pharmacologic Properties of Selected Anticoagulants Warfarin and other coumarin derivatives are structurally related to vitamin K. These drugs inhibit the synthesis of coagulation factors II (prothrombin), VII, IX, and X, whose carboxylation is dependent on a reduced form of vitamin K. As shown in Figure 16-3, warfarin blocks the reduction of oxidized vitamin K and thereby prevents the posttranslational (following protein synthesis) carboxylation of these four factors. Warfarin also inhibits the synthesis of proteins C and S, which are endogenous anticoagulants that inactivate factors V and VIII and promote fibrinolysis. It is possible that the inhibition of proteins C and S contributes to a transient procoagulant effect when warfarin is first administered. The most common adverse effect of warfarin is bleeding (Table 16-3), which can range in severity from mild nosebleed to life-threatening hemorrhage. Patients should be instructed to report any signs of bleeding, including hematuria and bleeding into the skin (ecchymoses). TABLE 16-3 Adverse Effects and Drug Interactions of Anticoagulant, Antiplatelet, and Fibrinolytic Drugs *Also alteplase, urokinase, anistreplase, reteplase, and tenecteplase. Warfarin is contraindicated in pregnancy because of its potential to cause fetal hemorrhage and various structural malformations referred to as the fetal warfarin syndrome. These malformations are partly a result of antagonism of vitamin K–dependent maturation of bone proteins during a process in which these proteins are carboxylated in the same manner as the nascent clotting factors. Warfarin and other vitamin K antagonists block this process and can cause bone deformities and various birth defects that are listed in Table 4-6. Warfarin has been primarily used in the long-term treatment of patients who have a thromboembolic disorder such as deep vein thrombosis (DVT) and patients who have atrial fibrillation or an artificial heart valve (Table 16-4). It has also been used with a heparin-type anticoagulant for the treatment of MI. The goals of warfarin therapy are to prevent thrombus formation or expansion and to prevent embolization and other potentially fatal consequences of thrombosis. TABLE 16-4 Clinical Uses of Antithrombotic Agents
Anticoagulant, Antiplatelet, and Fibrinolytic Drugs
Blood Coagulation
Normal Hemostasis
FACTOR*
COMMON SYNONYM
DEPENDENT ON VITAMIN K†
I
Fibrinogen
No
II
Prothrombin
Yes
III
Tissue thromboplastin
No
IV
Calcium
No
V
Proaccelerin
No
VII
Proconvertin
Yes
VIII
Antihemophilic factor
No
IX
Plasma thromboplastin component
Yes
X
Stuart factor
Yes
XI
Plasma thromboplastin antecedent
No
XII
Hageman factor
No
XIII
Fibrin stabilizing factor
No
Anticoagulant Drugs
PROPERTY
WARFARIN
HEPARIN AND RELATED DRUGS
DABIGATRAN ETEXILATE
RIVAROXABAN
Mechanism
Vitamin K antagonist
Potentiator of antithrombin III
Direct thrombin inhibitor
Active factor X inhibitor
Active in vitro
No
Yes
No (a prodrug)
Yes
Route of administration
Oral
Parenteral
Oral
Oral
Onset of action
Delayed
Immediate
Immediate
Immediate
Safe to take during pregnancy
No
Yes
Unknown
Use with caution
Antidote
Phytonadione (vitamin K1)
Protamine sulfate
Unknown
Active factor VII or prothrombin complex concentrate
Warfarin
Chemistry and Mechanisms
Adverse Effects and Interactions
DRUG
COMMON ADVERSE EFFECTS
COMMON DRUG INTERACTIONS
Anticoagulants
Warfarin
Birth defects and bleeding
Serum levels altered by drugs that induce or inhibit cytochrome P450, by drugs that inhibit gut absorption, and by drugs that directly increase or decrease the anticoagulant effect.
Rivaroxaban
Bleeding
Serum levels increased by cytochrome P450 3A4 inhibitors and by drugs that inhibit P-glycoprotein (Pgp) drug transport.
Dalteparin, enoxaparin
Bleeding and thrombocytopenia
Risk of bleeding increased by salicylates.
Heparin
Bleeding, hyperkalemia, and thrombocytopenia
Same as dalteparin.
Hirudin and related drugs
Bleeding
Same as dalteparin.
Antiplatelet Drugs
Abciximab
Bleeding, bradycardia, hypotension, and thrombocytopenia
Unknown.
Aspirin
Gastrointestinal irritation and bleeding, hypersensitivity reactions, and tinnitus
Increases hypoglycemic effect of sulfonylureas. Increases risk of gastrointestinal bleeding and ulceration associated with methotrexate, valproate, and other drugs. Inhibits uricosuric effect of probenecid.
Dipyridamole
Gastrointestinal distress, headache, mild and transient dizziness, and rash
Decreases metabolism of adenosine. Increases risk of bradycardia associated with β-adrenergic receptor antagonists.
Clopidogrel
Bleeding, diarrhea, gastrointestinal pain, increased cholesterol and triglyceride levels, nausea, and neutropenia
Increases levels of drugs metabolized by liver microsomal enzymes.
Fibrinolytic Drugs
Streptokinase*
Bleeding, hypersensitivity reactions, and reperfusion arrhythmias
Increases risk of bleeding associated with anticoagulant and antiplatelet drugs.
Indications
CLINICAL USE
PRIMARY DRUGS
Venous Thromboembolism
Acute
LMWH, fondaparinux
Surgical prophylaxis
LMWH, fondaparinux, or rivaroxaban
Long-term prophylaxis
Warfarin or rivaroxaban
Pulmonary embolism
Heparin, fibrinolytic drug
Acute Coronary Syndromes
Unstable angina and non-STE ACS
Aspirin ± clopidogrel or prasugrel; eptifibatide or tirofiban; LMWH or fondaparinux
STEMI
Fibrinolytic drug, aspirin, and LMWH
Percutaneous coronary interventions*
LMWH or bivalirudin ± abciximab, or eptifibatide; also aspirin, clopidogrel, or prasugrel
Stroke, Thrombotic
Acute
Fibrinolytic drug or aspirin
Prophylaxis, including transient ischemic attacks
Aspirin and dipyridamole combined; clopidogrel or prasugrel
Atrial fibrillation
Heparin or LMWH followed by warfarin, dabigatran, or rivaroxaban
Artificial heart valve
Warfarin, aspirin
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Anticoagulant, Antiplatelet, and Fibrinolytic Drugs
Only gold members can continue reading. Log In or Register to continue