Chapter 6 Antibodies and Biological Products
| Abbreviations | |
|---|---|
| APC | Antigen presenting cell |
| CNS | Central nervous system |
| CSF | Colony-stimulating factor |
| CTL | Cytolytic T lymphocyte |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IL | Interleukin |
| IM | Intramuscular |
| IV | Intravenous |
| M-CSF | Macrophage colony-stimulating factor |
| MHC | Major histocompatibility complex |
| MS | Multiple sclerosis |
| NFAT | Nuclear factor for activated T cells |
| SC | Subcutaneous |
| Th | T-helper (cells) |
| TNF | Tumor necrosis factor |
Therapeutic Overview
compounds such as monoclonal antibodies against cytokines, receptors, and specific immune cell antigens, as well as recombinant cytokines and cytokine receptor antagonists have provided greater selectivity and less toxicity. These drugs have made important contributions to the treatment of autoimmune diseases such multiple sclerosis (MS) and rheumatoid arthritis.
The benefits of these agents are shown in the Therapeutic Overview Box.
| Therapeutic Overview |
|---|
| Immunosuppressive/anti-inflammatory drugs |
| Prevent or modulate immune-mediated organ/tissue transplantation rejection |
| Inhibit initiation and/or progression of autoimmune diseases |
| Immunostimulatory drugs |
| Enhance immune responses against infectious disease (viral, bacterial, fungal) |
| Enhance immune responses against neoplastic cells |
| Stimulate development of immunocompetent cells from bone marrow |
Mechanisms of Action
The role of the immune system is to recognize and remove invading microorganisms and tumor cells, while ignoring host cells, through innate and acquired immune responses. When exposed to an invading organism, nonspecific innate immunity, comprising the first line of defense, is immediately called into action. The antigen-specific responses of acquired immunity also develop in tandem to more effectively remove the organism. When re-exposed to the invading organism, antigen-specific cells of the acquired immune response are called back into action for a more rapid and effective response. This memory and the ability to specifically recognize antigens differentiates acquired from innate immunity. Key differences between innate and acquired immune responses are listed in Table 6-1.
TABLE 6–1 Characteristics of Innate and Acquired Immune Responses
| Characteristic | Innate Immunity | Acquired Immunity |
|---|---|---|
| Onset of action | Immediate | Days to weeks |
| Mechanism of antigen recognition | Receptors that recognize common molecules on microbes and viruses | Unique antigen specific receptors (T-cell receptor, B-cell receptor) |
| Cell types/factors | Macrophages, neutrophils, mast cells, natural killer cells, complement, interferon | Antigen presenting cells, T lymphocytes, B lymphocytes |
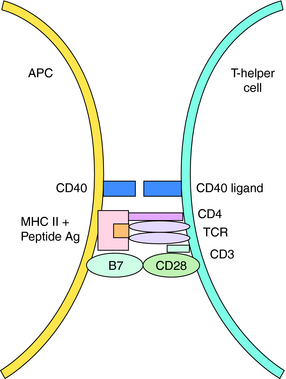
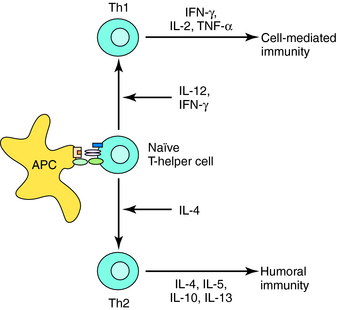
Acquired immunity is commonly divided into humoral and cell-mediated responses. Initiation of acquired immunity initially involves antigen-specific activation of naive T cells (CD4+, T-helper cells). This requires participation of antigen presenting cells (APC) (dendritic cells, macrophages, and B cells) that take up and process antigens into peptide fragments. Peptides bind to major histocompatibility complex (MHC) Class II molecules within the APC and are presented to CD4+ T cells that possess a T-cell receptor specific for the peptide-MHC complex. The naïve T cell requires two signals to be fully activated. The first is provided by the peptide binding to the T-cell receptor, and the second from interaction of costimulatory molecules of the APC and the T cell (Fig. 6-1). Signal One in the absence of Signal Two leads to tolerance, or functional silencing of the T cell. Fully activated T cells proliferate and differentiate into effector T-helper (Th) cells that produce cytokines, such as ILs. In general, there are two types of effector Th cells: Th1 and Th2 cells. The type of cytokines produced by each Th cell determines its function. Cytokines produced by Th1 cells (IFN-γ, IL- 2, tumor necrosis factor [TNF]-α) stimulate generation of cell-mediated immune responses (Fig. 6-2), whereas Th2 cells produce cytokines (IL-4, IL-5, IL-10, IL-13) that drive formation of an antibody response (humoral immunity).
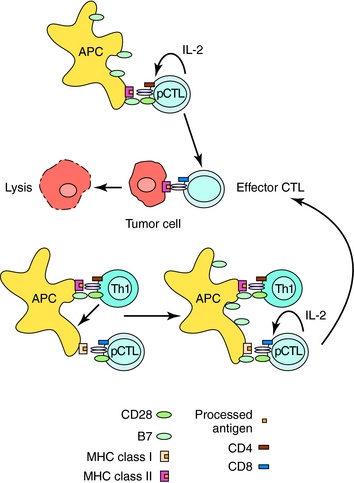
CTLs mediate antigen-specific lysis of tumor cells, virally infected cells, and graft/transplant cells. Generation of CTLs for all three functions generally involves similar mechanisms (Fig. 6-3). Naïve, precursor CTLs (pCTLs) require activation by two signals, as described for Th cells. The first is delivered by binding of peptide antigens associated with MHC Class I molecules on APCs to the T-cell receptor on CD8+ pCTLs. The second is provided by receptor-ligand interaction of costimulatory molecules. Th1 cells produce cytokines that stimulate dendritic cells to up regulate a costimulatory molecule that will activate antigen-stimulated CD8+ cells. Activated CTLs produce IL-2, which stimulates its own proliferation and differentiation. In certain situations, APCs that contain high levels of costimulatory molecules are able to activate CD8+ CTLs without the help of Th1 cells. Antigen recognition and binding of activated CTLs to antigen on cells result in cell lysis.
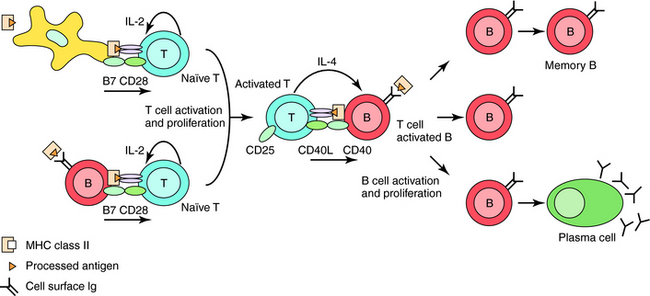
Th2 cells secrete cytokines that stimulate proliferation and differentiation of B cells to antibody-secreting plasma cells or to long-lived memory cells (Fig. 6-4). Specific antibodies can remove harmful foreign antigens (e.g., bacterial toxins) by binding to and neutralizing their effects. Antigen-antibody immune complexes can activate complement to elicit a local inflammatory reaction for further antigen removal by phagocytes. Once bound to foreign protein or bacteria, the Fc region of antibodies can bind to receptors on phagocytic cells, leading to internalization of the invading pathogens.
Pharmacological Immunosuppression
Pharmacological approaches to immunosuppressive therapy may involve selective eradication of immunocompetent cells, similar to the selective killing of tumor cells by antineoplastic drugs (see Chapter 54), or down regulation of the immune response without deleting the target cell. In both cases the goal is to balance the activity and selectivity of the drug to optimize clinical efficacy while preventing adverse effects. The principal drugs used currently to obtain immunosuppression include glucocorticoids, antiproliferative/antimetabolite agents, calcineurin inhibitors, and biologicals. Most of these compounds are highly effective in inhibiting the immune response. However, their usefulness is limited by their severe toxicities. Therefore the different drugs are used in combination at lower doses to obtain a synergistic effect on immune responses while minimizing adverse effects. Immunosuppressive drugs are used primarily to prevent transplant rejection and treat autoimmune diseases.
The actions of the corticosteroids are discussed in Chapter 39. Glucocorticoids are effective in treating autoimmune diseases and preventing graft rejection because of their immunosuppressive and anti-inflammatory effects. When glucocorticoids are administered, the numbers of circulating lymphocytes, basophils, and eosinophils decrease over a 24-hour period, whereas the number of neutrophils increases. In addition, changes in glucocorticoid concentrations during the normal diurnal cycle and in stressful situations correlate with decreases in circulating lymphocytes. Lymphopenia is attributed to migration of cells into extravascular spaces, with more T cells than B cells or monocytes migrating. Most cells migrate to bone marrow. High-dose glucocorticoid therapy is also known to reduce the size of lymphoid organs.
Antiproliferative/Antimetabolite Agents
The structure of cyclophosphamide, its activation to phosphoramide mustard and acrolein, and its antitumor actions are discussed in Chapter 54. The ways in which the active metabolites phosphoramide mustard and acrolein alter the immune response are unclear. The mustard is believed to alkylate DNA and mediate the antiproliferative and immunosuppressive effects. This is consistent with the hypothesis that selective cytotoxic effects on B cells are attributable to a greater proliferation rate. However, the highly reactive, sulfhydryl-binding acrolein may also play an important role in the drug’s action.
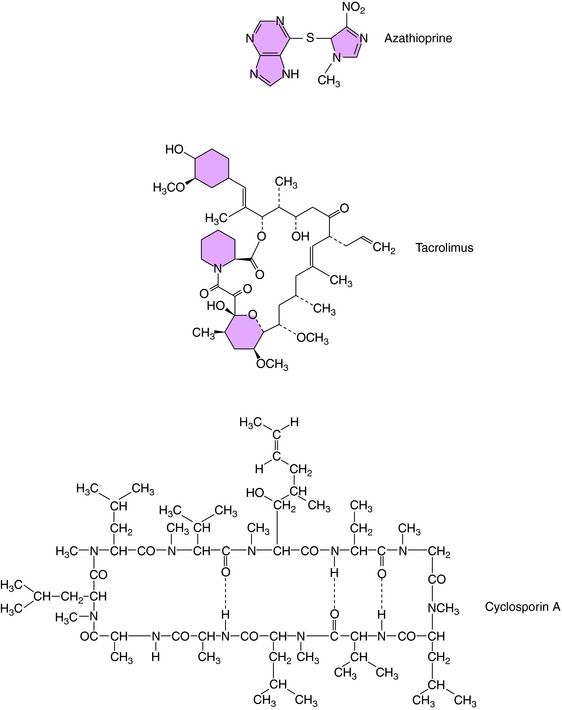
The structure of azathioprine is shown in Figure 6-5. This drug is metabolized to the antiproliferative drug 6-mercaptopurine (see Chapter 54), which is further metabolized to the active antitumor and immunosuppressive thioinosinic acid inhibiting hypoxanthine-guanine phosphoribosyltransferase, which catalyzes the conversion of purines to the corresponding phosphoribosyl-5′ phosphates and the conversion of hypoxanthine to inosinic acid. This leads to the inhibition of cellular proliferation. The immunosuppressive effects of azathioprine stem from its antiproliferative actions.
Methotrexate was originally developed as an anticancer drug (see Chapter 54) but is now being used widely at lower doses in several inflammatory diseases, including rheumatoid arthritis. The immunological and antitumor mechanisms are similar. An antimetabolite, methotrexate binds and inactivates dihydrofolate reductase, leading to inhibition of the synthesis of thymidylate, inosinic acid, and other purine metabolites. Methotrexate also stimulates the release of adenosine, which inhibits stimulated neutrophil function and has potent anti-inflammatory properties.
Calcineurin Inhibitors/Immunophilin Binding Agents
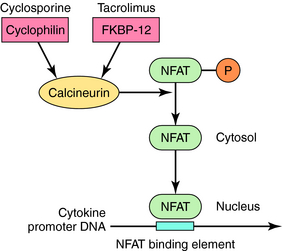
Calcineurin inhibitors down regulate immune responses by inhibiting the production of IL-2 in activated T cells. IL-2 is a key driver of many immune responses and especially important in mediating organ transplant rejection. The two calcineurin inhibitors cyclosporine and tacrolimus bind to cyclophilin and FK binding protein, respectively, and the drug-immunophilin complex binds to calcineurin. This leads to dephosphorylation of nuclear factor for activated T cells (NFAT) and prevention of its translocation to the nucleus, causing down regulation of cytokine transcription (Fig. 6-6).
Cyclosporine is a cyclic endecapeptide purified from fungi (see Fig. 6-5). It primarily affects T-cell–mediated responses, whereas most humoral immune responses not requiring T cells are spared. The effectiveness of cyclosporine stems from its selective inhibition of Th cell activation. Its major effect on Th cells is inhibition of cytokine production. Decreased IL-2 production in turn leads to a decrease in IL-2 receptors and in a lack of responsiveness of CTL precursor cells. Because there is positive feedback through IL-2 production and IL-2 receptors, the decreased IL-2 production of Th cells also leads to decreased IL-2 receptors. Cyclosporine does not, however, affect the proliferative response of activated CTLs to IL-2 or the lytic activity of CTLs. Consistent with this is the observation that cyclosporine is effective only during the very early stages of antigen activation of Th cells. There is also evidence for inhibition of macrophage antigen presentation and IL-1 production by macrophages.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree