Alterations in Musculoskeletal Function
Rheumatic Disorders
Carol L. Danning
Key Questions
• What are the infective organisms associated with joint inflammation and Lyme disease?
• What is the pathogenesis of gouty arthritis?
• How do the three subtypes of juvenile rheumatoid arthritis differ?
![]()
http://evolve.elsevier.com/Copstead/
Arthritis is the most common disabling musculoskeletal condition in the United States. The National Arthritis Foundation estimates that approximately 46 million people have arthritis with the numbers increasing yearly as the population ages. More than 150 defined rheumatologic diseases have been identified. This chapter discusses the more common rheumatologic diseases.1
Local Disorders of Joint Function
Osteoarthritis
Osteoarthritis (degenerative joint disease) is the most common arthritis worldwide. It is a progressive, noninflammatory disease of diarthrodial joints, especially those that bear weight. It is characterized by a progressive loss of articular cartilage and by formation of thick subchondral bone and new bone at the joint margins. Osteoarthritis (OA) becomes more prevalent with increasing age. Individuals older than 70 years have the highest incidence. In postmenopausal women, the knees and hands are most frequently affected by OA.1 It is difficult to estimate the exact prevalence of OA because of difficulties associated with diagnosis, lack of longitudinal data, and problems in defining disease onset.
Etiology and pathogenesis
The etiologic progression of OA varies widely. Development of OA may be related to factors that increase the likelihood of abnormal “wear and tear” on joints such as obesity, joint trauma, and congenital disorders (e.g., hip dysplasia, joint laxity, leg length discrepancy). Other predisposing conditions include lifestyle factors and occupation (stress to joints), genetic predisposition, and hormonal status (postmenopausal).1
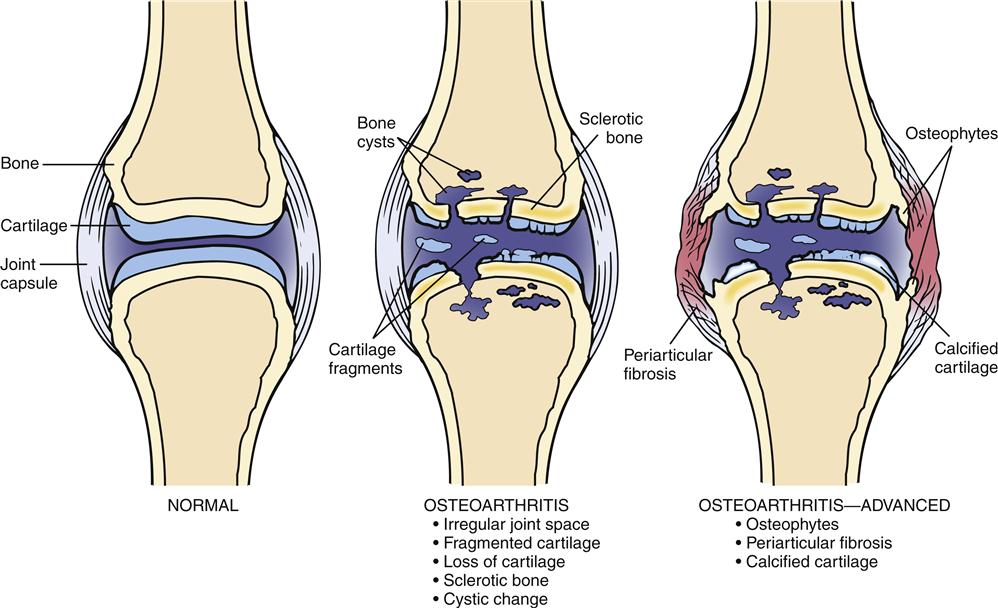
Biomechanical, biochemical, inflammatory, and immunologic factors may all be involved in the development of OA (Figure 52-1). An initial injury causes release of proteolytic and collagenolytic enzymes from chondrocytes. A breakdown of the matrix of proteoglycan and collagen occurs. The decreased hydration of cartilage that occurs with aging can increase the likelihood of wear and damage. Collagen fatigue and microfracture occur with the stress of weight bearing. The ability of the structure to absorb shock is decreased as a result of subcortical bone and cartilage microfractures. Breakdown of joint integrity overloads the capacity for repair, with resultant degenerative changes.
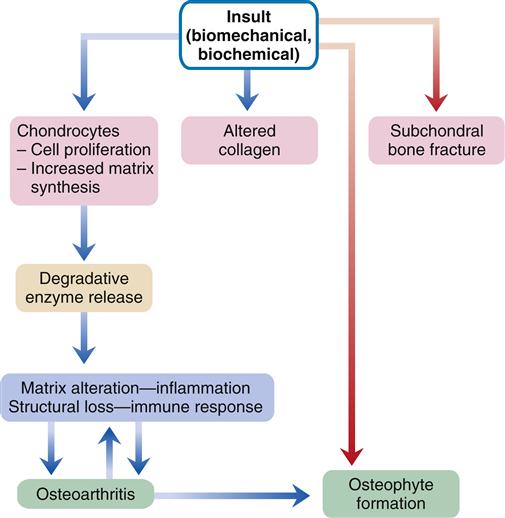
Structural deterioration of the cartilage involves fissuring, pitting, and erosion. Erosion can become so extensive that the articular surface denudes the full thickness of the cartilage. Osteophyte spur formation, subchondral bone sclerosis, and cyst formation are also examples of structural changes present in OA. Cartilage fragments may break off into joints and form “loose bodies” (Figure 52-2). Joint effusions are common in advanced cases. Synovium becomes inflamed and secretes an increased amount of synovial fluid, which causes the joint to distend.
Clinical manifestations
Bony enlargement of joints, crepitus with movement, morning stiffness lasting less than 30 minutes (that improves with joint mobility), and pain with function are typical clinical manifestations of OA. These signs and symptoms are usually local. Although any joint may be affected, weight-bearing joints such as the hips and knees, cervical and lumbosacral joints, and interphalangeal joints are most frequently involved. Degenerative arthritis or OA may occur in an isolated joint, or multiple joints can be involved, especially in the hand. Mechanical dysfunction, anatomic anomalies, or trauma may cause breakdown of the joint surface. It is imperative to establish a differential diagnosis and to eliminate a systemic or medical problem. Although OA is localized, rheumatoid arthritis (RA) is a systemic autoimmune disorder that causes a highly inflammatory, symmetric, peripheral arthritis.
Radiologic abnormalities are normally consistent with clinical symptoms. Classic findings include bony proliferation at the joint margins (i.e., osteophytes or bone spurs), asymmetric narrowing of the joint space, and sclerosis of the subchondral bone. Later, malalignment of the joints and cyst formation in subchondral bone can also be seen. When significant synovial fluid accumulates in the joint, it is usually translucent noninflammatory fluid containing less than 2000 white blood cells per cubic millimeter.1
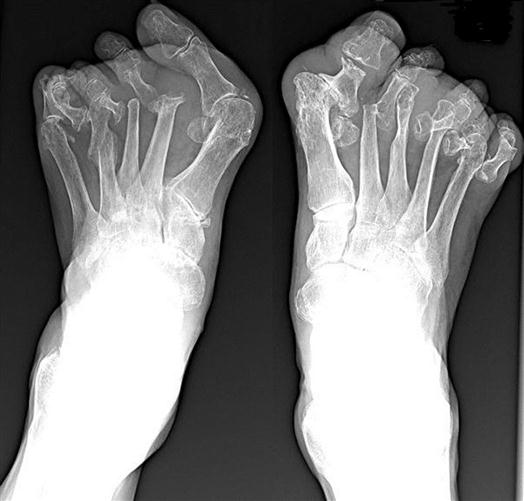
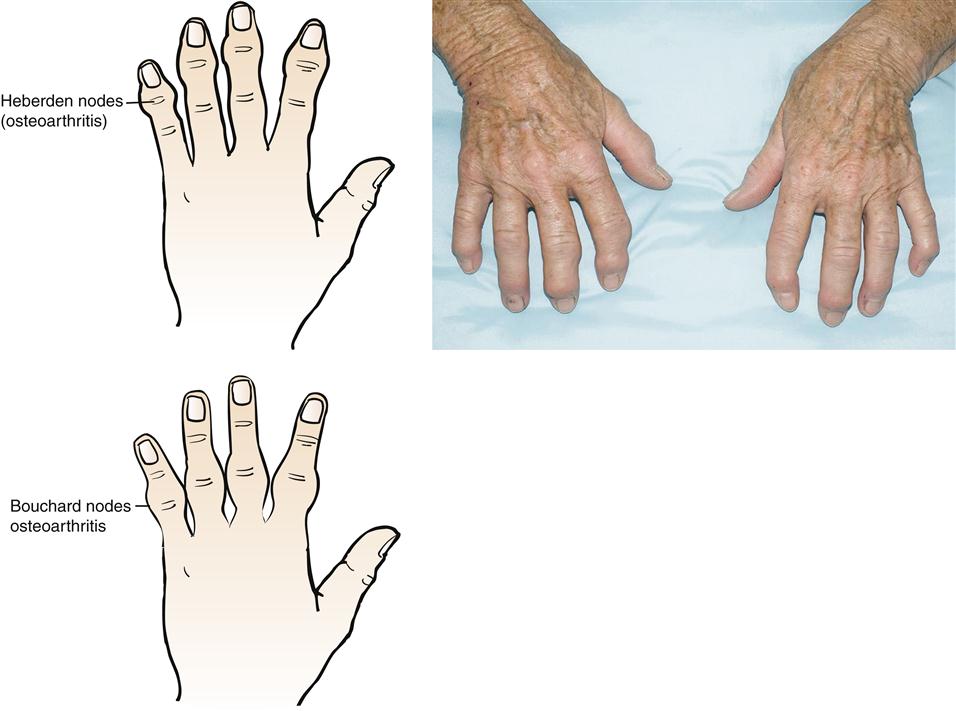
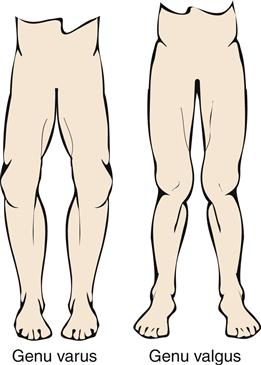
The most common deformity of the hands occurs in the distal interphalangeal (DIP) joints (Figure 52-3). Enlargement is caused by bone spurs (Heberden nodes) that form on the dorsolateral and medial aspects of the joint. Similar enlargements in the proximal interphalangeal (PIP) joints are called Bouchard nodes. The knees and hips are also common locations for OA. Local pain over joint margins, tenderness, crepitus, and muscle atrophy are common findings. Loss of cartilage in medial or lateral compartments of the knee may lead to such structural changes as genu valgus or varus (Figure 52-4).
Pain is relieved by rest during initial stages, though stiffness can be a complaint after prolonged sitting. Because cartilage does not contain nociceptors (pain receptors), pain originates from intraarticular and periarticular structures. Although an acute inflammatory response is often the result of a specific traumatic incident and may cause synovitis in the joint capsule, acute inflammation is not commonly associated with OA. As breakdown in structure progresses, even light activity elicits discomfort, and pain at night is common.
Treatment
Initial treatment is designed to decrease stress on the joint and protect it from additional trauma. Acetaminophen is often the initial analgesic agent recommended for management of mild OA symptoms. Nonsteroidal antiinflammatory drug (NSAID) therapy decreases swelling and pain. Most NSAIDs are nonselective, but antiinflammatory agents that target the cyclooxygenase-2 (COX-2) enzyme have clinical benefit equal to traditional NSAIDs and may have potentially fewer gastrointestinal side effects. The only COX-2 inhibitor presently available in the United States is celecoxib (Celebrex). All NSAIDs (selective or nonselective) have the potential for renal toxicity and possibly even cardiovascular side effects. Visco-supplementation, the intraarticular injection of hyaluronan or its derivatives, may increase joint lubrication, reduce inflammation, and alleviate pain. These agents are currently only available for use in knee OA. Intraarticular corticosteroid injections can provide temporary pain relief, but too frequent usage (i.e., more than three injections per year in the same joint) may accelerate cartilage deterioration.
Physical therapy to improve range of motion, muscle strength, and joint conditioning as well as weight reduction can improve symptoms and prevent loss of function. Assistive devices, such as a cane or walker, afford mechanical relief of weight-bearing stress as can supportive shoe wear. Surgical intervention may be necessary if the joint surface loses enough integrity to prevent joint function. OA is the most common cause for total hip and total knee replacement.
Infectious Arthritis
Infectious or septic arthritis may be defined as an invasion of the synovial membrane by bacteria or another pathogen, leading to a closed-space infection. A reported annual incidence of infection in a joint is 2 to 10 cases per 100,000, with much higher rates noted in patients with comorbid diseases such as rheumatoid arthritis, diabetes, osteoarthritis, chronic kidney disease, and others.2 The pathogen can invade the joint space via a hematogenous route, by extension of an adjacent infection, or from direct inoculation following trauma or an invasive procedure. Infection causes both synovium and cartilage to deteriorate. Joints with underlying disease or inflammation are more susceptible to infection because of increased vascularity and defective barrier effects of the synovial tissue.
Etiology and pathogenesis
The basic cause of bone and cartilage destruction is the interaction of antigenic bacterial cell wall components, the toxic effects of bacteria, the destruction caused by the purulent inflammatory exudate, and the local immune-mediated synovial or cartilage response. If the bacterial infection is not managed, cartilage can be destroyed, and this can lead to ankylosis of the joint.
In most adults and neonates, Staphylococcus aureus is the most common causative organism followed by Streptococcus pyogenes and Streptococcus pneumoniae. Also in neonates, gram-negative bacilli can be cultured, including Kingella kingae, which is an oral flora. In young children, Haemophilius influenzae type B (HIB) was a frequent cause of infection but this agent is now rare since the development of the HIB vaccine.2 Neisseria gonorrhoeae is a causative organism in some adults younger than 30 years of age.
Clinical manifestations
The patient with septic arthritis presents with joint pain, fever, chills, and leukocytosis. Fever may range from mild to high fever with shaking chills. A warm, red, and very swollen joint with limited range of motion attributable to pain is symptomatic of any type of infectious arthritis. Usually only a single joint is involved, but polyarticular joint infections can occur in 10% to 20% of cases, most often in debilitated or immunosuppressed individuals. Synovial fluid analysis reveals a very high white cell count (often >50,000 cells/mm3), and diagnosis is established by recovery of bacteria from synovial fluid. Blood cultures are also used to provide a medical diagnosis.2
Treatment
Treatment of a septic joint should include appropriate antibiotic therapy (often initially intravenous and then oral) with the average duration required being 4 to 6 weeks. This therapy is most effective when the bacteria can be isolated and identified from the synovial fluid and antibiotic sensitivities can be used to determine the most effective antibiotic to be used. In addition, the infected joint usually requires repetitive drainage to facilitate bacterial clearance, decrease pain, and prevent loss of function. This can be accomplished by repeated joint aspiration, arthroscopy with tidal lavage, or open surgical drainage.
Joint prosthesis infection
Any bacteria can lead to infection in a prosthetic joint via the hematogenous route. S. aureus is still common, but Staphylococcus epidermidis can also result in prosthetic joint infection and is rarely seen in a native septic joint. Generally, a prosthetic joint infection requires removal of the prosthesis followed by a rigorous course of intravenous antibiotic therapy, often for 6 weeks or longer. Antibiotic beads may also be placed in the wound. The prosthesis is replaced when cultures from the wound show no growth.2
Systemic Disorders of Joint Function
Immune-Mediated Disorders
Rheumatoid Arthritis
Rheumatoid arthritis is a systemic autoimmune inflammatory disease. In the United States, approximately 1% to 2% of the population is affected with a lower prevalence being reported in Asian countries.1 Women are two to three times more likely to develop RA than men, with a peak incidence in the fourth and fifth decades. Gender difference disappears in older age. RA affects all races, and its prevalence is not affected by climate. RA occurs two to three times more often in women with a familial history of RA.1
Etiology and pathogenesis
The specific cause of RA is unknown. An infectious agent has long been sought as a cause, but reproducible evidence of a particular bacterium or virus has not been found. A more likely theory is that RA is caused by an abnormal autoimmune response (possibly triggered by a bacterial or viral antigen) occurring in individuals who have a genetic predisposition to the disease.
Current research suggests that susceptibility to RA is influenced by the structure of class II major histocompatibility complex (MHC) molecules of antigen-presenting cells. β-Lymphocyte alloantigen human leukocyte antigen DR4 (HLA-DR4) has been noted in 70% of adult Caucasian patients with RA (compared with 30% in control patients), although it is likely that several genes are involved.3 These genes possibly control humoral and cell-mediated immune mechanisms believed to contribute to the pathogenesis of RA. A particular amino acid sequence of the third hypervariable region of DR β chains (called the “shared epitope”) is seen more commonly in RA patients. The cause and type of stimulus of immunologic abnormalities are not known, but they might be due to an infectious agent, environmental influences, or other lifestyle factors (such as tobacco use). An increase in physical and/or psychological stress has also been associated with precipitating acute exacerbation of the disease.
Initially, pathologic changes in RA occur when the immune response localizes in synovial tissue. Here lymphocytes (T and B cells) and macrophages are activated by an unknown antigen trigger. Activated B cells help to perpetuate the escalating inflammatory response by stimulating more lymphocytes and other immune cells. B cells also produce RF antibodies against immunoglobulin G (IgG) as well as anti–cyclic citrullinated peptide (anti-CCP) antibodies. Although immunoglobulins are natural human antibodies, the body produces an antibody (RF) against its own antibody (IgG). Anti-CCP antibodies target peptides modified by converting the amino acid arginine to citrulline.1 Activated lymphocytes, macrophages, and antigen-antibody complexes activate the complement system, stimulate recruitment of other immune cells into the synovium, and produce an extensive array of inflammatory cytokines, metalloproteinases, and other mediators. These products of macrophages and lymphocytes are believed to be critical in RA pathogenesis because they stimulate and perpetuate the inflammation in the joint. Key proinflammatory cytokines demonstrated in the synovium include tumor necrosis factor-α (TNF-α); interleukin-1β (IL-1β); and interleukins 6, 8, 15, 17, 18, and 23, although many more are thought to be involved. Newer biological therapies are designed to target these cytokines as well as activated inflammatory cells and cell costimulatory markers.
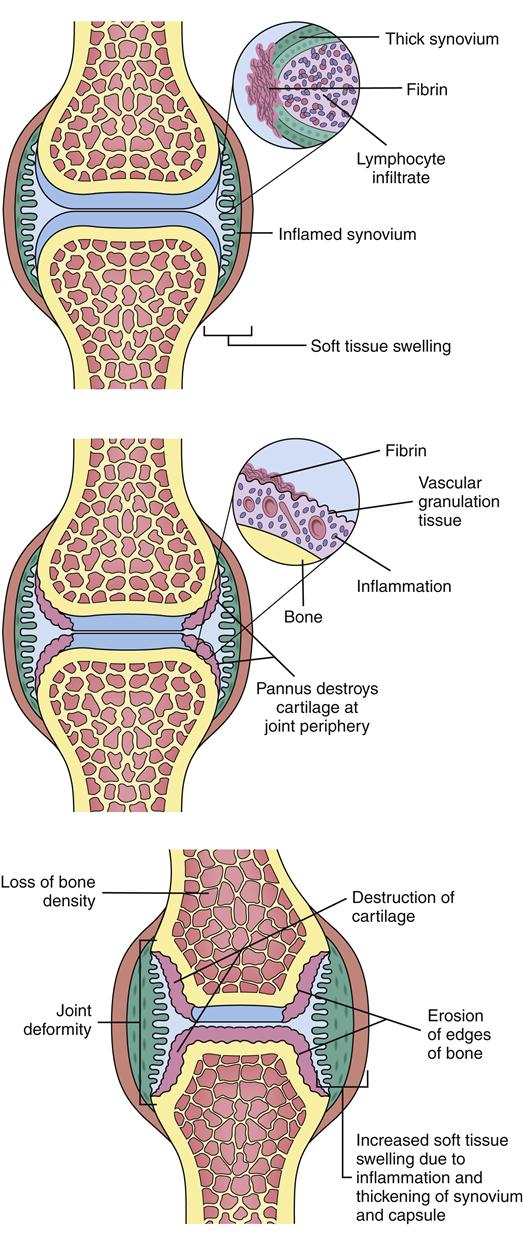
The escalating inflammatory response in the rheumatoid joint leads to accumulation of dense aggregates of immune cells and infiltration of the synovium. The cells produce more cytokines and growth factors, which also stimulate edema, neovascularization, and proliferation of the synovium (which expands in a tumorlike manner). This hypertrophied synovium invades such surrounding tissue as cartilage, ligaments, joint capsules, and tendons. Granulation tissue forms, covering articular cartilage and leading to pannus formation. Pannus is vascularized tissue composed of lymphocytes, macrophages, histiocytes, fibroblasts, and mast cells. Pannus can erode and destroy articular cartilage, resulting in bone erosion, bone cysts, and fissures (Figure 52-5). The expansion and destruction of joint structures can lead to inflammation, shortening, and even rupture of tendons as well as ligament laxity, joint subluxations, contractures, and deformities.1
Clinical manifestations
RA has a wide range of clinical features, but the classic presentation is that of a bilateral symmetric polyarthritis involving smaller joints. Malaise, fatigue, and diffuse musculoskeletal pain are common manifestations during acute exacerbations of the disease. It is interesting to note that symmetric patterns involving the joints of the hands, wrists, elbows, and shoulders are evident. DIP joints are usually spared. This symmetry and the noninvolvement of the DIP joint assist in making the diagnosis of RA.
The hands, wrists, knees, and feet are most commonly involved. In the spine, the upper cervical area is most often affected. However, any diarthrodial joint is potentially at risk. The development of pannus followed by inflammatory destruction of the soft tissue leads to laxity of the ligaments and tendons and results in biomechanical dysfunction. This mechanical stress causes the typical deformities of RA.
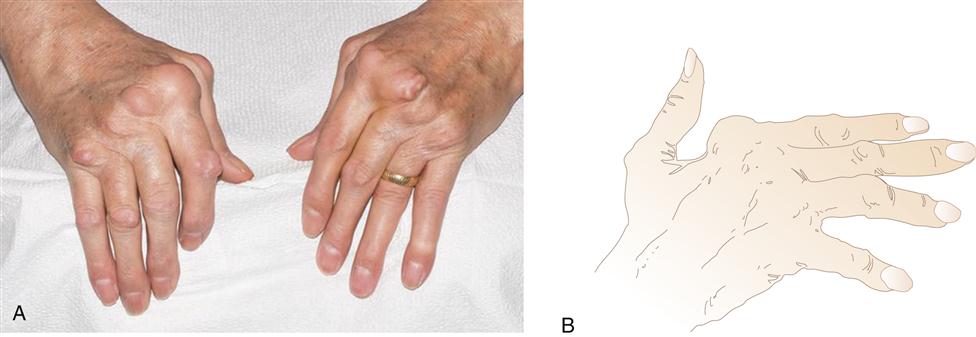
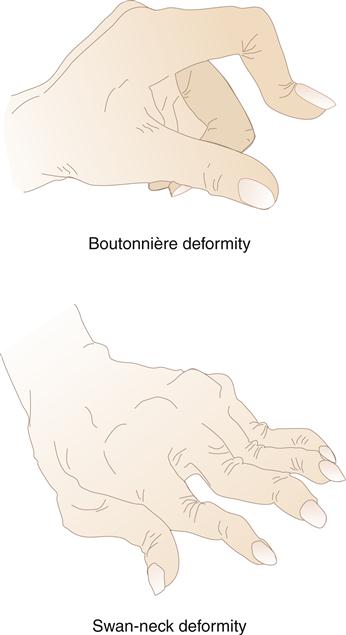
Swelling in the hands is a typical sign of metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joint involvement. Pain is elicited on palpation of the joints. Gradually, progressive synovial damage leads to characteristic ulnar deviation in the MCP joint (Figure 52-6). In advanced situations, a swan-neck deformity develops in the fingers. Swan-neck deformity is a hyperextension of the PIP joint with flexion of the MCP and DIP joints attributable to contractures of intrinsic muscles and tendons.4 A boutonnière deformity, consisting of flexion of the PIP joints and hyperextension of the DIP joints, is also a common dysfunctional position caused by rupture or excess laxity of extensor tendons over the fingers (Figure 52-7). Loss of strength and the ability to achieve a strong pinch is frequently noted in the hand affected by RA. A rupture of tendons and loss of the ability to extend the fingers are common findings in later stages of the disease.
The wrist is commonly involved. The synovium around the wrist becomes boggy and affects the tendon sheaths. Limitation of movement, especially dorsiflexion of the wrist, is often noted. Proliferation of the synovium on the volar or palmar aspect of the wrist may cause compression of the median nerve and development of carpal tunnel syndrome. Flexion contractures and swelling of the elbow are other common manifestations; in later stages of the disease, shoulder involvement may occur. Typical signs of shoulder involvement are limitations of movement and pain on palpation in the area of the coracoid process. Dislocation, subluxation, or rupture of the joint capsule may occur as the disease progresses.
Involvement of the upper cervical vertebrae is another common finding. Destruction of structures of the atlantoaxial vertebrae (such as the transverse ligament) creates the potential for subluxation of this joint and endangerment of spinal cord compression. Laxity in the cervical region may also allow compression of the vertebral artery, leading to vertebrobasilar insufficiency. Limitation of motion (especially rotation), pain on palpation, and headache in the occipital region are common.
Abnormalities in gait and limitations of movement are signs noted when RA affects the hip. Groin pain attributable to capsular involvement may be present. If synovitis of the hip becomes extensive, severe pain may be noted on evaluation. RA involvement in the knee is often extensive. Effusion, quadriceps atrophy, contractures, and synovitis of the semimembranous bursa (Baker cyst) may be observed. Destruction of the articular surface, bone, and soft tissue may result from joint instability. One common clinical sign of involvement of the foot is retrocalcaneal bursitis. Other features of foot involvement include swelling of joints, a cocking-up of the toes attributable to subluxation of the metatarsal heads (claw toes), and lateral deviation of the first through fourth toes.
These clinical manifestations may develop rapidly or progress over many years. Usually symptoms develop over weeks and months. Initially, the patient may feel fatigued or chronically tired and may complain of systemic aching in the musculoskeletal system. Specific joint pain, tenderness, swelling, redness, and nodules are quite common.
Prolonged inactivity, such as sitting, initiates complaints of stiffness and swelling. As the disease progresses, walking, climbing stairs, opening jars or doors, and precise movement of the digits become quite difficult. Weight loss, depression, and a low-grade fever often are noted in these patients.
Unlike OA, RA is a systemic autoimmune disease. It is imperative that a definitive differential diagnosis be developed. RA may be confused with a number of disease entities such as Lyme disease, systemic lupus erythematosus (SLE), gout, and other inflammatory conditions. RA may also cause subcutaneous nodules and be associated with cardiac, pulmonary, and ophthalmologic manifestations.
Cardiac manifestations may include pericarditis, myocarditis, mitral valve disease, and conduction system disease or complete heart block. Pulmonary manifestations may occur as pleuritis, pulmonary fibrosis, pleural effusions, or pulmonary nodules. Ophthalmic manifestations might include episcleritis, scleritis, or secondary Sjögren syndrome (dry eyes and mouth).
A positive RF is found in the sera of approximately 75% to 85% of patients with RA. The titer of the RF does not fluctuate with disease activity and is not essential for the diagnosis of RA. Antibodies against cyclic citrullinated peptide (anti-CCP antibody) can be found with a sensitivity of 70% to 80% and a high specificity of 90% to 95% for RA. The presence of both RF and anti-CCP antibodies in a patient may signify a risk of more aggressive disease. Inflammatory markers (sedimentation rate, C-reactive protein) are often elevated. Other laboratory features may include hypergammaglobulinemia, thrombocytosis, and hypochromic microcytic anemia.1
Radiography may demonstrate structural damage caused by RA. Typical findings include erosions on the bony margins of joints, joint space narrowing, periarticular osteopenia, and eventual malalignment and subluxation of the bones (Figure 52-8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree