Alterations in Metabolism and Nutrition
Daniel J. Guerra and Carrie W. Miller
Key Questions
• How do chronic and acute physiologic stress affect body metabolism?
• What are some useful biochemical diagnostic tests for malnutrition?
![]()
http://evolve.elsevier.com/Copstead/
Throughout its life span, the human body is differentially adjusted and maintained by a complex and connected network of biochemical reactions embedded in pathways that are involved in both energy balance and the interconversion of metabolites to meet ever-changing physiologic needs. This process, known as metabolism, uses energy to sustain the body’s vital functions.1 Metabolism utilizes the favorable thermodynamics of biochemical reactions to carry out the physiologic processes of the body in a systematic and tissue-specific manner. These interrelated and dynamic reactions meet the unique requirements of each independent cellular activity.2 To fuel this system, adequate supplies of digestible foodstuffs with the appropriate organic chemical structure must be acquired on a regular basis or malnutrition and eventual starvation will result. Adequate nutrition is needed for growth and metabolism, organ function, tissue repair, and response to infection. Excess nutrition without a compensatory energy expenditure leads to many diseases and may also make the body more susceptible to pathogens.
Many hospitalized patients enter the care center with a significant degree of physiologic stress and sometimes multiple organ dysfunctions, both of which can alter essential and general nutritional needs. Treatment modalities, altered intake, and restricted mobility may also require nutritional adjustment. In many cases, these problems can be averted, or at least addressed appropriately, if nutritional assessment and therapy are started early.3,4 This chapter reviews normal nutrient metabolism and nutritional assessment and then progresses to a discussion of physiologic and metabolic dysfunctions.
It is clear that many diseases are fundamentally metabolic, even those typically associated with biological clock mechanisms5 or ascribed to endocrine hormone dysfunction, such as insulin resistance in metabolic syndrome.6 Although not discussed here, heritable diseases are almost exclusively inborn errors of metabolism (see Chapter 6).
Metabolic Processes
Metabolism is the dynamic phenomenon in biological systems involving the physical and chemical processes that produce and maintain (anabolic), and also transform (catabolic), molecules into energy and waste products. Metabolism may be functionally examined along interconnecting biochemical pathways, which fall into three discrete cellular programs:
Nutrition and disease can impact all metabolic programs but the precise nature of this response must be carefully determined using the methods of biomedical screening and interpretation of results. Underlying these medical practices are the basic experimental sciences of biochemistry, genetics, cell physiology, pathology, and microbiology.
The key to metabolism is the control or regulation of the concentrations of metabolites in given cell types such that the system is poised to deal with nutritional and signaling phenomena. Cells must be receptive to membrane-associated signaling so that lipids (or glucose or amino acids) can be transferred or a suite of “messenger” molecules can be recruited and secreted by the activated cell. At the same time, the cell must be in an energetically favorable state; this requires both production and utilization of energy, usually in the form of adenosine triphosphate (ATP), although many nucleotides take part in this energy transfer (e.g., cyclic AMP [cAMP], guanosine triphosphate [GTP], reduced nicotinamide adenine dinucleotide phosphate [NAD(P)H], flavin adenine dinucleotide [FAD]).
The reactions leading to the assimilation of organic molecules are organized into metabolic pathways. A pathway can be considered a sequential vectorial transfer of substrate to product where the individual catalytic events are carried out by specific enzymatic reactions. The following is a brief account of how metabolic pathways are organized.
Pathways are functionally separated and regulated:
• Catabolism and degradative metabolism. Dietary foods are combusted or oxidized to the final end products CO2 and H2O. The electrons from these reactions are largely driven to molecular oxygen, and the production of ATP is coupled to this reduction of oxygen (see Chapter 3). Some of the electrons driven off during the oxidation of carbon are used to make NADPH that in turn provides reducing power for biosynthesis. Glycolysis and the TCA cycle are the major metabolic routes, as well as fatty acid β oxidation, amino acid catabolism to ketogenic (TCA) or glucogenic products, purine degradation to uric acid, and pyrimidine degradation to β-alanine, NH3, and CO2. Along these degradation pathways, intermediates for biosynthesis are formed and used (e.g., oxidative pentose phosphate pathway, flavin nucleotides).
Anabolism and Catabolism
Anabolism refers to the constructive phase of metabolism and involves the synthesis of organic molecules by cells. More complex or larger molecules are built from simple ones, and in the process energy is consumed. Anabolism occurs continuously along with catabolism, but is more prominent during times of rest, healing, pregnancy, lactation, and growth. Hormonal secretions such as insulin and sex hormones may also trigger anabolism. Obesity, with the accumulation of adipose tissue via net synthesis of lipids such as triacylglycerol, is a form of anabolism.7 Conversely, catabolism is the degradative phase of metabolism. Complex molecules are broken down into simpler substances, often with the concurrent release or production of chemical energy. During times of disease, stress, fever, or starvation or during the release of certain hormones such as thyroid hormone and cortisol, catabolism dominates the body’s metabolic processes. The resultant tissue wasting may lead to cellular injury or death if excessive catabolism is left unregulated.8 Anabolism and catabolism occur simultaneously and together create the dynamic, homeostatic balance of chemical substance and energy known as metabolism. In humans, both processes are most efficient in the presence of molecular oxygen although it is not required in active muscle. In some disease states such as cancer, oxygen may be present but is not used in the metabolism found in the tumor (anaerobic glycolysis, Warburg effect),9 although this has recently been challenged and may not be universal in breast cancer.10
The metabolic process requires nutrients in the form of carbohydrates, lipids, and proteins. Each of these three nutrients is altered or broken down into simpler substances. Lipids may be directly sequestered into adipose cells after limited lipolysis and synthesis into lipoproteins for transport. Enzymes and coenzymes mediate the metabolism of glucose, fatty acids, amino acids, and nucleic acids. In this fashion, the body’s continual cellular energy requirements are met.8 Energy produced by metabolic processes is used to create the energy currency of the body known as ATP (see Chapter 3).8
Cells use energy to perform essential physiologic and biochemical processes. Energy can be measured in kilocalories (kcal); 1 kcal represents the amount of energy required to raise the temperature of 1 kg of water from 15° to 16° C. For comparison, a medium-sized baked potato without butter is about 200 kcal whereas a glass of wine is about 100 kcal and a lean filet mignon (8 oz, 227 g) is about 400 kcal.
During catabolism of fuel molecules, approximately 40% of the available energy ultimately is converted to ATP or NADPH (nicotinamide adenine dinucleotide phosphate, reduced form), with the remaining 60% producing heat.2 The energy released as heat is important for maintaining body temperature.
Metabolic Rate
Several factors determine the body’s energy requirements or metabolic needs, including basal metabolic rate (BMR), growth, stress, activity level, and energy necessary for digestion.8
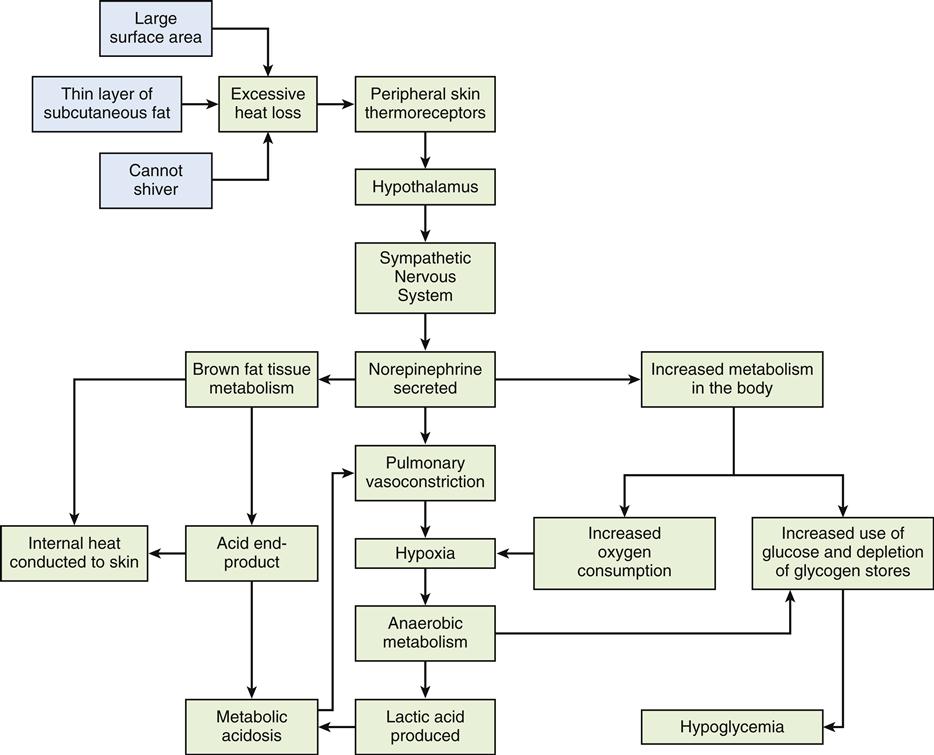
The basal metabolic rate refers to the rate of energy consumption by resting tissue. It is a measurement of the energy used in maintenance of the body at rest after a 12-hour fast.8 It represents the energy used in maintaining basic body processes such as respiration, cellular metabolism, circulation, glandular activity, and the maintenance of body temperature.8 The body’s BMR is determined by calculating oxygen use during a specific period. The normal range for BMR is generally between 0.8 and 1.43 kcal/min.8 Several factors that affect an individual’s BMR are described in Table 42-1. Body stature and size affect BMR by the amount of heat lost from the body surface. BMR also is an important determinant of total daily energy expenditure (TEE).5 Age is also an important determinant of BMR. A growing child’s BMR is significantly higher than an adult’s, primarily because of an increased rate of cellular activity, surface-to-volume ratio, and generation of new tissue.8 Conversely, as one ages, the BMR gradually declines by about 2% per decade. Body composition, determined by the amount of fat and lean tissue, also affects BMR. Muscle tissue requires more oxygen than adipose tissue, which explains why athletes have an approximately 5% higher BMR than nonathletes.2 One contribution to this observation among athletes is the enhanced β oxidation of fatty acids in this group.11 Women typically have a metabolic rate 5% to 10% less than that of men, probably because of differences in body mass. Women also tend to have more adipose tissue than men, and fat is less metabolically active than muscle.7 Pregnancy increases the BMR by about 20% to 28%, or 300 kcal/day, as a consequence of increased uterine and mammary gland size, fetal development, and additional cardiopulmonary workload.8 Other factors affecting BMR include nutritional status, muscle tone, sleep, fever, environmental temperature, and stress.2 Metabolic thermoregulation in newborns is described in the Pediatrics Consideration box.
TABLE 42-1
EXAMPLES OF FACTORS AFFECTING BASAL METABOLIC RATE
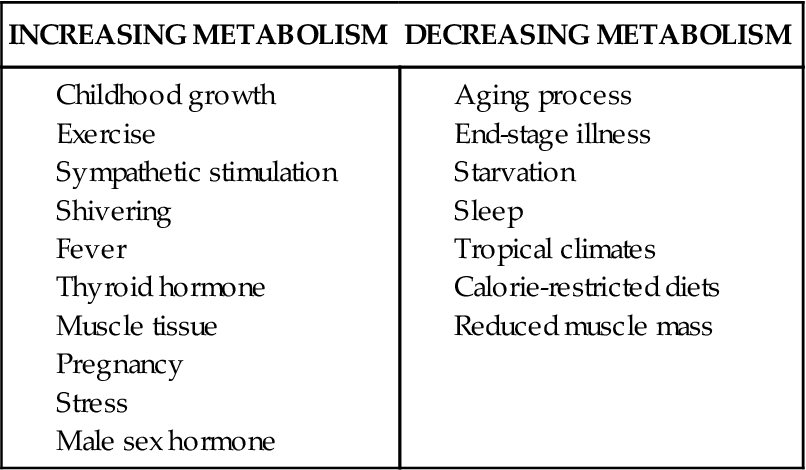
Almost any alteration in the body’s normal homeostatic state will alter its energy requirements and BMR. Many diseases are known to dramatically increase the body’s energy requirements, including chronic obstructive and restrictive pulmonary disease, hyperthermia, burns, cancer, diabetes, and Graves disease (hyperthyroidism).
Nutrient Metabolism
Metabolism is dependent upon energy balance, which is defined as the relationship between nutritional intake and expenditure. In general, metabolism is controlled by both the nervous system and the endocrine system. There are several hormones secreted from these systems that are triggered by the nutritional and energy status of the body, which may become rapidly altered during times of acute stress or chronic disease. Four major hormones involved in nutrient metabolism are insulin, glucagon, catecholamines, and cortisol. The effects of these four hormones on carbohydrate, fat, and protein metabolism are summarized
in Tables 42-2 to 42-4. Both the nervous system and the endocrine system directly affect metabolism by the release of the catecholamines epinephrine and norepinephrine, which during times of stress inhibit insulin activity. The pancreatic hormones insulin and glucagon have a crucial role in the metabolic processes that govern the body’s energy requirements. These hormones function in diametric opposition, with insulin lowering blood glucose levels and glucagon ultimately increasing blood glucose levels.2 Each of these hormones is controlled by another hierarchy of regulation. For example, growth hormone (GH) affects metabolism by decreasing cellular uptake and use of glucose. High levels of GH tend to decrease the affinity for insulin at the receptor site such that even increased secretion of insulin by the pancreas has diminutive effects on blood glucose levels. GH, in part, follows a circadian rhythm via stimulation by ghrelin, a gut peptide hormone.12 Ghrelin concentration is highest after several hours of fasting. Thus, after an extended period of fasting (as during normal sleep) ghrelin is released into the circulation, where it ultimately travels to the hypothalamus and triggers GH release. GH then travels to the periphery (e.g., muscle), where it stimulates lactic acid production for transport to the liver for gluconeogenesis. The release of ghrelin actually starts by catecholamine binding to adrenergic receptors in the gut.13
TABLE 42-2
HORMONAL ACTIONS ON CARBOHYDRATE METABOLISM
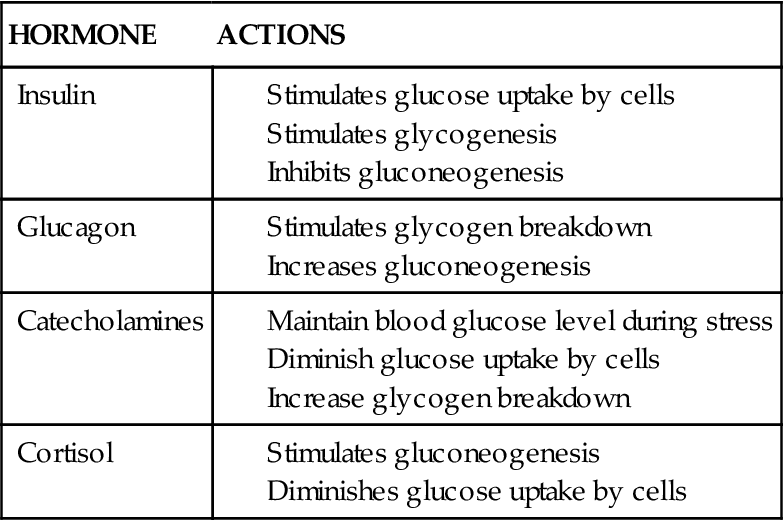
Glucocorticoid hormones, primarily cortisol, stimulate gluconeogenesis by the liver. Blood glucose levels 6 to 10 times normal may occur with significant cortisol secretion. Left uncorrected, diabetes mellitus type 2 may develop (see Chapter 41).7 Metabolic syndrome, which is well correlated to obesity, may be a precondition for type 2 diabetes (see Metabolic Syndrome section).
Carbohydrates
Carbohydrates are the main energy source for the body. Approximately 45% to 65% of the recommended diet should consist of carbohydrates.14 Dietary carbohydrates are starches or sugars. Carbohydrates are classified into the three categories of monosaccharides (simple sugars), oligosaccharides (2 to 10 joined monosaccharide units), and polysaccharides (10 to 10,000 monosaccharide units). They range from very simple sugars consisting of three to seven carbons to incredibly complex polymers made up of repeating units of thousands of monosaccharides.8
Dietary monosaccharides are the six-carbon sugars of glucose, mannose, fructose, and galactose. Glucose, the most physiologically important of the group, is the form of sugar normally found in the bloodstream. Glucose is derived from the catabolism of more complex carbohydrates during the process of digestion. Fructose and galactose are also eventually converted to glucose by the liver, although fructose can directly enter the metabolism via phosphorylation.15 Once in the bloodstream, glucose is transported throughout the body where (depending on the location) it is either oxidized to provide cellular energy and reducing power, metabolized to fatty acids, utilized in amino acid and nucleotide metabolism, or stored in the liver and muscles as glycogen. Blood sugar levels then reflect the difference between the amount of glucose released into the bloodstream by the liver and the amount of glucose taken up by the cells for energy.7
Intracellular Glucose Metabolism
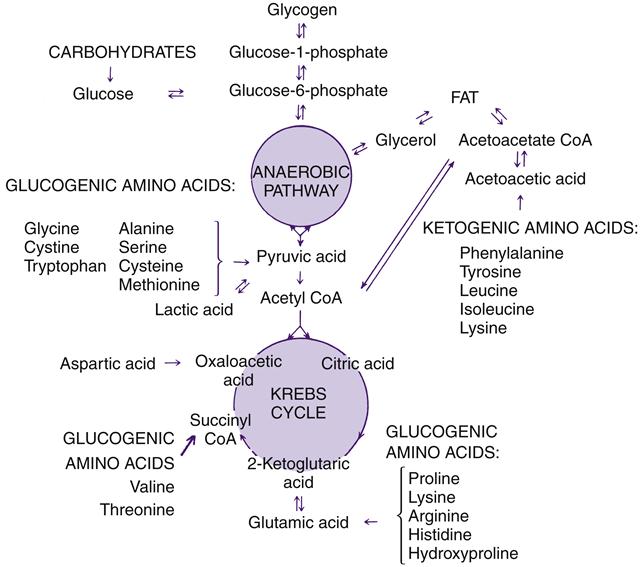
Once in the cell, glucose undergoes various biochemical transformations including a form of anaerobic oxidation called glycolysis (see Chapter 3). Glycolysis is the metabolic sequence that converts glucose to pyruvate and, depending on several variables including oxygen availability and energy needs, eventually yields the end products of carbon dioxide and water.8 Catabolism of glucose may occur anaerobically along the Embden-Meyerhof pathway (glycolysis), which is a 10-step process altering the chemical composition of glucose to pyruvic acid and results in a net gain of two ATP molecules for each molecule of glucose that enters the pathway. Pyruvic acid has two important roles in the catabolic process of carbohydrates. It provides the body with acetyl coenzyme A (acetyl CoA), which is required for conversion to fatty acids or to energy, and it is the initial step for the second stage of carbohydrate metabolism, the tricarboxylic acid (TCA) cycle (also called the citric acid cycle or the Krebs cycle) and oxidative phosphorylation. The TCA cycle occurs in the mitochondrial matrix. Oxidative phosphorylation (see Chapter 3) produces a total of about 30 molecules of ATP for each molecule of glucose. Although other pathways exist, the interrelated glycolytic, TCA cycle, and respiratory chain enzymes can produce nearly all of the energy required for cellular functioning.8 Figure 42-1 illustrates the metabolism of carbohydrate, fat, and amino acids through the anaerobic glycolytic pathway and the TCA cycle. The TCA cycle and the respiratory chain require molecular oxygen (aerobic) to function.
Depending on needs, glucose is catabolized for the production of energy, synthesized into lipid, stored as polymers of glycogen, or resynthesized. Gluconeogenesis refers to the process by which glucose is formed from non-carbohydrate sources, including amino acids or lactic acid supplied by muscle tissue and glycerol supplied from fat breakdown.14 The glucose made through this mechanism may be either stored in the liver as glycogen or released into the bloodstream. During periods of fasting, gluconeogenesis and glycogenolysis provide the necessary glucose to meet the metabolic requirements of the brain and other glucose-dependent tissues.7
Hormonal Control of Glucose Metabolism
Many hormones affect glucose levels by altering carbohydrate metabolism. The only hormone known to lower blood glucose levels is insulin. Hormones that tend to raise blood glucose levels include glucagon, growth hormone, glucocorticoid hormones, epinephrine and norepinephrine, and thyroid hormone. Table 42-2 describes the major hormonal effects on glucose metabolism.
Insulin, a peptide hormone formed from its precursor pre-proinsulin and synthesized by β cells in the pancreas, is secreted in response to increased blood glucose levels. Minutes after ingestion of a meal, insulin levels in the blood rise significantly, peak in 30 minutes, and plateau in about 3 hours. Between meals, when blood glucose levels tend to drop, insulin levels also remain low. At that time, glucose and amino acid stores are used for cellular energy requirements.7
Insulin directly affects glucose metabolism by promoting glucose uptake by the liver, which then favors the synthesis of glycogen. Glucose formation (gluconeogenesis) and the breakdown of glycogen to form glucose (glycogenolysis) are inhibited by insulin. The transport of glucose across cellular membranes into muscle and adipose tissue is facilitated by insulin and has a direct lowering effect on blood glucose levels.16
Glucagon is a peptide hormone secreted by α cells in the pancreas and also by some cells lining the gastrointestinal tract. Acting in a manner opposite that of insulin, glucagon increases blood glucose levels.17 As blood glucose levels begin to drop, plasma glucagon levels begin to rise. Therefore, the two primary effects of glucagon are to promote the breakdown of liver glycogen with subsequent release of glucose into the bloodstream and to promote liver gluconeogenesis. These actions tend to bring serum glucose levels back to normal. Conversely, as glucose levels rise, glucagon secretion is diminished and serum glucose levels drop toward normal. The diametric actions of insulin and glucagon partially explain why increased glucagon secretion may also have a role in the elevated blood glucose levels seen in people with diabetes mellitus.2
Catecholamines (i.e., epinephrine and norepinephrine) are derived from the amino acid tyrosine and serve a role in carbohydrate metabolism to maintain blood glucose levels during times of stress. As the stress response occurs, catecholamines stimulate the conversion of glycogen to glucose in the muscles and liver. Although muscles, unlike the liver, cannot release glucose into the general circulation, mobilization of muscle glycogen makes unused blood glucose available for other tissues such as the brain and the peripheral nervous system. The second primary action of epinephrine during the stress response is to stimulate glucagon secretion and prevent insulin release from the pancreas, thereby preventing glucose movement into muscle cells. Epinephrine also promotes glycogenolysis by the liver and muscles and reduces glucose uptake by muscle tissue. As mentioned previously, catecholamines also control ghrelin signaling. The role of catecholamines in glucose metabolism is very similar to that of glucagon and opposite that of insulin.2
Cortisol is derived from cholesterol and is the primary glucocorticoid hormone secreted from the adrenal cortex. Cortisol is an insulin antagonist and helps to maintain serum glucose levels. During fasting, cortisol permissively enables other hormonal changes to occur, such as decreased insulin production and increased glucagon and epinephrine secretion. The end result is promotion of gluconeogenesis and lipolysis. If cortisol deficiency occurs simultaneously with fasting, hypoglycemic reactions significant enough to alter brain functioning can occur. A recent study indicated that cortisol deficiency may be a significant cause of morbidity and mortality in critically ill surgical patients, who frequently are poorly nourished.18
Growth hormone has a role in carbohydrate metabolism, although it may be indirect in comparison with its role in growth regulation and protein anabolism; however, it can have a significant impact on glucose regulation under certain circumstances. Growth hormone’s effects parallel those of cortisol: growth hormone increases gluconeogenesis in the liver and inhibits glucose uptake by muscle cells.2 Growth hormone disinhibits gene expression in the liver in favor of transcription of phosphoenolpyruvate (PEP) carboxykinase, a key enzyme in hepatic gluconeogenesis.12 Elevated serum growth hormone levels tend to increase blood glucose levels. As a result, the insulin-secreting β cells in the pancreas are stimulated. If this process is not corrected, the β cells will eventually be exhausted. It is for this reason that diabetes mellitus eventually develops in individuals with excessive growth hormone, as in acromegaly.7
Thyroid hormone tends to raise blood glucose levels. In carbohydrate metabolism, the primary mode of action is to increase glucose absorption from the intestines and stimulate the release of epinephrine. Thyroid hormone also promotes the rate of insulin destruction. Ultimately, thyroid hormone causes an increase in cellular oxygen consumption and the basal metabolic rate of tissues. An alteration in thyroid hormone signaling because of activating mutations of its receptor causes a reduction in body weight and decreased amounts of adipose tissue.19
Lipids
Lipids or fats, the most concentrated form of energy, are derived de novo and from animal fats and vegetable oils. Fats supply 9 kcal of energy per gram, as compared with 4 kcal from glucose and 4 kcal from protein. Fats are 98% triacylglycerol (TAG). Like carbohydrates, fats are made up of carbon, hydrogen, and oxygen. The bulk of each TAG molecule in humans consists of fatty acids containing 12 to 22 carbon atoms. Fatty acids are categorized as saturated or unsaturated. The degree of hydrogen saturation refers to the number of double bonds between the carbon atoms in the chain. If a fatty acid chain contains all the hydrogen atoms possible with no double bonds, it is called a saturated fatty acid. Those fatty acids with one double bond are termed monounsaturated, and those with several double bonds are called polyunsaturated.8
Fats in the form of TAG supply approximately two thirds of the cell’s total energy requirements. Whereas the human body is able to economically store approximately 140,000 kcal of usable fats in adipose tissue, it can store only 24,000 kcal of protein and a mere 800 kcal of carbohydrate in an adult male.17 Carbohydrates and amino acids not immediately used by the tissues are converted to fat and stored, along with ingested fat, as adipose tissue. Fat deposits are extremely important in the economical use of metabolites. If intake of calories exceeds expenditure, obesity develops over time. During times of fasting, the body quickly reverts to the breakdown and use of fats as its energy source.18 All tissues in the body, with the exception of brain cells, can metabolize and use fatty acids as an energy source as effectively as glucose.17
Almost all fats are absorbed into the lymph system from the intestinal mucosa. They are then converted to a chylomicron consisting of 80% triglyceride, 9% cholesterol, 7% phospholipid, and 4% lipoprotein coat.20 Chylomicrons empty into the venous blood at the thoracic duct and are carried to the liver for metabolism or assimilated into adipose tissue. Once in the liver, TAG is stored and eventually mobilized via lipoprotein secretion. TAG can also be hydrolyzed to glycerol and free fatty acid in a process known as lipolysis. When released into the circulation, the fatty acids, bound to albumin, are quickly assimilated into tissue. Oxidation in tissue begins when coenzyme A forms a thioester bond with the free carbonyl of the fatty acid. Progressing through a series of reactions known as β oxidation, the fatty acid chain is shortened by two carbon units until all is converted to acetyl CoA. During this process the reduced form of flavin adenine dinucleotide (FADH2) and the reduced form of nicotinamide adenine dinucleotide (NADH) are formed, which can be used by the electron-transport chain to make ATP. Acetyl CoA can be used to make ketone bodies or it may enter the TCA cycle and oxidative phosphorylation, with each 2-carbon segment producing 2 molecules of carbon dioxide and 12 molecules of ATP. During prolonged fasting, the ketone bodies can traverse the blood-brain barrier and provide energy to the brain (see following paragraph). The average fatty acid contains approximately 18 carbon atoms, with 146 ATP molecules being produced during catabolism.2 Unlike fatty acids, glycerol (the other component of triacylglycerol) can be further metabolized (primarily in the liver and in adipose and muscle tissue).21,22 Free glycerol is generally carried to the liver, where it can be used to form glucose or recycled to generate new triglycerides.
As mentioned, within the liver, fatty acids are generally transformed to acetyl CoA, which is further processed into one of three compounds collectively known as ketone bodies. These are acetoacetate, β-hydroxybutyrate, and acetone. Once released into the bloodstream, ketone bodies have a critical role as an energy source for tissues able to oxidize them in the Krebs cycle. During the fasting state, tissues use ketone bodies as a primary energy source, with glucose reserved for brain metabolism. If the fasting state continues, many areas of the brain begin to use ketone bodies as an energy source. As the brain begins to use ketone bodies, less protein is broken down to provide glucose. For this reason, the body is able to withstand periods of fasting with minimal protein breakdown and associated lean body mass degeneration.2 However, excessive acute ketogenesis is harmful and produces a condition known as ketoacidosis, which is prevalent in type 1 diabetes (Chapter 41).
The liver is the major organ responsible for lipid metabolism and regulation of serum lipid levels. The five primary functions of the hepatic system in regard to lipid metabolism are: (1) synthesis of triacylglycerol from carbohydrates and protein, (2) synthesis of phospholipids and cholesterol from dietary TAG, (3) desaturation and elongation of fatty acids, (4) utilization of TAG as an energy source, and (5) transport of lipids to the periphery, especially to adipose tissue.8 Liver disease can significantly alter any of these processes and cause serious metabolic dysfunction. A fatty liver is characterized by fat deposits in the liver cells caused either by ingestion of hepatotoxic substances such as alcohol or halocarbons or by consumption of diets significantly low in protein for a prolonged period (see Chapter 38). Infections managed with protein synthesis–inhibiting antibiotics, such as tetracycline, and malignancies may also lead to increased fat deposits within the liver by adversely affecting the hepatic cells or biliary tract. Increased mobilization of fatty acids from adipose tissue to the liver occurs in certain conditions, such as metabolic syndrome, diabetes mellitus, starvation, and obesity, where lipogenesis exceeds the ability of the liver to export the fat as lipoproteins.23 Metabolic studies of critically ill patients indicate that fatty acid breakdown occurs at a much higher rate than required by patient caloric needs. This excess lipolysis may cause fatty liver.24
Hormonal Control of Lipid Metabolism
Carbohydrates can be metabolized along the anaerobic glycolytic pathway and this process can lead to mitochondrial production of citrate (via acetyl CoA and oxaloacetic acid [OAA]; both produced from pyruvate), which may reenter the cytosol for lipid synthesis. Therefore, hormones that affect carbohydrate metabolism also affect lipid metabolism. Table 42-3 describes the hormones considered to have the greatest effect on lipid metabolism. These hormones include insulin, thyroid hormone, glucocorticoids, mineralocorticoids, growth hormone, epinephrine, and norepinephrine. Insulin prevents fat utilization by indirectly causing fatty acids to be taken up by adipose tissue and by decreasing the activity of hormone-sensitive lipase, which promotes the movement of fat out of adipose tissue. Glucocorticoids increase fat cell membrane permeability, whereas mineralocorticoids and glucagon increase the activity of hormone-sensitive lipase. Epinephrine and norepinephrine increase fat mobilization by stimulating the activity of hormone-sensitive lipase, thus increasing the serum free fatty acid level. Growth hormone increases fatty acid mobilization and use by tissues as an energy source.8
TABLE 42-3
HORMONAL ACTIONS ON LIPID METABOLISM
| HORMONE | ACTIONS |
| Insulin | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|