33
Agents Used in Cytopenias; Hematopoietic Growth Factors
CASE STUDY
A 65-year-old woman with a long-standing history of poorly controlled type 2 diabetes mellitus presents with increasing numbness and paresthesias in her extremities, generalized weakness, a sore tongue, and gastrointestinal discomfort. Physical examination reveals a frail-looking, pale woman with diminished vibration sensation, diminished spinal reflexes, and a positive Babinski sign. Examination of her oral cavity reveals atrophic glossitis, in which the tongue appears deep red in color and abnormally smooth and shiny due to atrophy of the lingual papillae. Laboratory testing reveals a macrocytic anemia based on a hematocrit of 30% (normal for women, 37–48%), a hemoglobin concentration of 9.4 g/dL (normal for elderly women, 11.7–13.8 g/dL), an erythrocyte mean cell volume (MCV) of 123 fL (normal, 84–99 fL), an erythrocyte mean cell hemoglobin concentration (MCHC) of 34% (normal, 31–36%), and a low reticulocyte count. Further laboratory testing reveals a normal serum folate concentration and a serum vitamin B12 (cobalamin) concentration of 98 pg/mL (normal, 250–1100 pg/mL). Results of a Schilling test indicate a diagnosis of pernicious anemia. Once megaloblastic anemia was identified, why was it important to measure serum concentrations of both folic acid and cobalamin? Should this patient be treated with oral or parenteral vitamin B12?
Hematopoiesis, the production from undifferentiated stem cells of circulating erythrocytes, platelets, and leukocytes, is a remarkable process that produces over 200 billion new blood cells per day in the normal person and even greater numbers of cells in people with conditions that cause loss or destruction of blood cells. The hematopoietic machinery resides primarily in the bone marrow in adults and requires a constant supply of three essential nutrients—iron, vitamin B12, and folic acid—as well as the presence of hematopoietic growth factors, proteins that regulate the proliferation and differentiation of hematopoietic cells. Inadequate supplies of either the essential nutrients or the growth factors result in deficiency of functional blood cells. Anemia, a deficiency in oxygen-carrying erythrocytes, is the most common and several forms are easily treated. Sickle cell anemia, a condition resulting from a genetic alteration in the hemoglobin molecule, is common but is not easily treated. It is discussed in the Box: Sickle Cell Disease and Hydroxyurea. Thrombocytopenia and neutropenia are not rare, and some forms are amenable to drug therapy. In this chapter, we first consider treatment of anemia due to deficiency of iron, vitamin B12, or folic acid and then turn to the medical use of hematopoietic growth factors to combat anemia, thrombocytopenia, and neutropenia, and to support stem cell transplantation.
 AGENTS USED IN ANEMIAS
AGENTS USED IN ANEMIAS
IRON
Basic Pharmacology
Iron deficiency is the most common cause of chronic anemia. Like other forms of chronic anemia, iron deficiency anemia leads to pallor, fatigue, dizziness, exertional dyspnea, and other generalized symptoms of tissue hypoxia. The cardiovascular adaptations to chronic anemia—tachycardia, increased cardiac output, vasodilation—can worsen the condition of patients with underlying cardiovascular disease.
Iron forms the nucleus of the iron-porphyrin heme ring, which together with globin chains forms hemoglobin. Hemoglobin reversibly binds oxygen and provides the critical mechanism for oxygen delivery from the lungs to other tissues. In the absence of adequate iron, small erythrocytes with insufficient hemoglobin are formed, giving rise to microcytic hypochromic anemia. Iron-containing heme is also an essential component of myoglobin, cytochromes, and other proteins with diverse biologic functions.
Pharmacokinetics
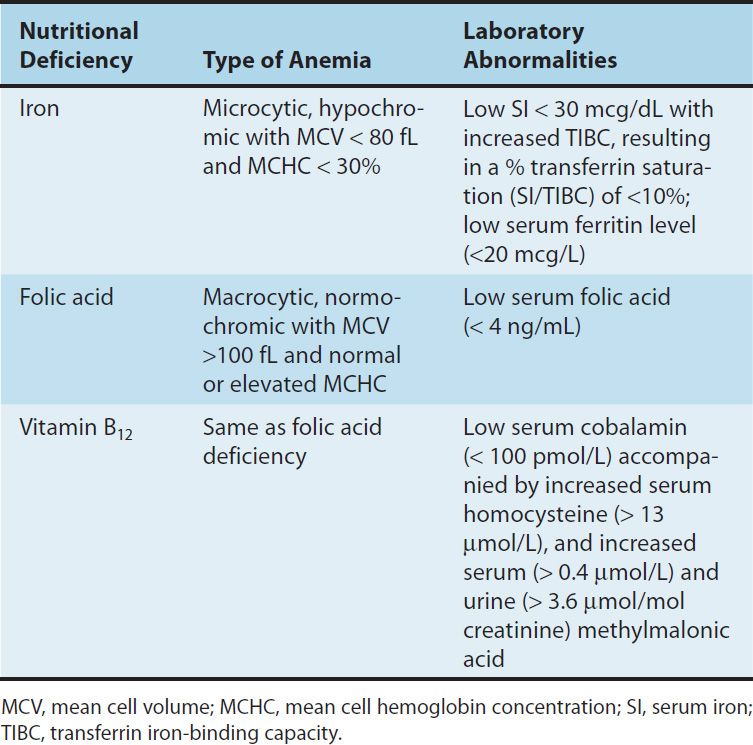
Free inorganic iron is extremely toxic, but iron is required for essential proteins such as hemoglobin; therefore, evolution has provided an elaborate system for regulating iron absorption, transport, and storage (Figure 33–1). The system uses specialized transport, storage, ferrireductase, and ferroxidase proteins whose concentrations are controlled by the body’s demand for hemoglobin synthesis and adequate iron stores (Table 33–1). A peptide called hepcidin, produced primarily by liver cells, serves as a key central regulator of the system. Nearly all of the iron used to support hematopoiesis is reclaimed from catalysis of the hemoglobin in senescent or damaged erythrocytes. Normally, only a small amount of iron is lost from the body each day, so dietary requirements are small and easily fulfilled by the iron available in a wide variety of foods. However, in special populations with either increased iron requirements (eg, growing children, pregnant women) or increased losses of iron (eg, menstruating women), iron requirements can exceed normal dietary supplies, and iron deficiency can develop.
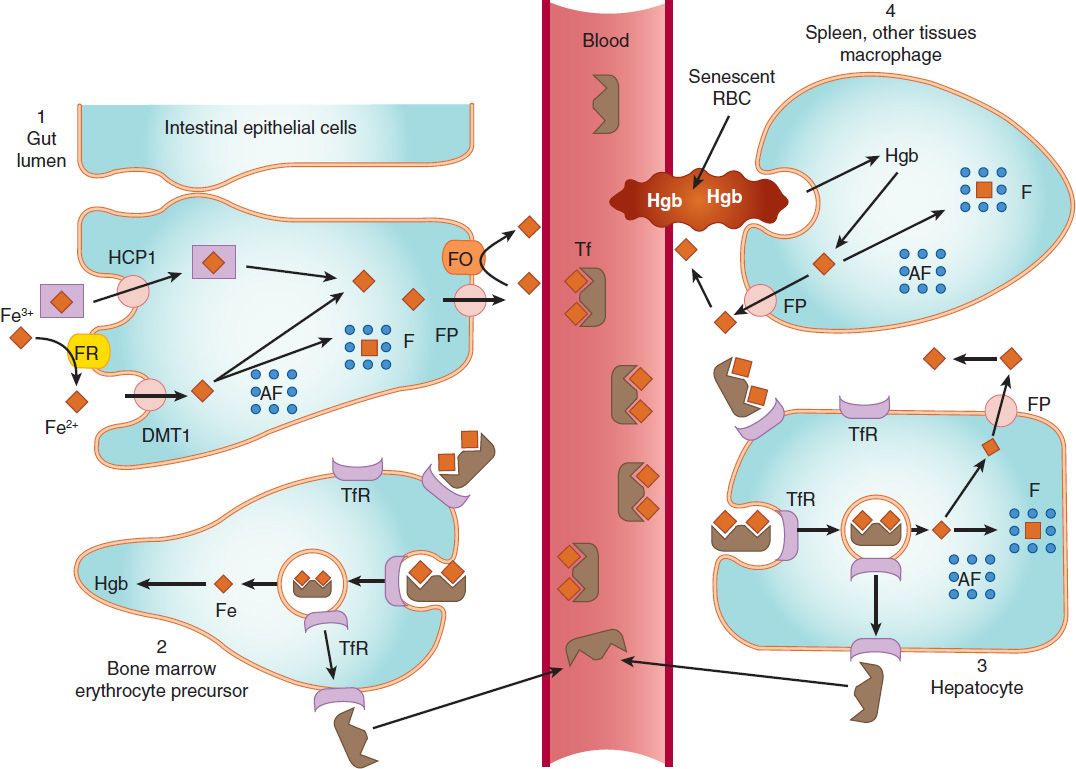
FIGURE 33–1 Absorption, transport, and storage of iron. Intestinal epithelial cells actively absorb inorganic iron via the divalent metal transporter 1 (DMT1) and heme iron via the heme carrier protein 1 (HCP1). Iron that is absorbed or released from absorbed heme iron in the intestine (1) is actively transported into the blood by ferroportin (FP) or complexed with apoferritin (AF) and stored as ferritin (F). In the blood, iron is transported by transferrin (Tf) to erythroid precursors in the bone marrow for synthesis of hemoglobin (Hgb) (2) or to hepatocytes for storage as ferritin (3). The transferrin-iron complex binds to transferrin receptors (TfR) in erythroid precursors and hepatocytes and is internalized. After release of iron, the TfR-Tf complex is recycled to the plasma membrane and Tf is released. Macrophages that phagocytize senescent erythrocytes (RBC) reclaim the iron from the RBC hemoglobin and either export it or store it as ferritin (4). Hepatocytes use several mechanisms to take up iron and store the iron as ferritin. FO, ferroxidase. (Reproduced, with permission, from Trevor A et al: Pharmacology Examination & Board Review, 9th ed. McGraw-Hill, 2010. Copyright © The McGraw-Hill Companies, Inc.)
TABLE 33–1 Iron distribution in normal adults.1
Sickle Cell Disease and Hydroxyurea
Sickle cell disease is an important genetic cause of hemolytic anemia, a form of anemia due to increased erythrocyte destruction, instead of the reduced mature erythrocyte production seen with iron, folic acid, and vitamin B12 deficiency. Patients with sickle cell disease are homozygous for the aberrant β-hemoglobin S (HbS) allele (substitution of valine for glutamic acid at amino acid 6 of β-globin) or heterozygous for HbS and a second mutated β-hemoglobin gene such as hemoglobin C (HbC) or β-thalassemia. Sickle cell disease has an increased prevalence in individuals of African descent because the heterozygous trait confers resistance to malaria.
In the majority of patients with sickle cell disease, anemia is not the major problem; the anemia is generally well compensated even though such individuals have a chronically low hematocrit (20–30%), a low serum hemoglobin level (7–10 g/dL), and an elevated reticulocyte count. Instead, the primary problem is that deoxygenated HbS chains form polymeric structures that dramatically change erythrocyte shape, reduce deformability, and elicit membrane permeability changes that further promote hemoglobin polymerization. Abnormal erythrocytes aggregate in the microvasculature—where oxygen tension is low and hemoglobin is deoxygenated—and cause veno-occlusive damage. In the musculoskeletal system, this results in characteristic, extremely severe bone and joint pain. In the cerebral vascular system, it causes ischemic stroke. Damage to the spleen increases the risk of infection, particularly by encapsulated bacteria such as Streptococcus pneumoniae. In the pulmonary system, there is an increased risk of infection and, in adults, an increase in embolism and pulmonary hypertension. Supportive treatment includes analgesics, antibiotics, pneumococcal vaccination, and blood transfusions. In addition, the cancer chemotherapeutic drug hydroxyurea (hydroxycarbamide) reduces veno-occlusive events. It is approved in the United States for treatment of adults with recurrent sickle cell crises and approved in Europe in adults and children with recurrent vaso-occlusive events. As an anticancer drug used in the treatment of chronic and acute myelogenous leukemia, hydroxyurea inhibits ribonucleotide reductase and thereby depletes deoxynucleoside triphosphate and arrests cells in the S phase of the cell cycle (see Chapter 54). In the treatment of sickle cell disease, hydroxyurea acts through poorly defined pathways to increase the production of fetal hemoglobin γ (HbF), which interferes with the polymerization of HbS. Clinical trials have shown that hydroxyurea decreases painful crises in adults and children with severe sickle cell disease. Its adverse effects include hematopoietic depression, gastrointestinal effects, and teratogenicity in pregnant women.
A. Absorption
The average American diet contains 10–15 mg of elemental iron daily. A normal individual absorbs 5–10% of this iron, or about 0.5–1 mg daily. Iron is absorbed in the duodenum and proximal jejunum, although the more distal small intestine can absorb iron if necessary. Iron absorption increases in response to low iron stores or increased iron requirements. Total iron absorption increases to 1–2 mg/d in menstruating women and may be as high as 3–4 mg/d in pregnant women.
Iron is available in a wide variety of foods but is especially abundant in meat. The iron in meat protein can be efficiently absorbed, because heme iron in meat hemoglobin and myoglobin can be absorbed intact without first having to be dissociated into elemental iron (Figure 33–1). Iron in other foods, especially vegetables and grains, is often tightly bound to organic compounds and is much less available for absorption. Nonheme iron in foods and iron in inorganic iron salts and complexes must be reduced by a ferrireductase to ferrous iron (Fe2+) before it can be absorbed by intestinal mucosal cells.
Iron crosses the luminal membrane of the intestinal mucosal cell by two mechanisms: active transport of ferrous iron by the divalent metal transporter DMT1, and absorption of iron complexed with heme (Figure 33–1). Together with iron split from absorbed heme, the newly absorbed iron can be actively transported into the blood across the basolateral membrane by a transporter known as ferroportin and oxidized to ferric iron (Fe3+) by the ferroxidase hephaestin. The liver-derived hepcidin inhibits intestinal cell iron release by binding to ferroportin and triggering its internalization and destruction. Excess iron is stored in intestinal epithelial cells as ferritin, a water-soluble complex consisting of a core of ferric hydroxide covered by a shell of a specialized storage protein called apoferritin.
B. Transport
Iron is transported in the plasma bound to transferrin, a β-globulin that can bind two molecules of ferric iron (Figure 33–1). The transferrin-iron complex enters maturing erythroid cells by a specific receptor mechanism. Transferrin receptors—integral membrane glycoproteins present in large numbers on proliferating erythroid cells—bind and internalize the transferrin-iron complex through the process of receptor-mediated endocytosis. In endosomes, the ferric iron is released, reduced to ferrous iron, and transported by DMT1 into the cytoplasm, where it is funneled into hemoglobin synthesis or stored as ferritin. The transferrin-transferrin receptor complex is recycled to the cell membrane, where the transferrin dissociates and returns to the plasma. This process provides an efficient mechanism for supplying the iron required by developing red blood cells.
Increased erythropoiesis is associated with an increase in the number of transferrin receptors on developing erythroid cells and a reduction in hepatic hepcidin release. Iron store depletion and iron deficiency anemia are associated with an increased concentration of serum transferrin.
C. Storage
In addition to the storage of iron in intestinal mucosal cells, iron is also stored, primarily as ferritin, in macrophages in the liver, spleen, and bone, and in parenchymal liver cells (Figure 33–1). The mobilization of iron from macrophages and hepatocytes is primarily controlled by hepcidin regulation of ferroportin activity. Low hepcidin concentrations result in iron release from these storage sites; high hepcidin concentrations inhibit iron release. Ferritin is detectable in serum. Since the ferritin present in serum is in equilibrium with storage ferritin in reticuloendothelial tissues, the serum ferritin level can be used to estimate total body iron stores.
D. Elimination
There is no mechanism for excretion of iron. Small amounts are lost in the feces by exfoliation of intestinal mucosal cells, and trace amounts are excreted in bile, urine, and sweat. These losses account for no more than 1 mg of iron per day. Because the body’s ability to excrete iron is so limited, regulation of iron balance must be achieved by changing intestinal absorption and storage of iron in response to the body’s needs. As noted below, impaired regulation of iron absorption leads to serious pathology.
Clinical Pharmacology
A. Indications for the Use of Iron
The only clinical indication for the use of iron preparations is the treatment or prevention of iron deficiency anemia. This manifests as a hypochromic, microcytic anemia in which the erythrocyte mean cell volume (MCV) and the mean cell hemoglobin concentration are low (Table 33–2). Iron deficiency is commonly seen in populations with increased iron requirements. These include infants, especially premature infants; children during rapid growth periods; pregnant and lactating women; and patients with chronic kidney disease who lose erythrocytes at a relatively high rate during hemodialysis and also form them at a high rate as a result of treatment with the erythrocyte growth factor erythropoietin (see below). Inadequate iron absorption can also cause iron deficiency. This is seen after gastrectomy and in patients with severe small bowel disease that results in generalized malabsorption.
TABLE 33–2 Distinguishing features of the nutritional anemias.
The most common cause of iron deficiency in adults is blood loss. Menstruating women lose about 30 mg of iron with each menstrual period; women with heavy menstrual bleeding may lose much more. Thus, many premenopausal women have low iron stores or even iron deficiency. In men and postmenopausal women, the most common site of blood loss is the gastrointestinal tract. Patients with unexplained iron deficiency anemia should be evaluated for occult gastrointestinal bleeding.
B. Treatment
Iron deficiency anemia is treated with oral or parenteral iron preparations. Oral iron corrects the anemia just as rapidly and completely as parenteral iron in most cases if iron absorption from the gastrointestinal tract is normal. An exception is the high requirement for iron of patients with advanced chronic kidney disease who are undergoing hemodialysis and treatment with erythropoietin; for these patients, parenteral iron administration is preferred.
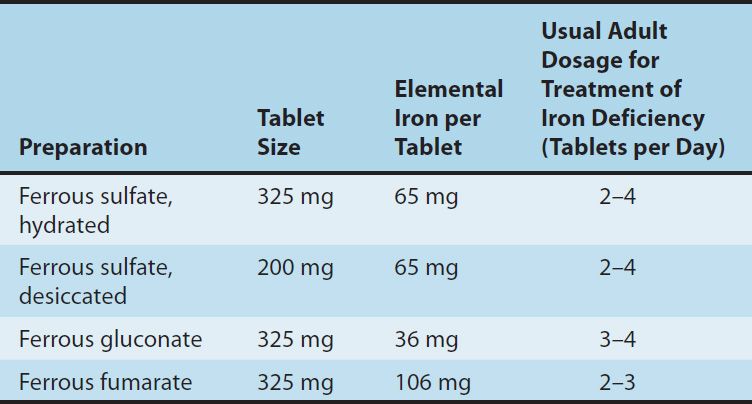
1. Oral iron therapy—A wide variety of oral iron preparations is available. Because ferrous iron is most efficiently absorbed, ferrous salts should be used. Ferrous sulfate, ferrous gluconate, and ferrous fumarate are all effective and inexpensive and are recommended for the treatment of most patients.
Different iron salts provide different amounts of elemental iron, as shown in Table 33–3. In an iron-deficient individual, about 50–100 mg of iron can be incorporated into hemoglobin daily, and about 25% of oral iron given as ferrous salt can be absorbed. Therefore, 200–400 mg of elemental iron should be given daily to correct iron deficiency most rapidly. Patients unable to tolerate such large doses of iron can be given lower daily doses of iron, which results in slower but still complete correction of iron deficiency. Treatment with oral iron should be continued for 3–6 months after correction of the cause of the iron loss. This corrects the anemia and replenishes iron stores.
TABLE 33–3 Some commonly used oral iron preparations.
Common adverse effects of oral iron therapy include nausea, epigastric discomfort, abdominal cramps, constipation, and diarrhea. These effects are usually dose-related and can often be overcome by lowering the daily dose of iron or by taking the tablets immediately after or with meals. Some patients have less severe gastrointestinal adverse effects with one iron salt than another and benefit from changing preparations. Patients taking oral iron develop black stools; this has no clinical significance in itself but may obscure the diagnosis of continued gastrointestinal blood loss.
2. Parenteral iron therapy—Parenteral therapy should be reserved for patients with documented iron deficiency who are unable to tolerate or absorb oral iron and for patients with extensive chronic anemia who cannot be maintained with oral iron alone. This includes patients with advanced chronic renal disease requiring hemodialysis and treatment with erythropoietin, various postgastrectomy conditions and previous small bowel resection, inflammatory bowel disease involving the proximal small bowel, and malabsorption syndromes.
The challenge with parenteral iron therapy is that parenteral administration of inorganic free ferric iron produces serious dose-dependent toxicity, which severely limits the dose that can be administered. However, when the ferric iron is formulated as a colloid containing particles with a core of iron oxyhydroxide surrounded by a core of carbohydrate, bioactive iron is released slowly from the stable colloid particles. In the United States, the three traditional forms of parenteral iron are iron dextran, sodium ferric gluconate complex, and iron sucrose. Two newer preparations are available (see below).
Iron dextran is a stable complex of ferric oxyhydroxide and dextran polymers containing 50 mg of elemental iron per milliliter of solution. It can be given by deep intramuscular injection or by intravenous infusion, although the intravenous route is used most commonly. Intravenous administration eliminates the local pain and tissue staining that often occur with the intramuscular route and allows delivery of the entire dose of iron necessary to correct the iron deficiency at one time. Adverse effects of intravenous iron dextran therapy include headache, light-headedness, fever, arthralgias, nausea and vomiting, back pain, flushing, urticaria, bronchospasm, and, rarely, anaphylaxis and death. Owing to the risk of a hypersensitivity reaction, a small test dose of iron dextran should always be given before full intramuscular or intravenous doses are given. Patients with a strong history of allergy and patients who have previously received parenteral iron dextran are more likely to have hypersensitivity reactions after treatment with parenteral iron dextran. The iron dextran formulations used clinically are distinguishable as high-molecular-weight and low-molecular-weight forms. In the United States, the InFeD preparation is a low-molecular-weight form while DexFerrum is a high-molecular-weight form. Clinical data—primarily from observational studies—indicate that the risk of anaphylaxis is largely associated with high-molecular-weight formulations.
Sodium ferric gluconate complex and iron-sucrose complex are alternative parenteral iron preparations. Ferric carboxymaltose is a colloidal iron preparation embedded within a carbohydrate polymer. Ferumoxytol is a superparamagnetic iron oxide nanoparticle coated with carbohydrate. The carbohydrate shell is removed in the reticuloendothelial system, allowing the iron to be stored as ferritin, or released to transferrin. Ferumoxytol may interfere with magnetic resonance imaging (MRI) studies. Thus if imaging is needed, MRI should be performed prior to ferumoxytol therapy or alternative imaging modality used if needed soon after dosing.
These agents can be given only by the intravenous route. They appear to be less likely than high-molecular-weight iron dextran to cause hypersensitivity reactions.
For patients treated chronically with parenteral iron, it is important to monitor iron storage levels to avoid the serious toxicity associated with iron overload. Unlike oral iron therapy, which is subject to the regulatory mechanism provided by the intestinal uptake system, parenteral administration—which bypasses this regulatory system—can deliver more iron than can be safely stored. Iron stores can be estimated on the basis of serum concentrations of ferritin and the transferrin saturation, which is the ratio of the total serum iron concentration to the total iron-binding capacity (TIBC).
Clinical Toxicity
A. Acute Iron Toxicity
Acute iron toxicity is seen almost exclusively in young children who accidentally ingest iron tablets. As few as 10 tablets of any of the commonly available oral iron preparations can be lethal in young children. Adult patients taking oral iron preparations should be instructed to store tablets in child-proof containers out of the reach of children. Children who are poisoned with oral iron experience necrotizing gastroenteritis with vomiting, abdominal pain, and bloody diarrhea followed by shock, lethargy, and dyspnea. Subsequently, improvement is often noted, but this may be followed by severe metabolic acidosis, coma, and death. Urgent treatment is necessary. Whole bowel irrigation (see Chapter 58) should be performed to flush out unabsorbed pills. Deferoxamine, a potent iron-chelating compound, can be given intravenously to bind iron that has already been absorbed and to promote its excretion in urine and feces. Activated charcoal, a highly effective adsorbent for most toxins, does not bind iron and thus is ineffective. Appropriate supportive therapy for gastrointestinal bleeding, metabolic acidosis, and shock must also be provided.
B. Chronic Iron Toxicity
Chronic iron toxicity (iron overload), also known as hemochromatosis, results when excess iron is deposited in the heart, liver, pancreas, and other organs. It can lead to organ failure and death. It most commonly occurs in patients with inherited hemochromatosis, a disorder characterized by excessive iron absorption, and in patients who receive many red cell transfusions over a long period of time (eg, individuals with β-thalassemia).
Chronic iron overload in the absence of anemia is most efficiently treated by intermittent phlebotomy. One unit of blood can be removed every week or so until all of the excess iron is removed. Iron chelation therapy using parenteral deferoxamine or the oral iron chelator deferasirox (see Chapter 57) is less efficient as well as more complicated, expensive, and hazardous, but it may be the only option for iron overload that cannot be managed by phlebotomy, as is the case for many individuals with inherited and acquired causes of refractory anemia such as thalassemia major, sickle cell anemia, aplastic anemia, etc.
VITAMIN B12
Vitamin B12 (cobalamin) serves as a cofactor for several essential biochemical reactions in humans. Deficiency of vitamin B12 leads to megaloblastic anemia (Table 33–2), gastrointestinal symptoms, and neurologic abnormalities. Although deficiency of vitamin B12 due to an inadequate supply in the diet is unusual, deficiency of B12 in adults—especially older adults—due to inadequate absorption of dietary vitamin B12 is a relatively common and easily treated disorder.
Chemistry
Vitamin B12 consists of a porphyrin-like ring with a central cobalt atom attached to a nucleotide. Various organic groups may be covalently bound to the cobalt atom, forming different cobalamins. Deoxyadenosylcobalamin and methylcobalamin are the active forms of the vitamin in humans. Cyanocobalamin and hydroxocobalamin (both available for therapeutic use) and other cobalamins found in food sources are converted to the active forms. The ultimate source of vitamin B12 is from microbial synthesis; the vitamin is not synthesized by animals or plants. The chief dietary source of vitamin B12 is microbially derived vitamin B12 in meat (especially liver), eggs, and dairy products. Vitamin B12 is sometimes called extrinsic factor to differentiate it from intrinsic factor, a protein secreted by the stomach that is required for gastrointestinal uptake of dietary vitamin B12.
Pharmacokinetics
The average American diet contains 5–30 mcg of vitamin B12 daily, 1–5 mcg of which is usually absorbed. The vitamin is avidly stored, primarily in the liver, with an average adult having a total vitamin B12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


