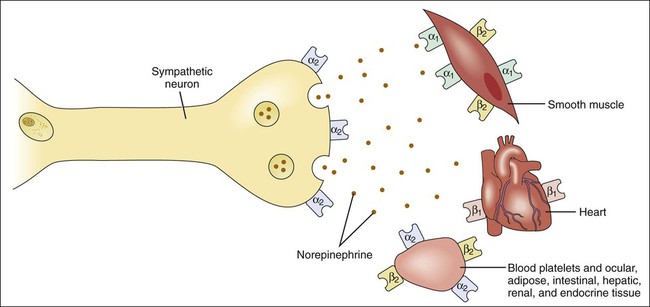
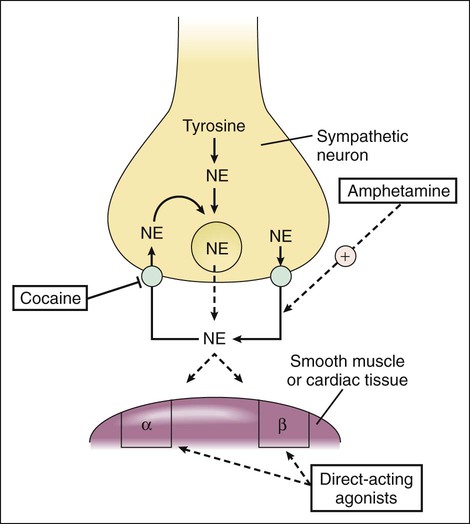
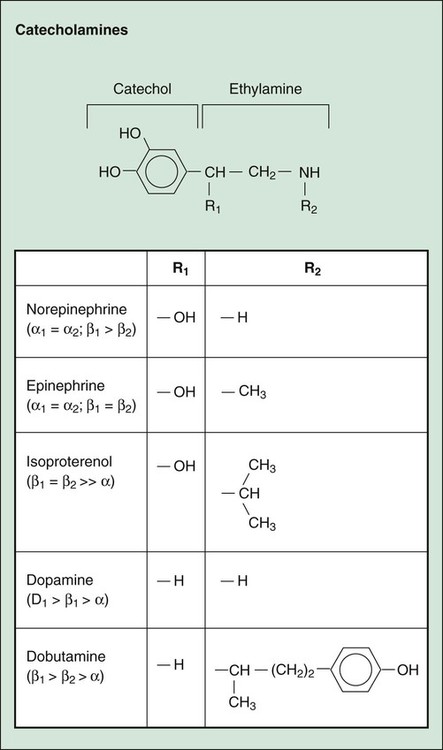
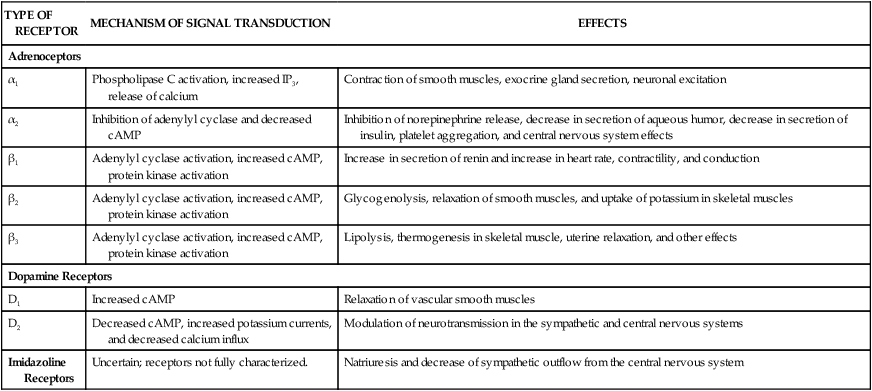
As shown in Table 8-1 and Figure 8-1, subtypes of α- and β-adrenoceptors have been identified on the basis of several criteria, including differences in signal transduction and physiologic effects and differences in agonist affinity for different receptors. The receptor subtypes have been cloned and their molecular structures determined. TABLE 8-1 Properties of Adrenergic, Dopamine, and Imidazoline Receptors cAMP, Cyclic adenosine monophosphate; IP3, inositol triphosphate. The α-adrenoceptors can be differentiated on the basis of their location and function. The α1-adrenoceptors are primarily located in smooth muscle at sympathetic neuroeffector junctions, but these receptors are also found in exocrine glands and in the central nervous system. Three main subtypes of α1-adrenoceptors have been identified (α1A, α1B, and α1D), but the functional roles of these receptors have not been clearly established. The α2-adrenoceptors are widely distributed in presynaptic neurons, various tissues, and blood platelets (see Fig. 8-1), and subtypes of these receptors have also been identified. Table 8-1 summarizes the effects of three subtypes of β-adrenoceptors. The β2-adrenoceptors mediate relaxation of bronchial, uterine, and vascular smooth muscle (see Fig. 11-3). In skeletal muscle, β2 receptors mediate potassium uptake. In the liver, they mediate glycogenolysis (breakdown of glycogen and release of glucose), which increases the glucose concentration in the blood. Whereas epinephrine and norepinephrine are equally potent at β1-receptors in cardiac tissue, epinephrine is more potent than norepinephrine at β2-receptors in smooth muscle. Dopamine receptors are activated by dopamine but not by other adrenoceptor agonists. The subtypes of dopamine receptors include D1 receptors, which mediate vascular smooth muscle relaxation, and D2-receptors, which modulate neurotransmitter release. Fenoldopam is a D1-receptor agonist used in treating acute severe hypertension (see Chapter 10). Imidazoline receptors are activated by adrenoceptor agonists that possess an imidazoline structure. These receptors, which are not cloned or fully characterized, are found in the central nervous system and in several peripheral tissues. The antihypertensive effect of clonidine appears to result from activation of both α2-adrenoceptors and imidazoline receptors in the central nervous system, leading to a reduced sympathetic outflow to the heart and vascular smooth muscle. The pharmacologic properties of clonidine and related drugs are discussed in Chapter 10. Activation of β-adrenoceptors and D1 receptors leads to stimulation of adenylyl cyclase and an increase in the levels of cAMP in cardiac tissue and smooth muscle. cAMP activates protein kinase A, which phosphorylates other proteins and enzymes. The cellular response depends on the specific proteins that are phosphorylated in each tissue. In cardiac tissue, calcium channels are phosphorylated, thereby augmenting calcium influx and cardiac contractility. In smooth muscle, cAMP produces muscle relaxation via effects on multiple targets; including potassium channels, calcium channels, and myosin light chain kinase (see Fig. 11-3). The adrenoceptor agonists mimic the effect of sympathetic nervous system stimulation and are sometimes called sympathomimetic drugs. These drugs are divided into three groups based on their mode of action. The direct-acting agonists bind and activate adrenoceptors. As shown in Figure 8-2, the indirect-acting agonists increase the stimulation of adrenoceptors by increasing the concentration of norepinephrine at sympathetic neuroeffector junctions in one of two ways. Cocaine inhibits the catecholamine transporter located in the plasma membrane of the presynaptic sympathetic neuron and thereby decreases the neuronal reuptake of norepinephrine and increases its synaptic concentration. Amphetamine and related drugs are transported into the sympathetic nerve terminal by the catecholamine transporter. Once inside the sympathetic neuron, amphetamines inhibit the storage of norepinephrine by neuronal vesicles. This increases the cytoplasmic concentration of norepinephrine, leading to reverse transport of norepinephrine into the synapse by the catecholamine transporter. The mixed-acting agonists (e.g., ephedrine) have both direct and indirect actions. Each catecholamine consists of the catechol moiety and an ethylamine side chain (Fig. 8-3). The catecholamines are rapidly inactivated by monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT), enzymes found in the gut, liver, and other tissues. For this reason, these drugs have low oral bioavailabilities and short plasma half-lives, and they must be administered parenterally when a systemic action is required (such as in the treatment of anaphylactic shock). As shown in Figure 8-3 and Table 8-2, the various catecholamines differ in their affinities and specificities for receptors. The size of the alkyl substitution on the amine nitrogen (R2) determines the relative affinity for α- and β-adrenoceptors. Drugs with a large alkyl group (e.g., isoproterenol) have greater affinity for β-adrenoceptors than do drugs with a small alkyl group (e.g., epinephrine). Epinephrine is a potent agonist at all α- and β-adrenoceptors. Norepinephrine differs from epinephrine only in that it has greater affinity for β1-adrenoceptors than for β2-adrenoceptors. Because of this difference, norepinephrine constricts all blood vessels, whereas epinephrine constricts some blood vessels but dilates others. Isoproterenol is a selective β1– and β2-adrenoceptor agonist because it has little affinity for α-receptors. Dobutamine primarily stimulates β1-receptors, with smaller effects on β2– and α-receptors. Dopamine activates D1 receptors, β1-receptors, and α-receptors. Unlike the other catecholamines, dopamine also stimulates the release of norepinephrine from sympathetic neurons. For this reason, dopamine is both a direct-acting and an indirect-acting receptor agonist. TABLE 8-2 Pharmacologic Effects and Clinical Uses of Adrenoceptor Agonists
Adrenoceptor Agonists
Adrenoceptors
TYPE OF RECEPTOR
MECHANISM OF SIGNAL TRANSDUCTION
EFFECTS
Adrenoceptors
α1
Phospholipase C activation, increased IP3, release of calcium
Contraction of smooth muscles, exocrine gland secretion, neuronal excitation
α2
Inhibition of adenylyl cyclase and decreased cAMP
Inhibition of norepinephrine release, decrease in secretion of aqueous humor, decrease in secretion of insulin, platelet aggregation, and central nervous system effects
β1
Adenylyl cyclase activation, increased cAMP, protein kinase activation
Increase in secretion of renin and increase in heart rate, contractility, and conduction
β2
Adenylyl cyclase activation, increased cAMP, protein kinase activation
Glycogenolysis, relaxation of smooth muscles, and uptake of potassium in skeletal muscles
β3
Adenylyl cyclase activation, increased cAMP, protein kinase activation
Lipolysis, thermogenesis in skeletal muscle, uterine relaxation, and other effects
Dopamine Receptors
D1
Increased cAMP
Relaxation of vascular smooth muscles
D2
Decreased cAMP, increased potassium currents, and decreased calcium influx
Modulation of neurotransmission in the sympathetic and central nervous systems
Imidazoline Receptors
Uncertain; receptors not fully characterized.
Natriuresis and decrease of sympathetic outflow from the central nervous system
α-Adrenoceptors
β-Adrenoceptors
Dopamine Receptors
Imidazoline Receptors
Signal Transduction
Classification of Adrenoceptor Agonists
Catecholamines
General Properties
Chemistry and Pharmacokinetics
Mechanisms and Effects
DRUG
PHARMACOLOGIC EFFECT (AND RECEPTOR)
CLINICAL USE
Direct-Acting Catecholamines
Dobutamine
Cardiac stimulation (β1) and vasodilation (β2)
Cardiogenic shock, acute heart failure, and cardiac stimulation during heart surgery
Dopamine*
Renal vasodilation (D1), cardiac stimulation (β1), and increased blood pressure (β1 and α1)
Cardiogenic shock, septic shock, heart failure, and adjunct to fluid administration in hypovolemic shock
Epinephrine
Vasoconstriction and increased blood pressure (α1), cardiac stimulation (β1), and bronchodilation (β2)
Anaphylactic shock, cardiac arrest, ventricular fibrillation, reduction in bleeding during surgery, and prolongation of the action of local anesthetics
Isoproterenol
Cardiac stimulation (β1) and bronchodilation (β2)
Atrioventricular block and bradycardia
Norepinephrine
Vasoconstriction and increased blood pressure (α1)
Hypotension and shock
Direct-Acting Noncatecholamines
Albuterol
Bronchodilation (β2)
Asthma
Apraclonidine
Decreased aqueous humor formation (α2)
Short-term control of intraocular pressure
Clonidine
Decreased sympathetic outflow from central nervous system (α2 and imidazoline)
Hypertension, opioid dependence
Dexmedetomidine
Sedation (α2)
Adjunct to anesthesia
Midodrine
Vasoconstriction (α1)
Orthostatic hypotension
Oxymetazoline
Vasoconstriction (α1)
Nasal and ocular decongestion
Phenylephrine
Vasoconstriction, increased blood pressure, and mydriasis (α1)
Nasal and ocular decongestion, mydriasis, maintenance of blood pressure, and treatment of shock
Terbutaline
Bronchodilation (β2)
Asthma, premature labor
Indirect-Acting Agents
Amphetamine
Increase in norepinephrine release, central nervous system stimulation
Narcolepsy, attention-deficit disorder
Cocaine
Inhibition of norepinephrine uptake
Local anesthesia
Mixed-Acting Agents
Ephedrine
Vasoconstriction (α1)
Nasal decongestion
Pseudoephedrine
Vasoconstriction (α1)
Nasal decongestion ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree