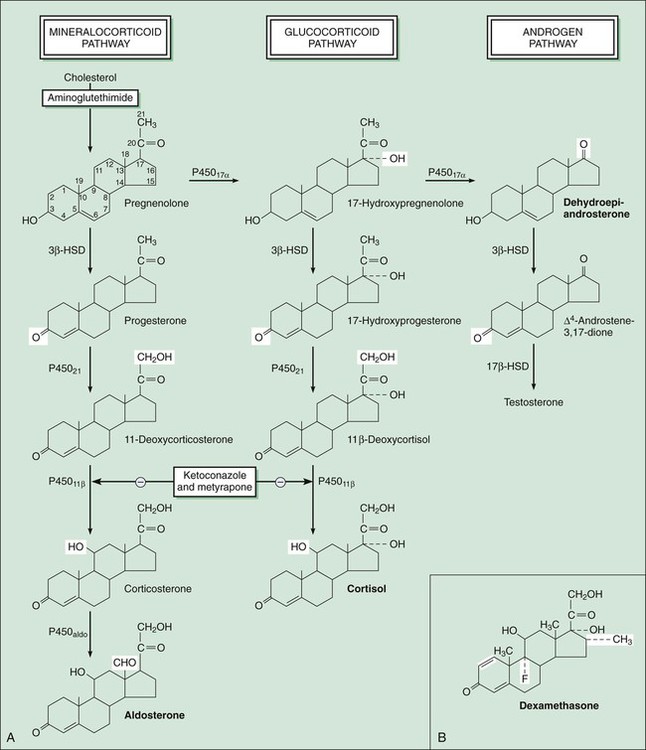
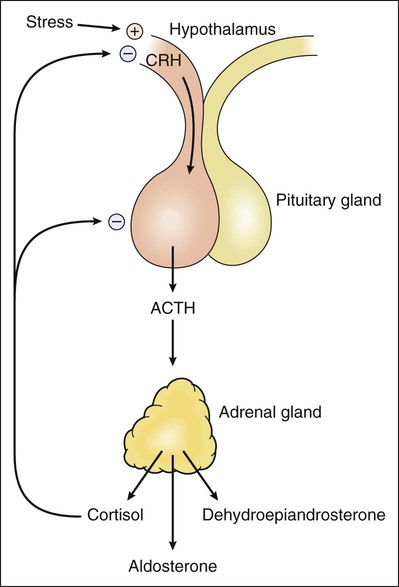
The adrenal glands are situated on top of the kidneys (as obvious from their name) and are essential for life. The adrenal glands are composed of two major parts: the adrenal cortex and the adrenal medulla, also called chromaffin tissue owing to its brightly staining characteristics. The adrenal medulla produces epinephrine (adrenaline) as its main hormone and is an integral part of the sympathetic nervous system (see Chapter 5). This chapter describes the drugs that serve as replacements or alter the effects of steroid hormones produced by the adrenal cortex. The major pathways for mineralocorticoid, glucocorticoid, and androgen biosynthesis are shown in Figure 33-1. Also shown is the site of action of drugs that inhibit adrenocorticoid synthesis. In humans, aldosterone is the major mineralocorticoid, cortisol is the major glucocorticoid, and dehydroepiandrosterone (DHEA) is the major adrenal androgen. The regulation of corticosteroid secretion is depicted in Figure 33-2. The secretion of cortisol and adrenal androgens is primarily controlled by corticotropin (ACTH) secreted by the pituitary gland, whereas the secretion of aldosterone is chiefly regulated by the renin-angiotensin system. Various types of physical and mental stress are powerful activators of corticotropin-releasing hormone secretion, leading to increased corticotropin and cortisol production. Cortisol exerts several effects that increase the body’s resistance to stress. As shown in Figure 33-2, cortisol and other glucocorticoids act as feedback inhibitors of both corticotropin-releasing hormone and corticotropin. This is why exogenously administered glucocorticoids can suppress the hypothalamic-pituitary-adrenal axis and inhibit endogenous cortisol production, leading to adrenal insufficiency when the exogenous glucocorticoid is withdrawn. The adrenal steroids act on target tissues by binding to specific cytoplasmic steroid receptors, which are then translocated to the cell nucleus, a common mechanism for all steroid hormones (see Fig. 3-4 in Chapter 3). In the nucleus the activated receptors stimulate the transcription of specific genes and thereby increase the translation of specific proteins. These actions lead to the various metabolic and antiinflammatory effects of glucocorticoids, which are described later. In the renal tubules, activation of the mineralocorticoid receptor stimulates the synthesis of sodium channels and sodium-potassium adenosine triphosphatase, which are needed for sodium reabsorption. This mechanism is responsible for the salt-retaining effects of mineralocorticoids. A large number of semisynthetic glucocorticoid drugs are available; they are most frequently employed to attain the antiinflammatory effects produced by supraphysiologic doses of these drugs. Less commonly, corticosteroids are used as replacement therapy in the treatment of adrenal insufficiency and in the treatment of adrenogenital syndromes that produce excessive quantities of adrenal androgens and insufficient quantities of other corticosteroids. Mineralocorticoids are primarily used as replacement therapy in persons with adrenal insufficiency. The properties of selected corticosteroids are listed in Table 33-1. TABLE 33-1 Pharmacologic Properties of Selected Corticosteroids *Fludrocortisone is classified as a mineralocorticoid; the other drugs are classified as glucocorticoids. A large number of glucocorticoid preparations are available for oral, parenteral, inhalational, or topical administration for the treatment of a wide range of inflammatory, allergic, autoimmune, and other disorders. Whenever possible, topical or inhalational administration is preferred because it is usually well tolerated and avoids most systemic adverse effects. Topical administration is widely used in the treatment of allergic or inflammatory conditions affecting the skin, mucous membranes, or eyes (see later). For example, topical ocular glucocorticoids are used to treat acute uveitis (inflammation of the iris, ciliary body, or choroid). Glucocorticoids are given by inhalation to treat allergic rhinitis, aspiration pneumonia, asthma, and other respiratory conditions (see Chapter 27). The glucocorticoids are usually classified according to their potency and duration of action (see Table 33-1). When given systemically, the duration of action of glucocorticoids is primarily determined by their potency at the glucocorticoid receptor, rather than by their elimination half-life. This is because the highly potent drugs evoke a longer-lasting stimulation of gene transcription than do less-potent glucocorticoids. The potency of specific topical corticosteroids is discussed under the treatment of dermatologic conditions (see later).
Adrenal Steroids and Related Drugs
Overview
Synthesis and Secretion of Adrenal Steroids
Physiologic Effects of Adrenal Steroids
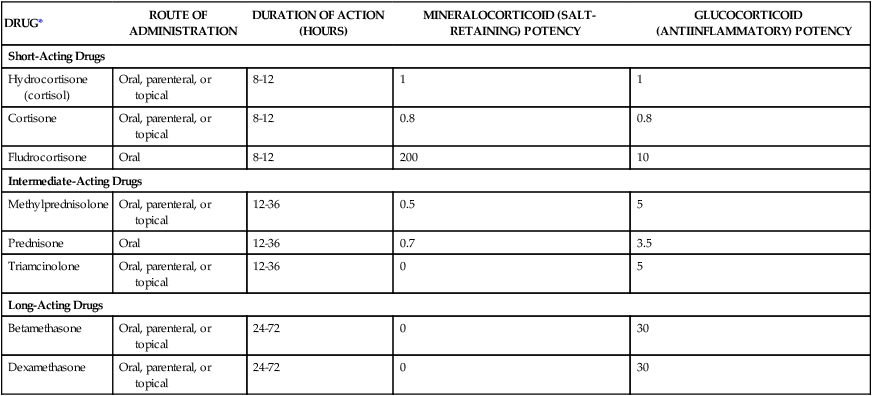
Corticosteroid Drugs
DRUG*
ROUTE OF ADMINISTRATION
DURATION OF ACTION (HOURS)
MINERALOCORTICOID (SALT-RETAINING) POTENCY
GLUCOCORTICOID (ANTIINFLAMMATORY) POTENCY
Short-Acting Drugs
Hydrocortisone (cortisol)
Oral, parenteral, or topical
8-12
1
1
Cortisone
Oral, parenteral, or topical
8-12
0.8
0.8
Fludrocortisone
Oral
8-12
200
10
Intermediate-Acting Drugs
Methylprednisolone
Oral, parenteral, or topical
12-36
0.5
5
Prednisone
Oral
12-36
0.7
3.5
Triamcinolone
Oral, parenteral, or topical
12-36
0
5
Long-Acting Drugs
Betamethasone
Oral, parenteral, or topical
24-72
0
30
Dexamethasone
Oral, parenteral, or topical
24-72
0
30
Glucocorticoids
Classification
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Adrenal Steroids and Related Drugs
Only gold members can continue reading. Log In or Register to continue