Malignant neoplasm arising from secretory cells
˜ 50% PCa harbor TMPRSS2 and ETS gene fusion
75-80% of PCas arise in PZ; ˜ 15-25% arise in TZ
Diagnosis based on constellation of architectural, nuclear, cytoplasmic, and intraluminal features
Crowded uniform glands that infiltrate between preexisting benign glands
Small caliber, crowded clusters, rigid or “sharp” lumina, tinctorial staining of cytoplasm distinct from adjacent benign glands
Malignant glands lack basal cells by immunohistochemistry
Nuclear enlargement and hyperchromasia with prominently enlarged &/or multiple and peripherally located nucleoli
Pathognomonic features for malignant glands
Glomerulations or collagenous micronodules
Circumferential perineural/intraneural invasion or growth within fat
Less differentiated tumors have poorly formed, fused, or large cribriform glands
Poorly differentiated tumors may grow as infiltrative single cells or solid sheets
Treated PCa glands usually poorly formed but retain infiltrative appearance
Prostate adenocarcinoma (PCa)
Prostatic adenocarcinoma
Malignant neoplasm arising from secretory cells
TMPRSS2 and ETS gene fusion
Most common recurrent arrangement identified
˜ 50% PCa harbor these recurrent gene fusions; individual estimates vary between 15-78%
TMPRSS2 encodes for serine protease secreted by prostatic cells in response to androgen exposure
ETS family of transcription factors include ERG, ETV1, ETV4, and ETV5
TMPRSS2:ERG gene fusion most common (˜ 90%)
ERG brought under control of androgen-regulated promoter causing protein overexpression
Intra- and interchromosomal genetic rearrangements lead to creation of fusion transcript
In ˜ 2/3 of cases, fusion results from deletion (intervening 3 Mb between TMPRSS2 and ERG)
Fusion may also occur by more complex rearrangement, such as translocation
Over 20 TMPRSS2:ERG variants now described
Morphological features of prostate cancer associated with TMPRSS2:ERG gene fusion
Blue-tinged mucin, cribriform pattern, intraductal spread, macronucleoli, and signet ring cells
Clinical significance of this gene fusion not yet fully understood
Conflicting reports in literature
Hereditary prostate cancer
Compelling evidence suggests familial predisposition to prostate cancer in some cases
High-risk alleles identified with either autosomal dominant or X-linked mode of inheritance
3 candidates genes identified: HPC2/ELAC2 on 17p, RNASEL on 1q25, and MSR1 on 8p22-23
Other genes and molecular alterations
Most common chromosomal alterations in prostate cancer are losses at 1p, 6q, 8p, 10q, 13q, 16q, and 18q and gains at 1q, 2p, 7, 8q, 18q, and Xq
Genes implicated in PCa include GST-pi, NKX3.1, PTEN, AMACR, hepsin, KLF-6, EZH2, p27, E-cadherin
Mutations in androgen receptor gene may promote cancer growth at lower circulating androgen levels
Hedgehog pathway has been shown to play a role in growth and metastasis of prostate cancer
Unlike most other visceral organ tumors, PCa often has no reliably distinguishable gross mass lesion
Grossly evident tumors are usually pT3, ≥ Gleason score (GS) 8, or ≥ 1 cm size tumors
Indurated yellow to yellow-tan homogeneous areas
More dense or firmer than surrounding benign spongy parenchyma
Typically lack necrosis or hemorrhage
Tumor border blends imperceptibly with benign parenchyma
Lesions < 5 mm generally inapparent
Tumors often larger when examined by microscopy than when measured grossly
False-positivity rate in gross identification up to 19%
Tumors usually in posterior or posterolateral aspect (peripheral zone [PZ]) of the gland
Anterior tumors more difficult to recognize as they are usually admixed with nodular hyperplasia
75-80% of PCas arise in PZ, and 15-25% arise in transition zone (TZ)
Central zone is usually only secondarily involved
Multifocal tumors present in more than 50% of PCas
Diagnosis based on constellation of architectural, nuclear, cytoplasmic, and intraluminal features
Some individual features may also be seen in benign glands
Architectural features
Better differentiated tumors consist of compact or loose collections of well-formed glands
Small crowded uniform glands infiltrate between preexisting benign glands
Malignant glands usually differ in appearance from surrounding benign glands
Smaller-caliber glands
Crowded or compact gland clusters
Rigid or “sharp” glandular lumina
May have periglandular clefts
Malignant glands should lack basal cells
Less differentiated tumors consist of poorly formed, fused, or large cribriform glands
Poorly differentiated tumors may grow as infiltrative single cells or solid sheets
Nuclear features
Nuclear enlargement and hyperchromasia
Prominently enlarged nucleoli
Multiple and peripherally located nucleoli
Parachromatin clearing
Mitoses are rare; highly suggestive of malignancy if present
Apoptotic bodies (rare)
Nuclei commonly uniform, nonpleomorphic
Cytoplasmic features
Typically cuboidal to columnar cells with modest cytoplasm
Amphophilic, clear or pale granular cytoplasm
Taller cells with clear to pale pink cytoplasm and basally located nuclei more common in TZ
Intraluminal features
Blue mucin
Usually prominent collection of wispy, bluetinged intracellular mucin
Eosinophilic amorphous secretions
Granular eosinophilic luminal material
Crystalloids
Geometric bright eosinophilic rhomboid to prismatic structures with sharp edges, usually associated with eosinophilic amorphous secretions
Present in up to 41% of PCas
Seen in atypical adenomatous hyperplasia and uncommonly in benign glands
Corpora amylacea are extremely rare in PCa, should strongly suggest benign glands
Intraluminal necrosis may be present in high-grade tumors, highly indicative of malignancy
Pathognomonic features for malignant glands
Glomerulations
Cribriform cellular luminal proliferations in otherwise well-formed glands attached to 1 pole
Collagenous micronodules (mucinous fibroplasia)
Hyalinized eosinophilic material usually associated with abundant intraluminal blue mucin
Often imparts an anastomosing epithelial pattern
Circumferential perineural or intraneural invasion
Gland should completely surround the nerve or be seen within the nerve
Benign glands may focally touch or indent a nerve; very rarely may be intraneural
Growth within adipose tissue
Intraprostatic fat is exceedingly rare
Indicates extraprostatic extension (EPE)
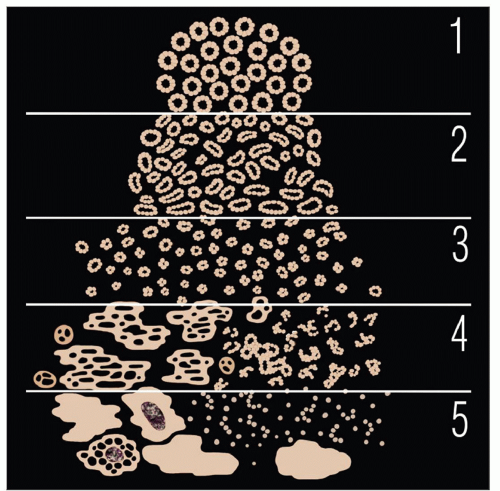
Universally accepted grading system for PCa
Assessment of glandular architecture at low/intermediate magnification: Classified into 5 basic patterns
Each pattern may arise de novo without progression from lower grade
In resection specimens, GS is sum of primary and secondary Gleason patterns
Primary pattern is most prevalent pattern and secondary is 2nd most common pattern
International Society of Urological Pathology (ISUP) 2005 consensus conference proposed several modifications and guidelines
In needle biopsies, include tertiary pattern in Gleason score if it is higher than secondary pattern
Similar rule applies for transurethral resection and enucleation (simple prostatectomy) specimens
In high-grade cancers, ignore lower grade pattern if < 5% (e.g., 4 + 4, if pattern 3 is < 5%)
For cancers with more than 1 pattern, include the higher grade even if it is < 5% (e.g., 3 + 4, even if pattern 4 is < 5%)
Assign individual GS to all cores as an aggregate if submitted in 1 container; assign GS to each core separately designated (e.g., ink or separately submitted) by urologist
In radical prostatectomy, provide GS (primary and secondary pattern); separately mention tertiary pattern
Assign separate GS to dominant tumor(s) for multifocal tumors in radical prostatectomy
Individualized Gleason grading approach for some PCa morphologic variants and subtypes
Gleason pattern 1
Circumscribed nodule of tightly packed, uniform, round to oval, well-formed glands, with no or minimal infiltration of adjacent parenchyma
Using these strict criteria, exceedingly rare and controversial in current practice
Most described pre-immunohistochemistry were likely atypical adenomatous hyperplasia
Gleason pattern 2
Nodular with minimal peripheral infiltration, less uniform and more loosely arranged glands
Also very rare and typically found in TZ
Usually incidental with associated higher grades, but occasionally secondary pattern in resection specimens
ISUP recommends that GS 3 or 4 should rarely, if ever, be diagnosed in needle biopsy specimens
Architecture cannot be assessed in its entirety
Poor reproducibility among experts
Poor correlation with grade in subsequent prostatectomy (i.e., undergrading)
May misguide clinicians and patients with assumption of indolent tumor
Gleason pattern 3
Most common pattern
Predominantly well-formed, individual glands that infiltrate between benign ducts and acini
Includes smaller but well-formed glands (microacini)
Glands typically smaller than in patterns 1 or 2
Inclusion of small cribriform structures is controversial, as most experts include all cribriform patterns in Gleason pattern 4
Should have perfectly round contours
Size similar to surrounding benign glands
Uniform round “punched-out” lumina with bridges not thicker than lumina
By this strict definition, cribriform Gleason pattern 3 is extraordinarily rare; we rarely designate cribriform patterns as Gleason 3
Gleason pattern 4
Most commonly fused, poorly formed glands
Tangentially sectioned pattern 3 glands may mimic fused pattern 4 glands
Common cause of overgrading
2nd most common pattern is cribriform structures with either regular or irregular outlines
Uncommon “hypernephromatoid” pattern, consists of solid sheets of cells with optically clear cytoplasm
Gleason pattern 5
Atrophic variant
PCa with glands lined by cells with scant cytoplasm, resembling atrophy
Infiltrative growth, cytology of malignancy
Usually admixed with nonatrophic PCa
In contrast, benign atrophic glands typically have dense hyperchromatic nuclei and lobular growth
Pseudohyperplastic variant
Large-sized or dilated glands, with branching and papillary infolding
Tall columnar cells with abundant pale to slight granular luminal cytoplasm
Basally located nuclei along basement membrane
Commonly with luminal eosinophilic amorphous secretions and may have crystalloids
Diagnostic malignant nuclear features retained, in contrast to benign hyperplastic glands
ISUP recommends grade of 3 + 3 = 6; if part of a large circumscribed nodule, grade may be 3 + 2 = 5
Foamy gland (xanthomatous) variant
PCa with abundant foamy cytoplasm
Malignant nuclear features not always present, as nuclei may be small and pyknotic
Presence of infiltrative pattern; may require immunostains
ISUP recommends discounting foamy cytoplasm and assigning grade based on architecture; most are 3 + 3 = 6 tumors
Mucinous (colloid) adenocarcinoma
≥ 25% of resected tumor shows extracellular mucin
Intraluminal mucinous material does not qualify, and extraprostatic origin must be excluded
Diagnosis should be made only in resection specimen, as needle biopsy specimen may not show exact proportion of mucinous component
In needle biopsy, may be diagnosed as “PCa with mucinous features”
With strict diagnostic criteria, suggested to have similar features and behavior with conventional PCa
ISUP recommend to grade irregular cribriform glands floating in mucin as 4 + 4 = 8; no consensus if individual discrete glands in mucin
Signet ring cell variant
≥ 25% of resected tumor shows signet ring cell (arbitrary definition)
Tumor cells contain optically clear vacuoles displacing nuclei and are widely infiltrative
Associated with typical high-grade PCa
May be mucin-producing PCa (“mucinous carcinoma with signet ring cells”)
Not clear if nonmucinous (mucicarmine negative) signet ring PCa is distinct clinically
Clinical presentation similar to conventional PCa, with tendency to present at higher stage
PCa with Paneth-cell-like differentiation
PCa containing neuroendocrine cells with bright eosinophilic cytoplasmic granules resembling Paneth cells of gastrointestinal tract
Suggested to have favorable prognosis, including those with nests, cords, or single cell morphology
Other rarer variants
Lymphoepithelioma-like variant
As in other organs, characterized by syncytial growth amid dense lymphocytic background
No Epstein-Barr virus association
Oncocytic variant
PCa with abundant granular eosinophilic cytoplasm
Like other oncocytic tumors, ultrastructurally contains abundant mitochondria
PCa with stratified epithelium (“PIN-like”)
Glands lined by ≥ 2 layers of malignant cells
May resemble flat or tufted high-grade PIN but lacks basal cells by immunohistochemistry
Radiation therapy
Treated PCa glands usually poorly formed but retain their infiltrative appearance
Foamy vacuolated cytoplasm and pleomorphic nuclei; changes vary from mild to marked
Marked radiation effect may artifactually produce architecture resembling GS 9 or 10 cancers
Luminal features of malignancy may be retained
Cytologic atypia and pleomorphism more pronounced in benign than malignant glands
Residual treated PCa with minimal or no radiation effect has higher chance of recurrence
PAN-CK(AE1/AE3) useful to highlight treated PCa
Hormonal therapy
Treated PCa may be shrunken glands or single cells
Glands show xanthomatous cytoplasm, pyknotic and fragmented nuclei, and mucin extravasation
Empty spaces representing remnants of shrunken glands may be present
Marked atrophy, basal cell hyperplasia, or squamous metaplasia in adjacent benign glands
PAN-CK(AE1/AE3) useful to highlight treated PCa
Given broad morphologic spectrum, differential diagnosis for PCa ranges from innocuous benign normal structures to secondary high-grade cancers
PCa most often mimicked by benign prostatic glandular lesions; difficulty enhanced in limited samples (e.g., biopsy)
Use of ancillary immunohistochemistry helpful in some scenarios
Pattern-based approach facilitates work-up and judicious selection of adjuvant stains
Focal atypical glands that are suspicious but quantitatively &/or qualitatively insufficient for diagnosis or exclusion of PCa
Most common differential diagnostic scenario for PCa
Differential diagnosis includes focal PCa and benign glandular lesions
Immunohistochemistry may be helpful
PCa lacks basal cell staining and often overexpresses AMACR
In some cases, definitive diagnosis of PCa is not possible even with a carcinoma staining pattern
Differential Diagnosis for Prostate Carcinoma | ||
Histologic Pattern | Prostate Carcinoma Main | Differential Diagnoses |
Small glandular proliferation | Glandular Gleason pattern 3 | Crowded benign glands, not otherwise specified |
Atrophic pattern | Simple atrophy | |
Post-treatment cancer | Outpouching of high-grade PIN | |
Partial atrophy | ||
Postatrophic hyperplasia (PAH) | ||
AAH (adenosis) | ||
Sclerosing adenosis | ||
Basal cell hyperplasia | ||
Seminal vesicle epithelium | ||
Ejaculatory duct | ||
Cowper glands | ||
Mesonephric remnants | ||
Nephrogenic adenoma | ||
Verumontanum mucosal gland hyperplasia | ||
Radiation atypia | ||
Atypical large glandular proliferation | Cribriform Gleason patterns 3, 4, and 5 | High-grade PIN |
Ductal adenocarcinoma | Urothelial carcinoma involving prostatic ducts and acini | |
Pseudohyperplastic pattern | Colorectal carcinoma involving prostate | |
Cribriform hyperplasia | ||
Squamous metaplasia | ||
Urothelial metaplasia | ||
Infiltrative single cell pattern | Single cell Gleason 5 pattern | Dense inflammation |
Post-treatment carcinoma | Granulomatous prostatitis | |
Lymphoma | ||
Small cell carcinoma | ||
Clear cell pattern | Hypernephroid Gleason pattern 4 | Prostatic xanthoma |
Glandular Gleason pattern 3 | ||
Oncocytic pattern | Gleason pattern 4 | Paraganglion/paraganglioma |
Carcinoid tumor | ||
Poorly to undifferentiated carcinoma | Solid Gleason pattern 5 | Urothelial carcinoma |
Spindle cell pattern | Sarcomatoid carcinoma | Pseudosarcomatous myofibroblastic proliferation |
Stromal sarcoma | ||
Leiomyosarcoma | ||
Small cell pattern | Small cell carcinoma | Lymphoma |
Rhabdomyosarcoma | ||
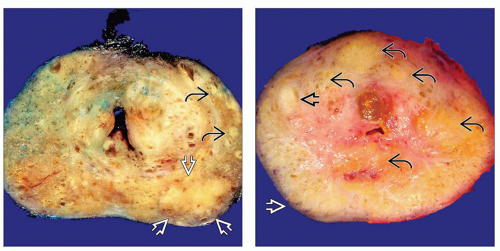 (Left) This coronal section of prostate shows multifocal PCa predominantly involving left lobe with a dominant nodule
 at the posterolateral aspect and additional smaller tumor foci at the posterolateral aspect and additional smaller tumor foci  at the lateral aspect of the peripheral zone. (Right) Coronal section of prostate shows a focus of PCa at the lateral aspect of the peripheral zone. (Right) Coronal section of prostate shows a focus of PCa  involving the anterior aspect of PZ. Several hyperplastic nodules involving the anterior aspect of PZ. Several hyperplastic nodules  are seen in the adjacent TZ. Note absence of grossly visible tumor in posterior and posterolateral aspect of the PZ are seen in the adjacent TZ. Note absence of grossly visible tumor in posterior and posterolateral aspect of the PZ  . .Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|