Acid-Base Homeostasis and Imbalances
Linda Felver
Key Questions
• What are the chemistry and functional importance of the bicarbonate buffer system?
• What is the role of the respiratory system in regulating carbonic acid (carbon dioxide)?
• What is the role of the kidneys in regulating bicarbonate ion and acids other than carbonic acid?
• How do the lungs compensate for acid-base imbalances caused by altered levels of metabolic acids?
• How do the kidneys compensate for acid-base imbalances caused by altered levels of carbonic acid?
![]()
http://evolve.elsevier.com/Copstead/
When the pH of body fluids becomes abnormal, cellular function is impaired. The pH of a fluid reflects its degree of acidity or alkalinity. Technically, pH is the negative logarithm of the hydrogen ion (H+) concentration. The normal hydrogen ion concentration of the blood is about 40 nmol/L (40 × 10−9 mol/L)—a very small number.1 The pH (negative logarithm) of this number is 7.40, which is easier to use in clinical settings. An alteration in pH is a change in the hydrogen ion concentration. A high pH indicates few hydrogen ions, meaning that the solution is alkaline (basic). A low pH indicates a large amount of hydrogen ions, meaning that the solution is acidic.
An acid releases hydrogen ions. The more hydrogen ions present, the more acidic the solution. The normal pH of adult blood ranges from 7.35 to 7.45 (may vary slightly with different laboratories). The range is somewhat wider in infants and children. Table 25-1 lists normal laboratory values for pH and other acid-base parameters. If the blood and other body fluids become too acidic (reflected by pH decreased below the lower limit of the normal range), dysfunction occurs; if the pH of the blood falls below 6.9, death is likely to occur. Similarly, if the body fluids become too alkaline, as reflected by pH increased above the upper limit of the normal range, dysfunction also occurs. If the pH of the blood rises above 7.8, death is likely.
TABLE 25-1
NORMAL LABORATORY VALUES FOR ACID-BASE PARAMETERS
| CHARACTERISTIC | NORMAL RANGE |
| PaCO2 (arterial blood) | 36-44 mm Hg (adults) |
| 30-34 mm Hg (infants) | |
| HCO3− (serum) | 22-26 mEq/L (adults) |
| 19-23 mEq/L (infants) | |
| pH (arterial blood) | 7.35-7.45 (adults) |
| 7.11-7.36 (neonates) | |
| 7.36-7.41 (infants) |
Normal cellular metabolism continually releases acids (carbonic and metabolic) that must be excreted from the body to prevent body fluids from becoming too acidic. This chapter discusses the normal mechanisms of acid-base homeostasis and the acid-base imbalances that arise when these homeostatic mechanisms become dysfunctional or overwhelmed.
Acid-Base Homeostasis
Three major mechanisms regulate the acid-base status of the body: buffers, the respiratory system, and the renal system. Laboratory measurements such as arterial blood gas values are useful indicators of the acid-base status of extracellular fluids. The partial pressure of carbon dioxide in arterial blood (PaCO2) is an indicator of the respiratory component of acid-base balance. The plasma bicarbonate ion (HCO3−) concentration is an indicator of the renal (metabolic) component of acid-base balance.1 The pH of the blood indicates the net result of normal acid-base regulation, any acid-base imbalance, and the body’s compensatory responses. It is important to remember that the pH measured clinically is that of the blood and may not reflect the pH inside cells or in cerebrospinal fluid.
Buffers
Buffers are chemicals that help control the pH of body fluids. Each buffer system consists of a weak acid, which releases hydrogen ions when the fluid is too alkaline, and a base, which takes up hydrogen ions when the fluid is too acidic. In this way, potential changes in pH are adjusted immediately by the action of buffers. All body fluids contain buffers. Chief among them are bicarbonate buffers (in the extracellular fluid), phosphate buffers (in intracellular fluid and urine), hemoglobin buffers (inside erythrocytes), and protein buffers (in intracellular fluid and the blood). These buffers are the first line of defense against pH imbalances.
The bicarbonate buffer system is the most important buffer in the extracellular fluid. Bicarbonate ion (HCO3−) is the base portion and carbonic acid (H2CO3) is the weak acid portion. These two components of the bicarbonate buffer system are in chemical equilibrium in the extracellular fluid.2 If too much acid (e.g., lactic acid) is present, the bicarbonate ions take up hydrogen ions (H+) released by the acid and become carbonic acid. Through the action of the enzyme carbonic anhydrase, the carbonic acid then is excreted through the respiratory system in the form of carbon dioxide and water. Thus the excess acid is neutralized when bicarbonate ions are used in the buffering process.
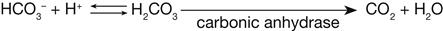
Conversely, if too little acid is present in the extracellular fluid, the carbonic acid portion of the bicarbonate buffer system releases hydrogen ions. This action helps to keep the pH from becoming too high or at least minimizes the increase.
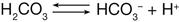
The pH of any fluid is determined by the relative amounts of acids and bases contained in it. For the pH of the blood to be within the normal range, the ratio of bicarbonate ions to carbonic acid must be 20:1, which means that 20 bicarbonate ions must be present for every carbonic acid molecule. This relationship is explained formally by the Henderson-Hasselbalch equation, which is a mathematical description of the pH of a buffered solution, here written specifically for the bicarbonate buffer system.
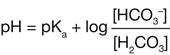
Square brackets, used throughout this chapter, are a standard notation for concentration. pKa is the dissociation constant for any particular acid; it equals 6.1 for carbonic acid.2 If the normal 20:1 ratio of bicarbonate ions to carbonic acid is present, the pH will be 7.4.
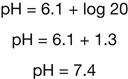
The 20:1 ratio of bicarbonate ions to carbonic acid necessary for a normal pH is an important concept in understanding the compensatory mechanisms for acid-base imbalances that are discussed later in this chapter.
Respiratory Contribution
The respiratory system is the second defense against acid-base disorders. Body cells continuously produce carbon dioxide (CO2). Together, CO2 and water (H2O) make carbonic acid (H2CO3). The lungs excrete carbon dioxide and water from the body. Therefore, during the process of exhalation the lungs effectively excrete carbonic acid. The respiratory system adjusts the amount of carbonic acid that remains in the body by altering rate and depth of respiration.
The rate and depth of respiration are influenced strongly by chemoreceptors that sense the PaCO2, PaO2, and pH of the blood. If too much carbonic acid begins to accumulate in the blood of a healthy person, the rate and depth of respiration increase and excess carbonic acid is removed. This response corrects the imbalance and restores blood chemistry to normal. If, on the other hand, too little carbonic acid is present in the blood, the rate and depth of respiration decrease to retain carbonic acid until it once more is present in normal amounts. Again, the imbalance is corrected and the blood chemistry returns to normal. Thus the body’s correction of a carbonic acid excess or deficit is dependent on normal function of all components of the respiratory system, including the chemoreceptors, respiratory neurons in the brainstem, motor nerves to respiratory muscles, diaphragm and other respiratory muscles, chest wall, and, of course, the airways, lungs, and pulmonary circulation. In older adults, the chemoreceptor response to increased PaCO2 may occur more slowly.
The PaCO2 indicates how effectively the respiratory system is excreting carbonic acid. If the PaCO2 is elevated above the upper limit of the normal range, carbonic acid has accumulated in the blood. In other words, the respiratory rate and depth have been insufficient or lung disease has prevented sufficient carbonic acid (carbon dioxide and water) excretion.3 Similarly, if the PaCO2 is decreased below the lower limit of the normal range, the lungs have excreted more carbonic acid than usual. In other words, the respiratory rate and depth have been excessive.4
Carbonic acid is known as a volatile acid because it can be excreted as gases (CO2 and H2O). It is the only volatile acid in the body. Other acids that accumulate in the body, such as lactic acid and acetoacetic acid, are nonvolatile. They are organic acids that have no gaseous form. The lungs can excrete only carbonic acid; they cannot excrete nonvolatile acids that may accumulate in the body. If a nonvolatile acid (such as lactic acid) accumulates in the blood, the rate and depth of respiration will increase because the excess hydrogen ions stimulate the chemoreceptors. This hyperventilation does not excrete lactic acid (which would correct the problem), but it does remove carbonic acid from the blood. Removing carbonic acid from the blood when another acid is present in excess helps keep the pH from dropping too low. However, this response makes other values abnormal. The respiratory response to an imbalance of any acid except carbonic acid is called compensation. A compensatory response does not correct a pH disorder but it does compensate for it by adjusting the pH back toward normal, even though other blood chemistry values are made abnormal in the process.
The compensatory response to a deficit of any acid except carbonic acid is hypoventilation.1,5 By decreasing rate and depth of respiration, the body retains carbonic acid. This carbonic acid accumulation helps keep blood pH from rising to a fatal level when another acid is deficient in the body. Respiratory compensation for an imbalance of metabolic acid begins in minutes but requires at least several hours for full effectiveness. Respiratory responses to changes in carbonic and metabolic acids are summarized in Table 25-2.
TABLE 25-2
RESPIRATORY RESPONSES TO CHANGES IN CARBONIC AND METABOLIC ACIDS
| STIMULUS | RESPIRATORY RESPONSE | RESULT |
| Increased PaCO2, decreased pH | Hyperventilation | Correction of imbalance |
| Decreased PaCO2, increased pH | Hypoventilation | Correction of imbalance |
| Decreased pH from excess of metabolic acids | Hyperventilation | Compensation for imbalance |
| Increased pH from deficit of metabolic acids | Hypoventilation | Compensation for imbalance |
Renal Contribution
The third defense against acid-base disorders is the kidneys. The kidneys can excrete any acid from the body except carbonic acid (which is excreted by the lungs). These acids that are not carbonic acid are called metabolic acids because cells continuously produce them during normal metabolism. The kidneys normally excrete metabolic acids. If a metabolic acid begins to accumulate in the blood, the kidneys increase their acid excretion mechanisms to correct the problem. If a metabolic acid is deficient in the blood, the kidneys slow their acid excretion mechanisms to allow acid to accumulate to normal levels. The body’s ability to correct an excess or deficit of a metabolic acid depends on normal function of the renal system. Infants excrete more bicarbonate in their urine than do older children or adults; their kidneys are less effective in excreting acid. The renal response to a large acid load also is less efficient in older adults.
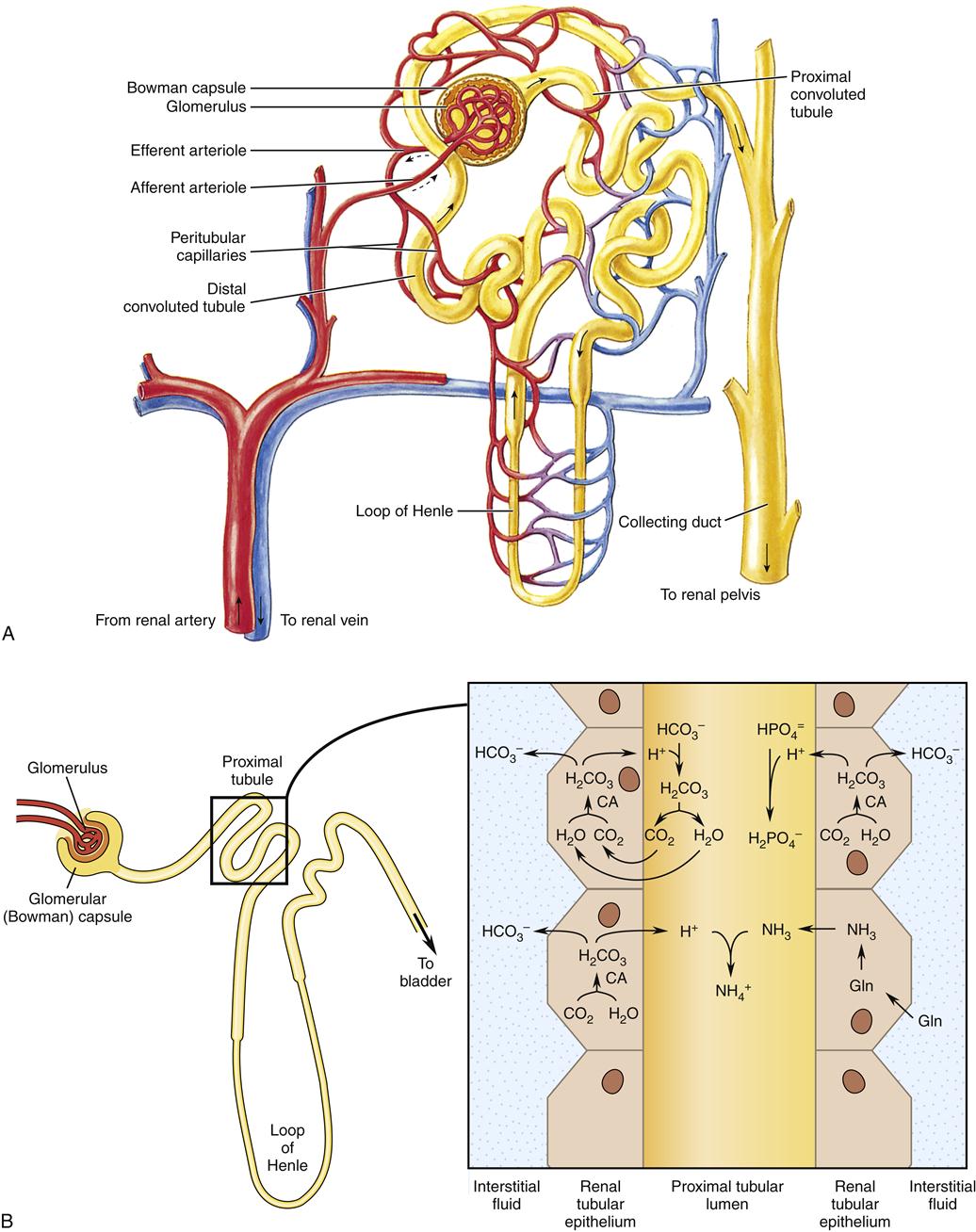
The kidneys have several mechanisms that accomplish acid excretion.2 Understanding these mechanisms requires a knowledge of basic renal physiology. Briefly, at the glomerulus, fluid filtered from the blood enters the glomerular (Bowman) capsule, which is the beginning of the nephron. The cells that line the lumen of the renal tubule modify the fluid inside the nephron (renal tubular fluid). Renal tubular fluid that passes through the entire nephron becomes the urine. Renal tubular epithelial cells have different membrane structures on opposite sides of the cells. The luminal membrane (next to the renal tubular fluid) contains different transporter proteins than the basolateral membrane (next to the interstitial fluid). This structure allows these cells to secrete certain substances into the renal tubular fluid and move other substances into the interstitial fluid.
In the proximal tubules, renal tubular epithelial cells excrete metabolic acid by secreting both the anion portion of the metabolic acid (e.g., lactate) and the hydrogen ions into the tubule lumen. For every hydrogen ion (H+) that is secreted into the renal tubular fluid, one bicarbonate ion (HCO3−) is moved into the interstitial fluid.6 The fluid filtered from the blood at the glomerulus contains many bicarbonate ions and most or all of that bicarbonate is reabsorbed (returned to the blood) during secretion of hydrogen ions. Renal tubular cells are able to secrete additional hydrogen ions into the tubular fluid to excrete large amounts of hydrogen ions from metabolic acid.
Once the H+ are in the renal tubular fluid, most of them combine with other chemicals: bicarbonate ions, which were filtered at the glomerulus, as described previously; urine buffers, such as phosphate, which were filtered at the glomerulus; or ammonia (NH3), which is produced by renal tubular cells. Net H+ excretion occurs after HCO3− has been reabsorbed in the amount that was filtered at the glomerulus. Thus net H+ excretion is accomplished in the form of buffered H+ (called titratable acidity) and H+ attached to ammonia (ammonium ions, NH4+). Figure 25-1 illustrates these processes. Some of these processes operate also in the thick ascending limb of the loop of Henle and the distal nephron, where the intracellular chemistry differs slightly but the overall processes are the same.
When the kidneys need to excrete more hydrogen ions, renal tubular cells increase their production of ammonia (NH3). Ammonia, a gas, moves easily into the renal tubular fluid where it combines with hydrogen ions to become ammonium ions (NH4+). Ammonium ions are not lipid soluble, so they do not cross easily from the renal tubular fluid back to the blood. Only free hydrogen ions contribute to the acidity of the urine, not those that are part of ammonium ions. Consequently, increased production of ammonia is an effective way of excreting more hydrogen ions in the renal tubular fluid without making the urine too acidic.
The concentration of HCO3− in plasma reflects the effectiveness of renal regulation of metabolic acids. If metabolic acids are accumulating in the blood, they will be buffered by HCO3− and the HCO3− concentration will drop below normal. Thus, a decreased concentration of HCO3− in plasma indicates a relative excess of metabolic acids. An increased HCO3− concentration in the plasma indicates a relative deficit of metabolic acids (in other words, a relative excess of base).
Although the kidneys are unable to excrete carbonic acid, they can compensate for carbonic acid imbalances by adjusting the excretion of metabolic acids.7 For example, if carbonic acid accumulates in the blood, the kidneys can increase the excretion of metabolic acids. This compensatory action helps keep the pH of the blood from becoming too abnormal. Similarly, if a deficit of carbonic acid in the blood is prolonged, the kidneys will decrease the excretion of metabolic acids. As these metabolic acids accumulate in the blood, they will compensate for the lack of carbonic acid and return the pH of the blood toward normal. The body’s compensatory response to an imbalance of one kind of acid thus returns the pH of the blood toward normal by creating an imbalance of another kind of acid. The renal compensatory response to an imbalance of carbonic acid requires several days to be fully operative. Renal responses to changes in metabolic and carbonic acids are summarized in Table 25-3.