FIGURE 7–1. Stages of poliovirus pathogenesis. The diagram illustrates multiple steps of poliovirus pathogenesis, starting from virus entry through oropharynx (fecal-oral transmission), virus multiplication at the site of entry (gut), invasion of the virus to the regional lymph nodes, development of viremia, virus shed in feces, virus crossing the blood–brain barrier; virus replication in anterior horn cells, cell destruction, motor neurons are damaged, and development of paralysis.
The process by which viruses cause disease in the host is called viral pathogenesis
Complex interactions between the virus and susceptible host result in disease
An important aspect of viral pathogenesis involves viral epidemiology, enabling physicians to study the distribution and determinants of disease in human populations. Understanding factors that influence acquisition and spread of infectious disease are essential for developing methods of prevention and control. Infection in a population can be endemic (disease present at fairly low, but constant, level), epidemic (infection greater than normally occurs in the population), or pandemic (infections that are spread worldwide involving a novel virus and person-to-person spread). Infection can be direct, for instance, respiratory spread of influenza virus, or indirect, for example, arboviruses (West Nile virus, yellow fever virus, Dengue virus) transmission involving a mosquito vector
Epidemiology deals with distribution and determinants of disease in human populations
Understanding the distribution and spread of disease involves several epidemiologic measures. Infectivity is the frequency with which an infection is transmitted when there is contact between a virus and a susceptible host, and represents the ability of the virus to infect an individual. Measures of infectivity are generally expressed as attack rates (number of persons infected after exposure/the number of susceptible persons). Disease index or pathogenicity is the ability of a pathogen to produce infection and cause disease (number of persons with clinical disease/total number infected). Virulence is a measure of severity of disease when infection occurs and represents the degree of damage done by a pathogen (number of persons with fatal or severe disease/total number infected). Incidence is the number of new cases of a disease among persons at-risk within a specified time period (new cases of disease/population at-risk) and reflects the risk of disease in a population. Attack rates are incidence rates calculated during epidemic outbreaks. Prevalence is the total number of cases of disease in a population during a defined time period (number of new and old cases of disease/population at-risk), and represents the burden of disease in a population. Propagation of epidemics involves multiple factors such as infectivity, pathogenicity, virulence, incidence, as well as host factors such as age, sex, race, ethnicity, genetic predisposition, and immune status including the degree and duration of immunity. Natural selection and prior exposure influence susceptibility of a population to new infection. Infectious disease pandemics occur when immunity is low or absent because of little or no prior exposure or introduction of a novel virus into a population. Cross-immunity may be lost by antigenic shifts. Therefore, immunization is the most effective method of specific individual and community protection against many infectious diseases.
Several viral factors such as infectivity and virulence and host factors, including age, genetic predisposition, and immune status significantly influence manifestations of disease
Immunization is the most effective way in preventing infectious disease in individuals and populations
TRANSMISSION AND ENTRY
Viruses are transmitted via horizontal (common route of transmission: person to person), and vertical (mother-to-child transmission) routes or vector transmission (from mosquitoes, animals; Tables 7–1 and 7–2). Human viruses cause either systemic or localized infections by entering the host through a variety of routes, including direct inoculation as well as respiratory, conjunctival, gastrointestinal, and genitourinary routes (Figure 7–2). In addition, viruses can enter the host through a break in the skin or via mucosal surfaces of various routes such as respiratory, gastrointestinal, and genitourinary tracts. Mother-to-child transmission (vertical transmission) can occur in utero, during delivery (via birth canal), and through breast-feeding.
TABLE 7–1 Common Routes of Transmission
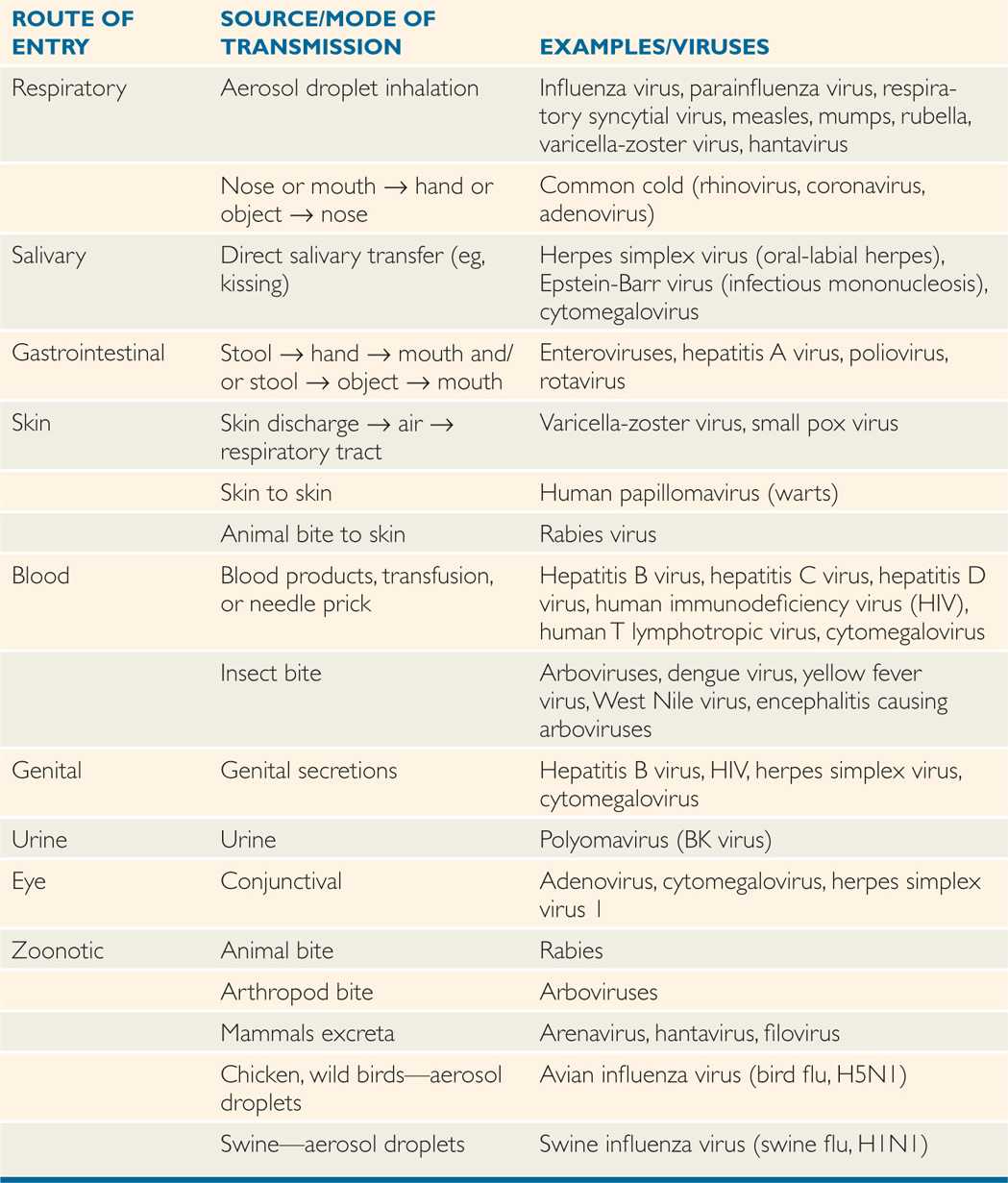
TABLE 7–2 Vertical Transmission of Viruses
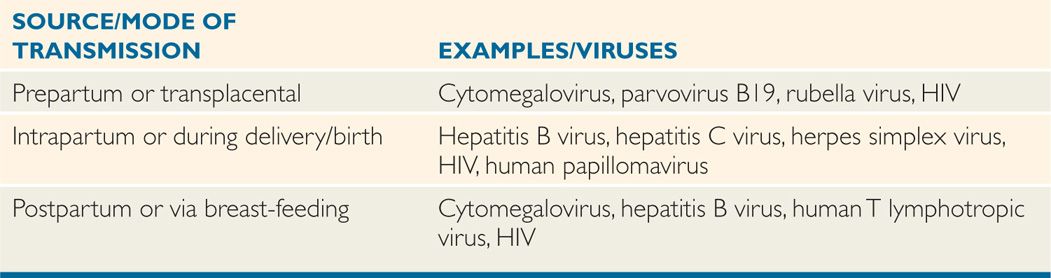
FIGURE 7–2. Routes and sites of entry of viruses into hosts. In this schematic diagram, various routes and sites of viral entry are shown, including food and water, aerosol, respiratory, gastrointestinal, break in the skin, via mucosal or blood, insect or animal bite, and urogenital, anal or sexual routes.
Viruses are transmitted horizontally (common routes) and vertically (mother to child)
Some viruses are transmitted through sexual routes
Zoonotic (animal-to-human) transmission of viral infections can occur from the bite of animals (eg, rabies) or insects (eg, dengue, yellow fever, West Nile) or from inhalation of animal excreta (eg, hantavirus, arenavirus; Table 7–2). In some cases, avian flu virus (bird flu) can be transmitted from birds or poultry to humans, and swine flu virus can also be transmitted to humans.
Some viruses are transmitted through mosquito or animal bites
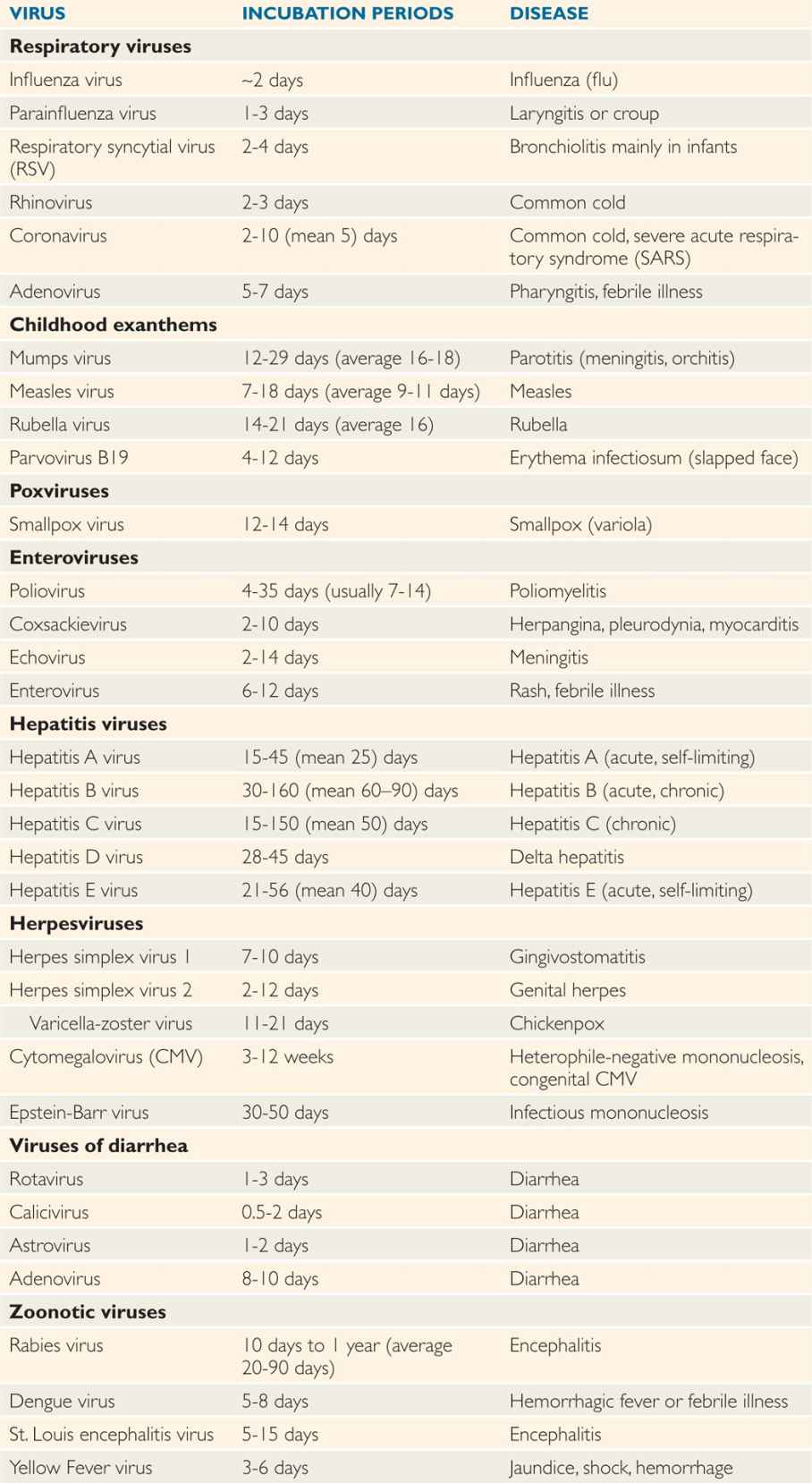
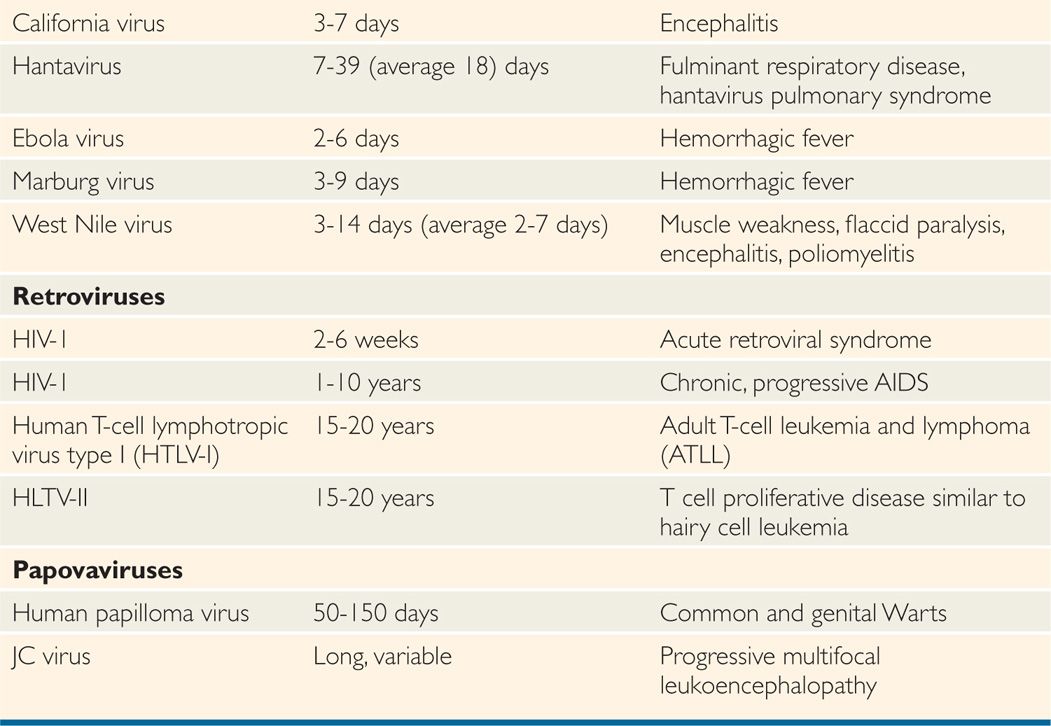
After virus entry into the host, viruses have variable incubation periods. Incubation period is the time between exposure to the organism and appearance of the first symptoms of the disease. Viruses generally multiply at the site of entry in order to establish infection in the host. Some of the examples include: respiratory viruses multiplying in the upper respiratory tract just after entry; rabies virus multiplying in the muscle cells after animal bite; and West Nile virus multiplying in Langerhans cells of skin after mosquito bite. Some viruses have short incubation periods (influenza—2-4 days), whereas others have long incubation periods (eg, hepatitis B virus—weeks to several months). Incubation periods of common viral infections are shown in Table 7–3. Communicability of a disease is the ability of the organism to shed in secretions, which may occur early in the incubation period. Some viruses can integrate into the host genome (HIV), survive by slow replication in the presence of an immune response (hepatitis B and C viruses [HBV, HCV]) or stay latent (herpes simplex virus [HSV]). This dormancy or latency is dangerous because the virus may emerge long after the original infection has occurred and potentially infect others.
TABLE 7–3 Incubation Periods of Human Pathogenic Viruses
SPREAD IN THE HOST
Viral infections produce either localized infection at the site of entry or disseminated infection spread throughout the body. Localized infections include influenza, parainfluenza, common cold (rhinoviruses, coronaviruses), gastrointestinal infections (rotaviruses, Norwalk viruses), and skin infections (papillomaviruses). In localized infections, the virus spreads mainly by infecting adjacent or neighboring cells
Viral infections cause either localized or systemic disease
Several viruses that cause systemic disease in the host spread from the site of entry to the target tissue, where they cause cell injury after multiplication. Viruses use two major routes to spread and cause systemic infection, that is, hematogenous (via the bloodstream) and neural (via neurons) spread. Some of the viruses that cause systemic or disseminated infection are poliovirus, flavivirus, rabies virus, HBV, HCV, HIV, measles, varicella zoster virus, and others. Pathogenesis of poliovirus can be cited as an example of disseminated infection in which poliovirus is transmitted via the fecal–oral route, and the disease (paralytic poliomyelitis) is caused in the central nervous system (CNS; Figure 7–1). Poliovirus replicates at the sites of entry in the small intestine and spreads to the regional lymph nodes where it multiplies again and enters the bloodstream, resulting in primary viremia. The virus is spread via the bloodstream to other organs (liver, spleen), where it multiplies and enters the bloodstream causing secondary viremia followed by transmission to, and replication in, the CNS and resultant damage to motor neurons. The development of viremia allows the immune system to mount humoral and cell-mediated responses to control the poliovirus infection.
Poliovirus enters by the fecal–oral route and multiplies in the small intestine, but causes major disease in the central nervous system
Viremia develops when the virus is detected in blood
Some of the viruses that are spread by the neural route are HSV, poliovirus, rabies virus, and certain arboviruses including West Nile virus and St. Louis encephalitis virus. HSV is transmitted in vesicle fluids, saliva, and vaginal secretions and replicated in the mucoepithelial cells, causing primary infection and then traveling via sensory neurons to nerve bundles called ganglia where they establish latent infection. HSV can also travel into the CNS and infect the brain causing herpes encephalitis.
Some viruses are spread via nerves to the target tissue
TROPISM
Tropism is the capability of viruses to infect a discrete population of cells within an organ. Cellular or tissue tropism is most often determined by the specific interaction of viral surface proteins (spikes) and cellular receptors on the host cells. Some of the identified cellular receptors for viruses are shown in Table 6–5. However, it should be kept in mind that the presence of a receptor for a virus is not always sufficient for viral infection in the target cells. For example, the presence of CD4 (HIV receptor) alone on target cells does not allow virus entry into these cells, but it requires that target cells also express coreceptors, CXCR4 or CCR5 (chemokine receptors) for efficient viral attachment. Tropism can also be determined by intracellular factors including host transcription factors and other factors necessary for viral replication.
Tropism is the capacity of viruses to infect a specific cell type within a tissue or organ
Tropism is most often determined by the specific interaction between viral surface proteins and cellular receptors
Some other viruses, such as HSV-1 and HSV-2, also use a receptor and coreceptor. Heparan sulfate serves as a receptor for HSV, whereas HveA, HveB, and HveC have been identified as coreceptors. Different viruses may use the same cellular molecule as receptors. Some examples are sialic acid residues functioning as important components of the receptor for influenza, corona, and reoviruses. Similarly, heparan sulfate is the receptor for HSV, cytomegalovirus (CMV), and adeno-associated virus (AAV).
Some viruses such as HIV use a receptor (CD4) and coreceptor (CCR5 or CXCR4)
Different viruses may use the same receptor on host cells
After attachment of viral surface proteins to the cellular receptor, the viral genome-protein complex is released in the cytoplasm followed by transcription, replication, and virus assembly. Whereas enveloped viruses use two mechanisms for entry—receptor-mediated endocytosis (viropexis) and fusion—naked capsid viruses use viropexis without membrane–membrane fusion. Influenza virus is tropic to cells that express sialic acid residues containing glycoproteins where the influenza virus attachment protein, hemagglutinin (HA), binds to the receptor, following which the virion is internalized into an endosomal vacuole and the viral envelope membrane fuses with the vacuole membrane. For other enveloped viruses such as HIV, viral envelope gp120 binds to the cellular receptor (CD4) and coreceptor (CXCR4 or CCR5) for attachment, and envelope gp41 fuses the viral envelope with the plasma membrane. Naked capsid viruses, such as poliovirus and hepatitis A virus, use outer capsid spikes to begin attachment to the cellular receptor; the virion is internalized and the viral genome is released in the cytoplasm without membrane–membrane fusion.
Enveloped viruses enter cells via receptor-mediated endocytosis (viropexis) and/or fusion
Naked capsid viruses enter cells via viropexis without membrane–membrane fusion
Both RNA and DNA viruses undergo genetic changes, including mutation and recombination (see Chapter 6). Viral tropism can be altered in the case of some viruses because of genetic variation in the viral surface proteins. Avian influenza virus (H5N1) does not bind to the receptor of human influenza virus (H1N1), but mutation or reassortment in H5N1 may allow binding of H5N1 to H1N1 receptor (see Chapter 9). Similarly, genetic changes in HIV-1 Env gp120 during infection in patients switch the coreceptor requirement from CCR5 to CXCR4. CCR5 is predominantly found on macrophages and also on T lymphocytes, whereas CXCR4 is mainly expressed on T lymphocytes (see Chapter 18).
Genetic changes in viral surface proteins alter viral tropism
Although interaction of the viral surface proteins with the receptors on the host cell plays a critical role in determining the tropism, other factors such as viral gene expression, especially in the case of retroviruses, hepatitis B viruses, and papillomaviruses, contribute to tropism. For example, HBV replicates more efficiently in liver cells, and papillomavirus in skin cells, because of regulation of individual viral promoter transcriptions.
Besides viral surface protein–cellular receptor interactions, viral gene expression also contributes to tropism
VIRULENCE AND CYTOPATHOGENICITY
The ability of a virus to cause disease in an infected host is called pathogenicity. Virulence is the relative ability of a virus to cause disease. Viral virulence is, basically, the degree of pathogenicity of a virus. A virus may be of high or low virulence for a particular host. Different strains of the same virus may differ in the degree of pathogenicity. The ability of a virus to cause degenerative changes in cells or cell death is called cytopathogenicity. Viral strains that kill target cells and cause disease are called virulent viruses, but other strains that have mutated and lost their ability to cause cytopathic effects (CPE) and disease are termed as avirulent or attenuated strains. Some attenuated strains can be used as live vaccines. Examples are MMR (measles, mumps, rubella), smallpox, poliovirus (not used in the United States), and yellow fever.
Pathogenicity is defined as the ability of a virus to cause disease in an infected host
Virulence is the relative ability of a virus to cause disease
Virulence can be measured as the degree of pathogenicity between closely related viruses to cause disease
Three major outcomes can be attributed to a viral infection: abortive infection, in which no progeny virus particles are produced, but the cell may die because early viral functions can occur; (2) lytic infection, in which active virus production is followed by cell death; and (3) persistent infection, in which small numbers of virus particles are produced with little or no CPE. Persistent infections include latent infection, in which viral genetic material remains in host cell without production of virus and may be activated at a later time to produce virus and/or transform the host cell; chronic infection, which involves low level of virus production with little or no CPE; and viral transformation, in which viral infection or viral gene product induces unregulated cellular growth, and cells form tumors in the host. If two closely related viruses infect a host, then infection by the first virus can inhibit the function of the second virus; this is termed interference.
Cytopathogenicity is the ability of a virus to cause degenerative changes in cells or cell death
Viruses can cause abortive, lytic, or persistent infections
Persistent infections could be latent or chronic infection
Virulence and cytopathogenicity depend on the nature of viruses and the characteristics of cells such as permissive and nonpermissive cells. A permissive cell permits production of progeny virus particles and/or viral transformation. A nonpermissive cell does not allow virus replication, but it may permit transformation of the cell. Replication of the virus results in alterations of cellular morphology and function as well as antigenicity of the virus. When a lytic virus infects a permissive cell, lots of daughter viruses are produced and this is followed by lysis of the infected cells, called cytopathic effects (CPE) of the virus (Figure 4–9). The features of CPE are morphologic changes of the cell organelles, including nucleus (inclusion bodies, thickening of the nucleus, swelling, nucleolar changes, margination of chromatin), cytoplasm (inclusion bodies, vacuoles), and membranes (cells round up, loss of adherence, cell fusion [syncytia]), followed by cellular lysis (disintegration).
Cytopathic effects (CPE) caused by a virus include morphologic changes of the cell followed by cell death
The molecular and genetic determinants of viral virulence are complex. Viral gene products influence pathogenesis and virulence. As previously described, viral surface proteins, both in enveloped and naked capsid viruses, determine tropism and spread, and alterations in these surface proteins may result in changes in tropism, spread, and virulence. However, other regions of the viral genome contribute to pathogenicity and virulence. There is no single master gene or protein that determines virulence. For example, live attenuated vaccine of poliovirus, also called oral polio vaccine (OPV), contains all three serotypes of poliovirus that are attenuated and have markedly reduced neurovirulence compared with wild-type polioviruses. The neurovirulence determinants are located in the 5′ untranslated region of the genome involved in initiation of translation and an internal ribosomal entry site, structural capsid proteins (VP1-VP4), and nonstructural proteins such as viral polymerase.
Molecular and genetic determinants of viral virulence are located throughout the viral genome
Some viruses encode a new class of proteins called virokines and viroreceptors, which contribute to viral virulence by mimicking cellular proteins. It is believed that some large DNA viruses, such as poxviruses and herpesviruses, have acquired these genes by recombination from the cells in which they replicate. Virokines are secreted from infected cells and act as cytokines, helping the cells to proliferate and increase virus production. Viroreceptors resemble cytokine receptors and attract cellular cytokines. In addition, some viruses encode proteins that bind antibodies or components of complement pathways to avoid lysis of virus-infected cells. For example, a member of the poxvirus family, vaccinia virus (strain used in smallpox vaccine), encodes a vaccinia complement control protein (VCP) that abrogates the complement-mediated killing of virus-infected cells. Similarly, two glycoproteins of HSV act together as a receptor for the Fc domain of immunoglobulins to avoid antibody-directed cell-mediated cytotoxicity (ADCC).
Some viruses such as poxviruses and herpesviruses encode virokines and viroreceptors to help cells proliferate and avoid host defenses, respectively
PATTERNS OF VIRAL INFECTION AND DISEASE
Not every viral infection results in a disease. Infection involves multiplication of the virus in the host, whereas disease represents a clinically apparent response. Infections are much more common than disease; unapparent infections are termed subclinical, and the individual is referred to as a carrier. Although some primary infections are invariably accompanied by clinical manifestations of the disease (influenza, measles), other infections may propagate and spread for long periods before the extent of problem is recognized (HIV-1, HBV, and HCV).
Infections are more common than disease
Infection involves multiplication of virus in the host, and disease represents clinical manifestations
Relative susceptibility of a host for a viral infection in terms of severity of the disease depends on several factors such as virulence, molecular and genetic determinants of the virus, and host factors (immune status of the host, age, health, and genetic background). After viral transmission, the virus multiplies in the host; this phase is referred to as the incubation period, which varies for different viruses (Table 7–3). Initial virus replication generally results in viremia, which allows the virus to travel to the target tissues and replicate further to cause cell damage and clinical symptoms. The host immune system plays a pivotal role in determining the course of infection and progression of disease.
The severity of the disease depends on the role of both viral and host factors in influencing viral infection and disease progression
Viral infection results in either a lytic or persistent (latent or chronic) infection. Lytic infections are those in which productive virus replication results in cell death because viral replication is not compatible with essential cellular functions. Several viruses interfere with the synthesis of cellular macromolecules and other factors that prevent cellular growth, maintenance, and repair, thus leading to cell death. For example, poliovirus blocks the synthesis of cellular proteins by inhibiting the translation of cellular mRNA and competing for ribosomes. Accumulation of progeny viruses and viral proteins can destroy the structure and function, and enhance the process of apoptosis, resulting in cell death. In enveloped viruses such as respiratory syncytial virus (RSV), HIV, and HSV, replication of the virus and cell surface expression of the envelope glycoproteins (spikes) cause cell-to-cell spread and formation of multinucleated giant cells (syncytia) causing cell death (cytopathic effect).
Viral infections could be lytic, latent, or chronic
Persistent viral infections are those in which the infected cells survive the effect of viral replication. Persistent infections are of two kinds: latent (viral genome without virus production) and chronic (low level of virus production without immune clearance). In addition, some persistent viruses cause oncogenic transformations. Several DNA viruses have the potential to cause oncogenic transformation; some viruses can cause tumors in their natural hosts (human papillomaviruses, HPV; HBV), whereas others can cause tumors in other species or only transform cells in vitro (human adenoviruses, human polyomaviruses). Some RNA viruses, such as retroviruses (human T lymphotropic virus, HTLV) and HCV can cause oncogenic transformation in infected hosts. In these human oncogenic viruses, viral gene products transform the cells either by interfering with the tumor suppressor gene pathways (eg, HPV) or increasing the expression of protoonco-genes (HTLV).
Persistent infection could be either latent or chronic
Some persistent viruses can cause oncogenic transformation
Based on patterns and levels of detectable infectious virus in the host and the role of immune response in clearing the virus, viral infections can be divided into five categories: (1) acute infection that is cleared by the immune response; (2) acute infection that becomes latent and periodically reactivated; (3) acute infection that becomes chronic; (4) acute infection followed by persistent infection (viral set point) established by immune response and followed by virus overproduction, immune dysfunction, and opportunistic infections; and (5) slow chronic infections. These patterns are shown in Figure 7–3 A-E. In acute infection, the virus enters the host, then multiplies at the site of entry and in the target tissue, and this is followed by viremia and cytopathic effects. This type of infection is a lytic infection. The immune system mounts both cellular and humoral responses and successfully eliminates the virus from the host. Examples of acute viral infections followed by clearance of the virus from the host by immune responses are hepatitis A, influenza, parainfluenza, rhino-, and coronaviruses. After causing acute or lytic infection, some viruses are not eliminated by the immune response but persist in the host either in a noninfectious latent form or an infectious chronic form. Most of the viruses opting to persist in the host have evolved various mechanisms for persistence, including restriction of viral cytopathic effects, infection of immunologically privileged sites, maintenance of viral genomes without full viral gene expression, antigenic variation, suppression of immune components, and transformation of host cells.
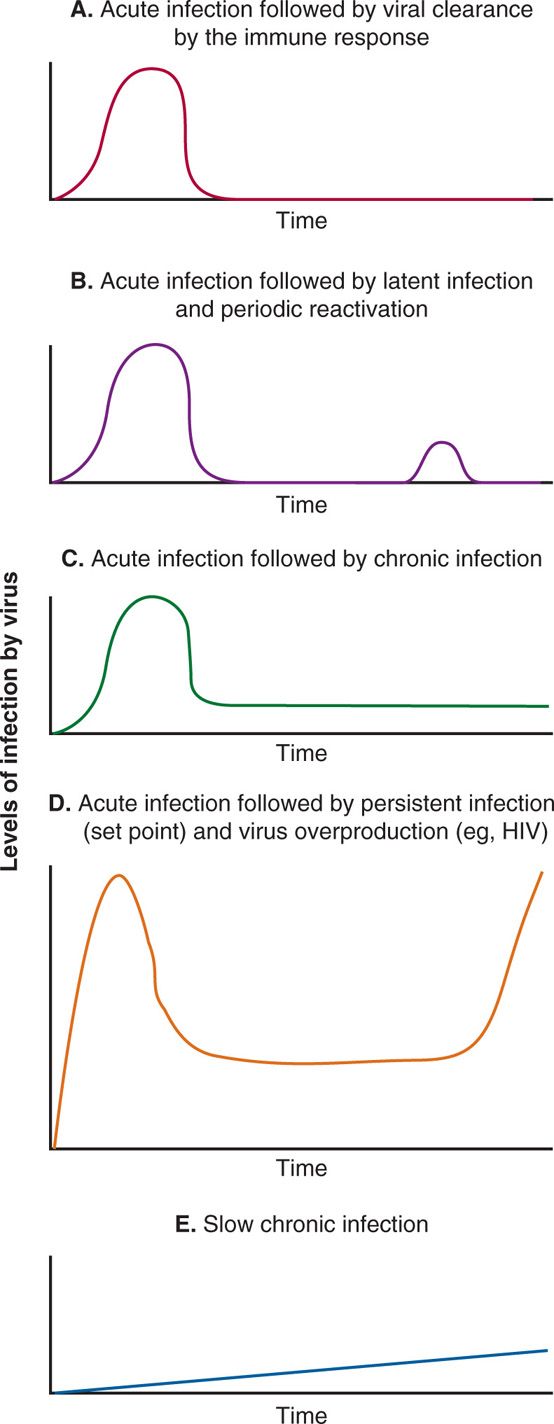
FIGURE 7–3. Patterns of viral infection. In these line diagrams, various patterns of viral infection are shown, including: A. Acute viral infection followed by viral clearance by the immune response (eg, Hepatitis A virus, influenza virus, parainfluenza virus, rhinovirus). B. Acute viral infection followed by viral latency and periodic reactivation (eg, herpes simplex viruses). C. Acute viral infection followed by chronic infection (eg, HBV and HCV). D. Acute viral infection followed by persistent infection (viral set point) and clinical latency followed by virus overproduction, immune dysfunction, and opportunistic infections (eg, HIV), and E. Slow chronic infections (eg, prions).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


