FIGURE 43–1. Fungi system view. Localized disease (left) is caused by local trauma or the superficial invasion of flora resident on the oropharyngeal (thrush), gastrointestinal, or vaginal mucosa. Systemic disease (right) begins with inhalation of conidia followed by dissemination to other sites.
Environmental conidia are inhaled or injected
Endogenous yeasts may invade
PATHOGENESIS
Compared with bacterial, viral, and parasitic disease, less is known about the pathogenic mechanisms and virulence factors involved in fungal infections. Analogies to bacterial diseases come the closest because of the apparent importance of adherence to mucosal surfaces, invasiveness, extracellular products, and interaction with phagocytes (Figure 43–2). In general, the principles discussed in Chapter 22 apply to fungal infections. Most fungi are opportunists, causing serious disease only in individuals with impaired host defense systems. Only a few fungi are able to cause disease in previously healthy persons.
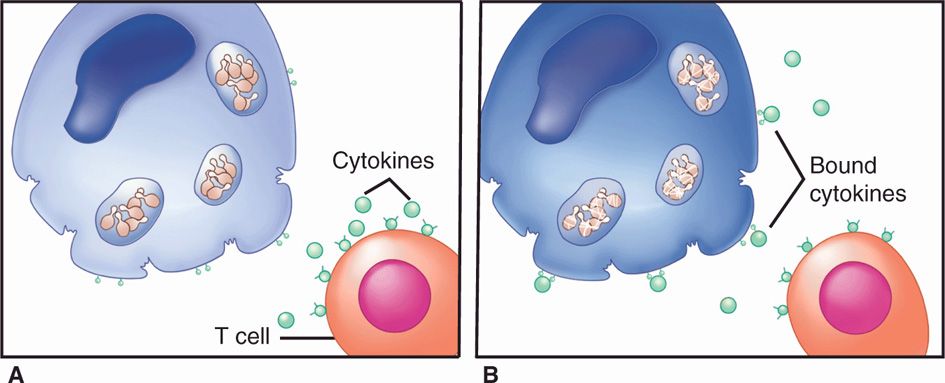
FIGURE 43–2. Immunity to fungal infections. A. Pathogenic fungi are able to survive and multiply slowly in nonactivated macrophages. B. When macrophages are activated by cytokines from T-cells the growth is restricted and the fungi digested.
Fungal pathogenesis is similar to bacteria
Most fungi are opportunists
 Adherence
Adherence
Several fungal species, particularly the yeasts, are able to colonize the mucosal surfaces of the gastrointestinal and female genital tracts. It has been shown experimentally that the ability to adhere to buccal or vaginal epithelial cells is associated with colonization and virulence. Within the genus Candida, the species that best adhere to epithelial cells are those most frequently isolated from clinical infections. Adherence usually requires a surface adhesin on the fungus and a receptor on the epithelial cell. In the case of C albicans, mannoprotein components extending from the cell wall have been implicated as the adhesin and fibronectin, and components of the extracellular matrix as the receptor(s). A few binding mediators have been identified for other fungi, usually a surface mannoprotein.
Adherence is mediated by fungal adhesins and host cell receptors
Mannoprotein is an adhesin, and fibronectin a receptor
 Invasion
Invasion
Passing an initial surface barrier—skin, mucous membrane, or respiratory epithelium—is an important step for most successful pathogens. Some fungi are introduced through mechanical breaks. For example, Sporothrix schenckii infection typically follows a thorn prick or some other obvious trauma. Fungi that initially infect the lung must produce conidia small enough to be inhaled past the upper airway defenses. For example, arthroconidia of Coccidioides immitis (2-6 μm) can remain suspended in air for a considerable period of time and can reach the terminal bronchioles to initiate pulmonary coccidioidomycosis.
Traumatic injection is linked to trauma
Small conidia may pass airway defenses
Triggered by temperature and possibly other cues, dimorphic fungi from the environment undergo a metabolic shift similar to the heat shock response and completely change their morphology and growth to a more invasive form. Invasion directly across mucosal barriers by the endogenous yeast C albicans is similarly associated with a morphologic change, the formation of hyphae. The triggering mechanisms of this change are unknown, but the new form is able to penetrate and spread. Extracellular enzymes (eg, proteases, elastases) are associated with the advancing edge of the hyphal form of Candida and with the invasive forms of many of the dimorphic and other pathogenic fungi. Although these enzymes must contribute to some aspect of invasion or spread, their precise role is unknown for any fungus.
Invasion across mucosal barriers may involve enzymes
 Injury
Injury
None of the extracellular products of opportunistic fungi or dimorphic pathogens has been shown to injure the host directly during infection in a manner analogous to bacterial toxins. Although the presence of necrosis and infarction in the tissues of patients with invasion by fungi such as Aspergillus suggests a toxic effect, direct evidence is lacking. Several fungi do produce exotoxins, called mycotoxins, in the environment but not in vivo. The structural components of the cell do not cause effects similar to those of the endotoxin of Gram-negative bacteria, although mannan is known to circulate widely in the body. The circulating products of Cryptococcus neoformans have been shown to downregulate immune functions. The injury caused by fungal infections seems to be due primarily to the destructive aspects of delayed-type hypersensitivity (DTH) responses as a result of the inability of the immune system to clear the fungus. In this respect, fungal infections resemble tuberculosis more than any other disease.
No classic exotoxins are produced in vivo
Injury is due to inflammatory and immunologic responses
IMMUNITY
 Innate Immunity
Innate Immunity
Considerable evidence exists indicating that healthy persons have a high level of innate immunity to most fungal infections. This is particularly true of opportunistic molds. This resistance is mediated by the professional phagocytes (neutrophils, macrophages, and dendritic cells), the complement system, and pattern recognition receptors. For fungi, the most important receptors include a lectin-like structure on phagocytes (dectin-1) that binds glucan, and Toll-like receptors (TLR2, TLR4). In most instances, neutrophils and alveolar macrophages are able to kill the conidia of fungi if they reach the tissues. A small number of species, all of which are dimorphic, are able to produce mild to severe disease in otherwise healthy persons. In vitro studies have shown these fungi to be more resistant to killing by phagocytes than the opportunists possibly because of a change in surface structures subject to pattern recognition. Candida albicans is able to bind complement components in a way that interferes with phagocytosis.
Most fungi are readily killed by neutrophils
Coccidioides immitis, one of the best-studied species, has been shown to contain a component in the wall of its conidial (infective) phase that is antiphagocytic. As the hyphae convert to the spherule (tissue) phase, they also become resistant to phagocytic killing because of their size and surface characteristics. Some fungi produce substances such as melanin, which interfere with oxidative killing by phagocytes. The tissue yeast form of Histoplasma capsulatum multiplies within macrophages by interfering with lysosomal killing mechanisms in a manner similar to that of some bacteria. These mechanisms of avoiding phagocytic killing appear to allow many dimorphic fungi to multiply sufficiently to produce an infection that can be controlled only by the immune response.
Tissue phases of dimorphic fungi resist phagocytic killing
 Adaptive Immune Response
Adaptive Immune Response
A recurrent theme with fungal infections is the importance of an intact immune response in preventing infection and progression of disease. Most fungi are incapable of producing even a mild infection in immunocompetent individuals. A small number of species are able to cause clinically apparent infection that usually resolves once there is time for activation of normal immune responses. In most instances in which it has been investigated, the actions of neutrophils and TH1-mediated immune responses have been found to be of primary importance in this resolution. Progressive, debilitating, or life-threatening disease with these agents is commonly associated with depressed or absent cellular immune responses, and the course of any fungal disease is worse in immunocompromised than previously healthy persons.
T-cell–mediated responses of primary importance
Progressive fungal diseases occur in the immunocompromised
 Humoral Immunity
Humoral Immunity
Antibodies can be detected at some time during the course of almost all fungal infections, but for most there is little evidence that they contribute to immunity. The only encapsulated fungus, C neoformans, is an example of a fungus against which antibody plays a role in controlling infection. Although the polysaccharide capsule of C neoformans has antiphagocytic properties similar to those of encapsulated bacterial pathogens, it is less antigenic. Anticapsular antibody plays a role in resolving cryptococcal infection, but TH1 responses are still dominant. Antibody also plays a role in control of C albicans infections by enhancing fungus–phagocyte interactions, and this is probably true for other yeasts. In some other fungal infections, the lack of protective effect of antibody is striking. In coccidioidomycosis, for example, high titers of C immitis-specific antibodies are associated with dissemination and a worsening clinical course.
Opsonizing antibody is effective in some yeast infections
 Cellular Immunity
Cellular Immunity
Considerable clinical and experimental evidence points toward the importance of cellular immunity in fungal infections. Most patients with severe systemic disease have neutropenia, defects in neutrophil function, or depressed TH1 immune reactions. These can result from factors such as steroid treatment, leukemia, Hodgkin disease, and AIDS. In other cases, an immunologic deficit can usually be demonstrated by absence of delayed-type hypersensitivity responses or TH1-stimulated cytokines specific to the fungus in question. In the latter case, it is possible that hyporesponsiveness is due at least, in part, to activation of suppressor cells or continued circulation of fungal antigen.
Systemic disease associated with deficiencies in neutrophils and T-cell–mediated immunity
Although not all fungi have been studied to the same extent, a unified picture emerging from clinical and experimental animal studies is illustrated in Figure 43–2. When hyphae or yeast cells of the fungus reach deep tissue sites, they are either killed by neutrophils or resist destruction by one of the antiphagocytic mechanisms described earlier. Surviving cells continue to grow slowly or, if they are dimorphic, convert to their yeast, hyphal, or spherule tissue phases. The growth of these invasive forms may be slowed but not killed by macrophages, which ingest them. A feature of the fungal pathogens is to resist the killing mechanisms of the macrophage and continue to multiply. In healthy persons, the extent of infection is minimal, and any symptoms are caused by the inflammatory response.
Fungi that escape neutrophils grow slowly in macrophages
Everything awaits the specific adaptive immune response to the invader. In fungal infections, it is the interaction between dendritic cells and macrophages that favors production of interleukin 12 (IL-12) and interferon (INF-γ) leading the CD4 cells to differentiate to TH2 cells that has the dominant effect. The turning point comes when local macrophages containing multiplying fungi are activated by cytokine mediators produced by T lymphocytes that have interacted with the fungal antigen. The activated macrophages are then able to restrict the growth of the fungus, and the infection is controlled. Defects that disturb this cycle lead to progressive disease. To the extent that they are known, the specifics of these reactions are discussed in the following chapters.
Growth is restricted when macrophages activated by cytokines
Immune defects lead to progressive disease
LABORATORY DIAGNOSIS
 Direct Examination
Direct Examination
Fungi often demonstrate distinctive morphologic features on direct microscopic examination of infected pus, fluids, or tissues owing to their large size. The simplest method is to mix the specimen with a 10% solution of potassium hydroxide (KOH) and place it under a coverslip. The strong alkali digests the tissue elements (epithelial cells, leukocytes, debris), but not the rigid cell walls of either yeasts or molds. After digestion of the material, the fungi can be observed under the light microscope with or without staining (Figure 43–3). Direct examinations can be aided by the use of calcifluor white, a dye that binds to polysaccharides in cellulose and chitin. Under ultraviolet light, calcifluor white fluoresces, enhancing detection of fungi in fluids or tissue sections. A few yeasts take the Gram stain, including C albicans (Gram positive).
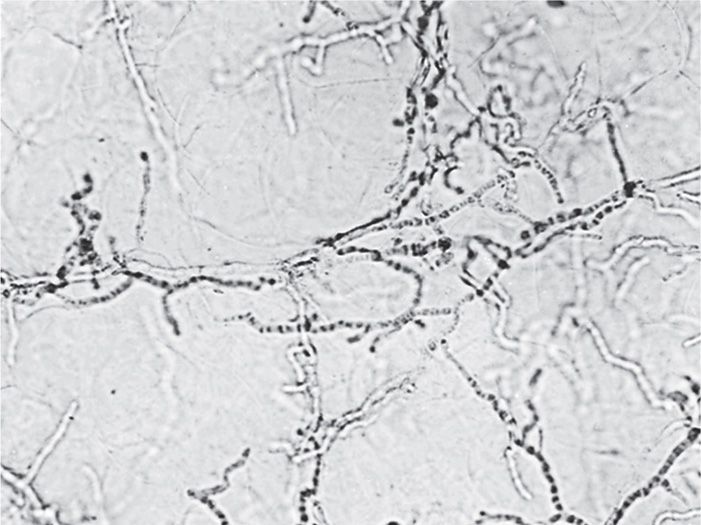
FIGURE 43–3. KOH (potassium hydroxide) preparation. Scalp scrapings from a suspected ringworm lesion have been mixed with 10% KOH and viewed under low power. The skin has been dissolved, revealing tubular branching hyphae.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


