Found in flora of vertebrates
These organisms are elongated or oval and typically measure 10 to 20 μm in length. They often possess a rudimentary cytostome (mouth aperture) and organelles, such as ventral discs or axostyles, which help maintain their intraluminal position. They are readily recognized in body fluid or excreta by their rapid motility and some can be specifically identified in unstained preparations. All can be cultivated on artificial media.
Morphology and rapid motility are distinctive
Some luminal flagellates, most notably T vaginalis, possess only a trophozoite stage and are sexually transmitted. Most, including G duodenalis, possess both trophozoite and cyst forms. The latter, which is the infective form, is transmitted via the direct or indirect fecal–oral route. Human-to-human infection is thus found in populations where inadequate sanitation or poor personal hygiene favors spread.
May or may not have the cyst stage
Trichomonas vaginalis
![]() PARASITOLOGY
PARASITOLOGY
Three members of the genus Trichomonas parasitize humans (Table 53–1), but only T vaginalis is an established pathogen. The three species closely resemble one another morphologically, but confusion in identification is rare because of the specificity of their habitats.
Three Trichomonas species have similar morphology
The T vaginalis trophozoite (Figure 53–1) is oval and typically measures 7 by 15 μm. Organisms up to twice this size are occasionally recovered from asymptomatic patients and from cultures. In stained preparations, a single, elongated nucleus and a small cytostome are observed anteriorly. Five flagella arise nearby. Four immediately exit the cell. The fifth bends back and runs posteriorly along the outer edge of an abbreviated undulating membrane. Lying along the base of this membrane is a cross-striated structure known as the costa. A conspicuous microtubule containing a supporting rod or axostyle bisects the trophozoite longitudinally and protrudes through its posterior end. It is thought that the pointed tip of this structure is useful for attachment. In unstained wet mounts, T vaginalis is identified by its axostyle and jerky, nondirectional movements.
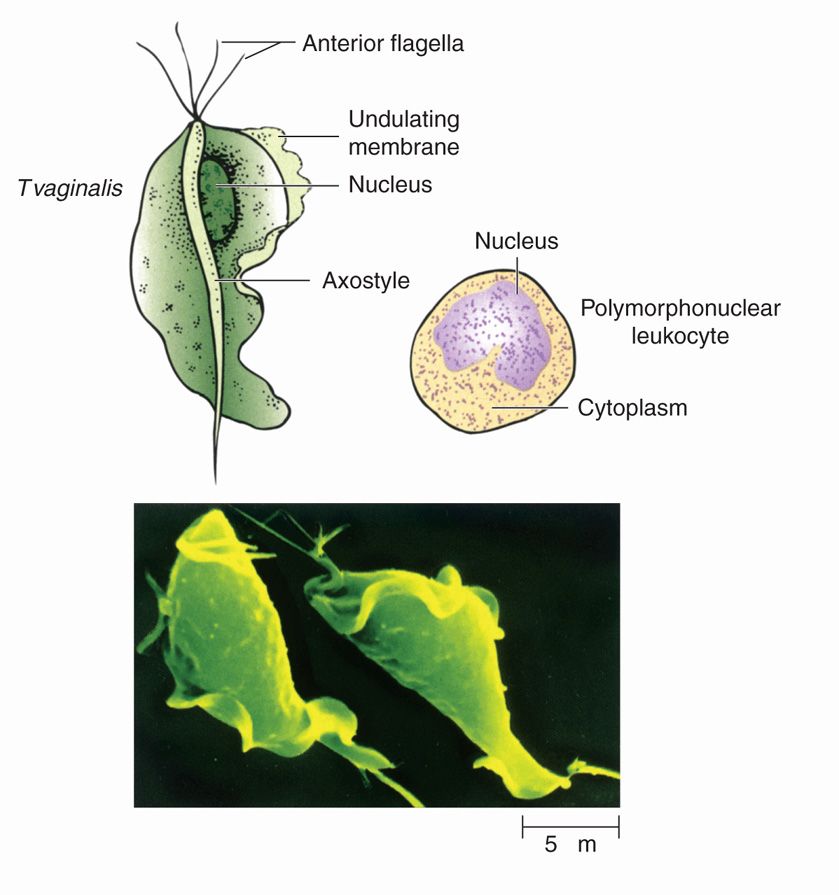
FIGURE 53–1. Trichomonas vaginalis. The parasite and its structures are shown in relation to the size of a polymorphonuclear leukocyte (top). The micrograph below illustrates their use for motility. (Reproduced with permission from Nester EW: Microbiology: A Human Perspective, 6th edition. 2009.)
Protruding axostyle may mediate attachment
The organism can be grown on artificial media under anaerobic conditions at pH 5.5 to 6.0. Soluble nutrients are absorbed across the cell membrane. A variety of carbohydrates are degraded to short-chained organic acids. Pyruvate is produced via glycolysis and reduced to lactate, part of which enters structures called hydrogenosomes. Molecular hydrogen and ATP are produced in the hydrogenosomes. These structures are analogous to mitochondria, which T vaginalis lacks. Although T vaginalis lacks a cyst form, the trophozoite can survive outside of the human host for 1 to 2 hours on moist surfaces. In urine, semen, and water, it may be viable for up to 24 hours, making it one of the most resistant of protozoan trophozoites. Attempts to infect laboratory animals have met with limited success.
Cultivable in vitro
Lacks cyst form, but may survive a few hours outside host
![]() TRICHOMONIASIS
TRICHOMONIASIS
EPIDEMIOLOGY
Trichomoniasis is a cosmopolitan disease usually transmitted by sexual intercourse. An estimated 8 million infections occur in the United States annually. Worldwide this figure reaches 180 million cases. Twenty-five percent of sexually active women become infected at some time during their lives and 30% to 70% of their male sexual partners are also parasitized, at least transiently. As would be expected, the likelihood of acquiring the disease correlates directly with the number of sexual contacts. Infection is rare in adult virgins, whereas rates as high as 70% are seen among prostitutes, sexual partners of infected patients, and individuals with other venereal diseases. In women, the peak incidence of trichomoniasis is between 16 and 35 years of age, but there is still a relatively high prevalence in the 30- to 50-year age group.
Transmission usually sexual
Prevalence linked to sexual activity
Nonvenereal transmission is uncommon
Nonvenereal transmission is uncommon. Transfer of organisms on shared washcloths may explain, in part, the high frequency of infection seen among institutionalized women. Female neonates are occasionally noted to harbor T vaginalis, presumably acquiring it during passage through the birth canal. High levels of maternal estrogen produce a transient decrease in the vaginal pH of the child, rendering it more susceptible to colonization. Within a few weeks, estrogen levels drop, the vagina assumes its premenarcheal state, and the parasite is eliminated.
PATHOGENESIS AND IMMUNITY
Direct contact of T vaginalis with the squamous epithelium of the genitourinary tract results in destruction of the involved epithelial cells and the development of a neutrophilic inflammatory reaction and petechial hemorrhages. Attachment appears to be mediated by adhesins, laminin-binding proteins, and lectin-binding carbohydrates. Trophozoites are capable of secreting a variety of proteinases that undoubtedly help initiate contact-dependent cytolytic events. These proteinases are also capable of degrading immunoglobulin-G (IgG) and IgA. A contact-independent mechanism of cell damage has also been shown to correlate with the presence of a 200 kDa glycoprotein that is heat and acid labile. Changes in the microbial, hormonal, and pH environment of the vagina as well as factors inherent to the infecting parasite are thought to modulate the severity of the pathologic changes.
Infection of the vaginal epithelium triggers innate responses by stimulating Toll-like receptors that trigger secretion of proinflammatory cytokines. This brings about a neutrophil and CD4+ response. Humoral and cellular immune responses follow, although they do not appear to result in clinically significant immunity. Because of the proinflammatory response produced, women with this infection are at greater risk of human immunodeficiency virus (HIV) infection. Trichomonas vaginalis is also capable of phenotypically varying surface antigenic determinants to help it escape immune detection. Parasite damages epithelial cells on contact.
 TRICHOMONIASIS: CLINICAL ASPECTS
TRICHOMONIASIS: CLINICAL ASPECTS
MANIFESTATIONS
In women, T vaginalis produces a persistent vaginitis. Although up to 50% are asymptomatic at the time of diagnosis, most develop clinical manifestations within 6 months. Approximately 75% develop a discharge, which is typically accompanied by vulvar itching or burning (50%), dyspareunia (50%), dysuria (50%), and a disagreeable odor (10%). Although fluctuating in intensity, symptoms usually persist for weeks or months. Commonly, manifestations worsen during menses and pregnancy. Eventually, the discharge subsides, even though the patient may continue to harbor the parasite. In symptomatic patients, physical examination reveals reddened vaginal and endocervical mucosa. In severe cases, petechial hemorrhages and extensive erosions are present. A red, granular, friable endocervix (strawberry cervix) is a characteristic but uncommon finding. An abundant discharge is generally seen pooled in the posterior vaginal fornix. Although classically described as thin, yellow, and frothy in character, the discharge more frequently lacks these characteristics. Trichomoniasis may increase the risk of preterm birth and enhance susceptibility to HIV infections.
Chronic vaginitis lasting weeks to months
The urethra and prostate are the usual sites of trichomoniasis in men; the seminal vesicles and epididymis may be involved on occasion. Infections are usually asymptomatic, possibly because of the efficiency with which the organisms are removed from the urogenital tract by voided urine. Symptomatic men complain of recurrent dysuria and scant, nonpurulent discharge. Acute purulent urethritis has been reported rarely. Trichomoniasis should be suspected in men presenting with nongonococcal urethritis, or a history of either prior trichomonal infection or recent exposure to trichomoniasis.
Urethral and prostatic infection in men usually asymptomatic
DIAGNOSIS
The diagnosis of trichomoniasis rests on the detection and morphologic identification of the organism in the genital tract. Identification is accomplished most easily by examining a wet mount preparation for the presence of motile organisms. In women, a drop of vaginal discharge is the most appropriate specimen; in men, urethral exudate or urine sediment after prostate massage may be used. Although highly specific when positive, wet mounts have a sensitivity of only 50% to 60%. They are most likely to be negative in asymptomatic or mildly symptomatic patients and in women who have douched in the previous 24 hours. Giemsa- and Papanicolaou-stained smears provide little additional help. The recent introduction of a commercial system that allows direct, rapid microscopic examination without the need for daily sampling may ameliorate this situation. Direct immunofluorescent antibody staining has a sensitivity of 70% to 90%. Parasitic culture, though more sensitive, requires several days to complete and is frequently unavailable. Nucleic acid amplification (NAA) methods have been shown to be the most sensitive for diagnosis.
Wet mount examination for motile trophozoites sufficient in most symptomatic cases
TREATMENT
Oral metronidazole is extremely effective in recommended dosage, curing more than 95% of all Trichomonas infections. It may be given as a single dose or over 7 days. Simultaneous treatment of sexual partners may minimize recurrent infections, particularly when single-dose therapy is used for the index case. Because of the disulfiram-like activity of metronidazole, alcohol consumption should be suspended during treatment. The drug should never be used during the first trimester of pregnancy because of its potential teratogenic activity. Use in the last two trimesters is unlikely to be hazardous but should be reserved for patients whose symptoms cannot be adequately controlled with local therapies. High-dose, long-term metronidazole treatment has been shown to be carcinogenic in rodents. No association with human malignancy has been described to date, and in the absence of a suitable alternative drug, metronidazole continues to be used. NAA-based studies have shown that this infection is under diagnosed, and therefore infections are undertreated contributing to the continued high incidence of this parasite.
Metronidazole cures 95% of cases
Giardia lamblia
![]() PARASITOLOGY
PARASITOLOGY
Giardia lamblia was first described by Anton von Leeuwenhoek 300 years ago when he examined his own diarrheal stool with one of the first primitive microscopes. It was not until the last several decades, however, that this cosmopolitan flagellate became widely regarded in the United States as a pathogen. Of the six other flagellated protozoans known to parasitize the alimentary tract of humans, only one, Dientamoeba fragilis, has been credibly associated with disease. Definitive confirmation or refutation of its pathogenicity will, it is hoped, not require the passage of another three centuries.
Unlike T vaginalis, Giardia possesses both a trophozoite and a cyst form (Figure 53–2). It is a sting-ray–shaped trophozoite 9 to 21 μm in length, 5 to 15 μm in width, and 2 to 4 μm in thickness. When viewed from the top, the organism’s two nuclei and central parabasal bodies give it the appearance of a face with two bespectacled eyes and a crooked mouth. It is uncertain why this organism has two nuclei, but both are transcriptionally active. Four pairs of flagella—anterior, lateral, ventral, and posterior—reinforce this image by suggesting the presence of hair and chin whiskers. These distinctive parasites reside in the duodenum and jejunum, where they thrive in the alkaline environment and absorb nutrients from the intestinal tract. They move about the unstirred mucous layer at the base of the microvilli (Figure 53–3) with a peculiar tumbling or “falling leaf” motility or, with the aid of a large ventral disk, attach themselves to the brush border of the intestinal epithelium. The exact molecular mechanism by which the ventral disk mediates attachment has not been resolved but is thought, in part, to involve flagellar motility. Unattached organisms may be carried by the fecal stream to the large intestine.
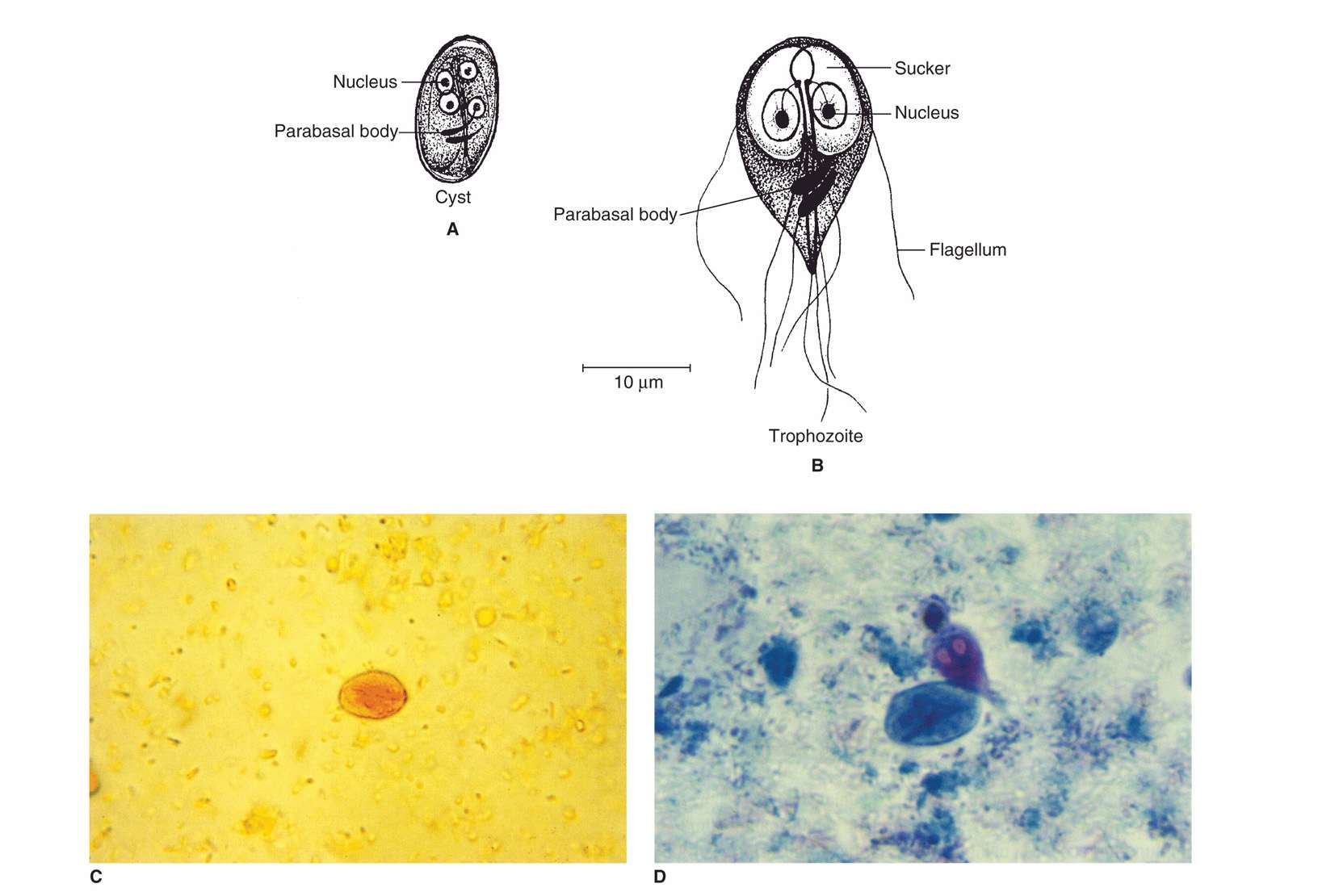
FIGURE 53–2. Giardia lamblia. A. Cyst structures. B. trophozoite structures. C. Cyst in stool iodine preparation. D. trophozoite in stool. (C and D, reproduced with permission from Connor Dh, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford Ct: appleton & Lange, 1997.)
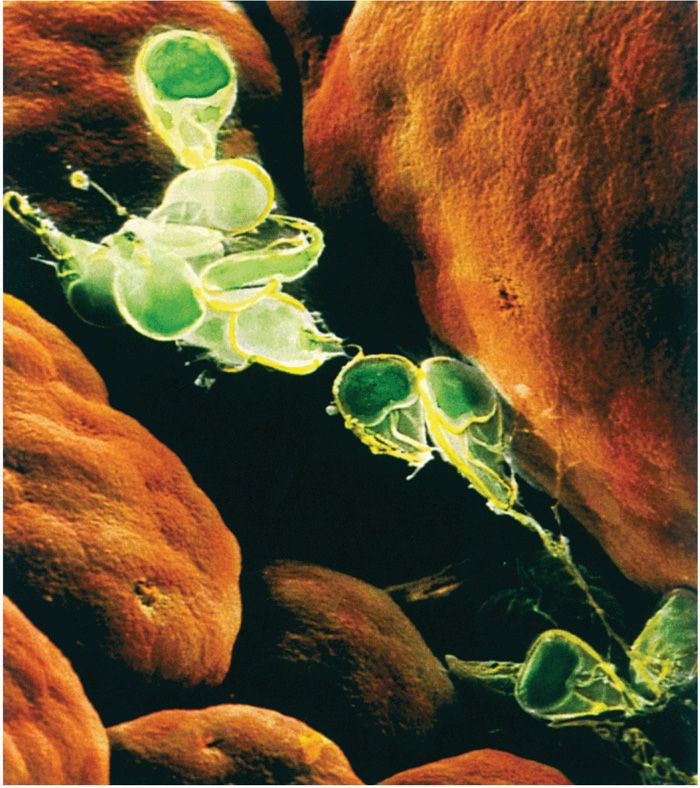
FIGURE 53–3. Giardiasis. Scanning electron micrograph of G lamblia trophozoites in human intestine. (Reproduced with permission from Nester EW: Microbiology: A Human Perspective, 6th edition. 2009.)
Trophozoite and cyst stages
Move about duodenum and jejunum with tumbling motility
In the descending colon, if transit time allows, the flagella are retracted into cytoplasmic sheaths and a smooth, clear cyst wall is secreted. These forms are oval and somewhat smaller than the trophozoites. With maturation, the internal structures divide, producing a quadrinucleate organism harboring two ventral discs, four kinetosomes, and eight axonemes. When fixed and stained, the cytoplasm pulls away from the cyst wall in a characteristic fashion. The mature cysts, which are the infective form of the parasite, may survive in cold water for more than 2 months and are resistant to concentrations of chlorine generally used in municipal water systems. They are transmitted from host to host by direct and indirect fecal–oral routes. In the duodenum of a new host, the cytoplasm divides to produce two binucleate trophozoites.
Cystic forms develop in colon
Resistant cysts transmitted from host to host
Giardia is amitocondriate like Trichomonas. Instead, Giardia possesses mitosomes, which like the hydrogenosomes of Trichomonas are thought to represent mitochondrial adaptations in these aerotolerant anaerobe parasites. Giardia can respire aerobically or anaerobically with glucose as the main substrate for respiration. Axenic cultivation of this organism has been achieved in vitro. Bile salts enhance the parasite’s growth. Although Giardia has largely been thought to be an asexual parasite, evidence for genetic recombination, hinting at a form of sexual recombination, has recently been reported.
Organisms of the genus Giardia are among the most widely distributed of intestinal Protozoa; they are found in fish, amphibians, reptiles, birds, and mammals. At first, it was assumed that Giardia strains found in different animals were host specific; on this basis, some 40 different species were described. Since it is now recognized that some strains can infect multiple animal hosts, the practice of assigning species status by the host from which the parasite was recovered is considered invalid. At present, only five species are considered valid and of these, only G duodenalis infects humans. This parasite is also commonly referred to as G lamblia or G intestinalis in much of the current literature.
![]() GIARDIASIS
GIARDIASIS
EPIDEMIOLOGY
Giardiasis has a cosmopolitan distribution; its prevalence is highest in areas with poor sanitation and among populations unable to maintain adequate personal hygiene. In developing countries, infection rates may reach 25% to 30%; in the United States, G lamblia is found in 4% of stools submitted for parasitologic examination, making it, along with Cryptosporidium, this country’s most frequently identified intestinal parasite. All ages and economic groups are represented, but young children and young adults are preferentially involved. Children with immunoglobulin deficiencies are more likely to acquire the flagellate, possibly because of a deficiency in intestinal IgA. Giardiasis is also common among attendees of day care centers. Attack rates of over 90% have been seen in the ambulatory non–toilet-trained population (age 1-2 years) of these institutions, suggesting direct person-to-person transmission of the parasite. The frequency with which secondary cases are seen among family contacts reinforces this probability. Undoubtedly, direct fecal spread is also responsible for the high infection rate among male homosexuals. In several recent studies, the prevalence of giardiasis and/or amebiasis in that population has ranged from 11% to 40% and is correlated closely with the number of oral–anal sexual contacts.
Transmission facilitated by poor hygiene and IgA deficiency
High attack rates in day care centers
Giardiasis common among male homosexuals
Waterborne and, less frequently, foodborne transmission of G lamblia has also been documented, and probably accounts for the frequency with which American travelers to Third World nations acquire infection. Unlike the typical bacterial diarrhea syndrome seen in travelers, the diarrhea begins late in the course of travel and may persist for several weeks. More than 20 waterborne outbreaks of giardiasis have also been reported in the United States. The sources have included swimming pools, untreated pond or stream water, sewage-contaminated municipal water supplies, and chlorinated but inadequately filtered water. In a few of these outbreaks, epidemiologic data have suggested that wild mammals, particularly beavers, served as the reservoir hosts. In spite of the evidence for zoonotic transmission, this remains a controversial topic. In some areas of the world, where different animals, including man’s closest friend, the dog, and many have been shown to be infected with Giardia, the infecting genotypes differed. In others, the same genotypes were demonstrated in man and animals. In most cases, humans sampled were shown to predominantly harbor human genotypes. Extensive infectivity studies using human genotypes have not been conducted.
Water- or foodborne traveler’s diarrhea lasts for weeks
Beavers and other mammals possible sources
PATHOGENESIS
Disease manifestations appear related to intestinal malabsorption, particularly of fat and carbohydrates. Disaccharidase deficiency with lactose intolerance, altered levels of intestinal peptidases, and decreased vitamin B12 absorption have been demonstrated. The precise pathogenetic mechanisms responsible for these changes remain poorly understood. Mechanical blockade of the intestinal mucosa by large numbers of Giardia, damage to the brush border of the microvilli by the parasite’s ventral disc, organism-induced deconjugation of bile salts, altered intestinal motility, accelerated turnover of mucosal epithelium, and mucosal invasion have all been suggested. None of these correlates well with clinical manifestations. Patients with severe malabsorption have jejunal colonization with enteric bacteria or yeasts, suggesting that these organisms may act synergistically with Giardia. Eradication of the associated microorganism, however, has not uniformly resulted in clinical improvement. Jejunal biopsies sometimes reveal a flattening of the microvilli and an inflammatory infiltrate, the severity of which correlates roughly with that of the clinical disease. Generally, both malabsorption and the jejunal lesions have been reversed with specific treatment. The demonstration of occasional trophozoites in the submucosa raises the possibility that these changes reflect T-lymphocyte–mediated damage.
Basis for malabsorption and jejunal pathology remains uncertain
IMMUNITY
Susceptibility to giardiasis has been related to several factors, including strain virulence, inoculum size, achlorhydria or hypochlorhydria, and immunologic abnormalities. In one experimental study, humans were challenged with varying doses from as few as 10 cysts. They were uniformly parasitized when 100 or more were ingested. Several workers have noted the frequency with which giardiasis occurs in achlorhydric and hypochlorhydric individuals. Giardia infection produces little or no host inflammation suggesting that local responses may help control the infection. Both innate responses involving nitric oxide, defensins, phagocytic, mast and dendritic cells, and adaptive responses involving IgA and T cells have been identified in mouse models of infections and are thought to operate in human infections as well. Animal studies have demonstrated that Giardia-specific, secretory IgA (sIgA) antibodies inhibit attachment of trophozoites to intestinal epithelium, perhaps by blocking parasite surface lectins. Moreover, antitrophozoite IgM or IgG antibodies, plus complement, are known to be capable of killing Giardia trophozoites. Another indication that antibodies play a role in controlling infections is that humans with immunodeficiencies involving antibody production are more likely to suffer from chronic giardiasis. Giardia trophozoites are also capable of changing their surface coat variant surface proteins (VSPs). VSP switching appears to be transcriptionally controlled. Over 200 VSP genes have been identified for this organism. This process occurs once every 6 to 16 generations. The process of VSP switching undoubtedly helps the organism evade host responses.
Predisposing factors include hypochlorhydria and immunocompromise
![]() GIARDIASIS: CLINICAL ASPECTS
GIARDIASIS: CLINICAL ASPECTS
MANIFESTATIONS
In endemic situations, over two-thirds of persons infected with giardiasis are asymptomatic. In acute outbreaks, this ratio of asymptomatic to symptomatic patients is usually reversed. When they do occur, symptoms begin 1 to 3 weeks after exposure and typically include diarrhea, which is sudden in onset and explosive in character. The stool is foul-smelling, greasy in appearance, and floats. It is devoid of blood or mucus. Upper abdominal cramping is common. Large quantities of intestinal gas produce abdominal distention, sulfuric eructations, and abundant flatus. Nausea, vomiting, and low-grade fever may be present. The acute illness generally resolves in 1 to 4 weeks; in children, however, it may persist for months, leading to significant malabsorption, weight loss, and malnutrition.
Subclinical infections common in endemic areas
Diarrhea, cramping, flatus, and greasy stools
In many adults, the acute phase of giardiasis is often followed by a subacute or chronic phase characterized by intermittent bouts of mushy stools, flatulence, “heartburn,” and weight loss that persist for weeks or months. At times, patients presenting in this fashion deny having experienced the acute syndrome described previously. In the majority, symptoms and organisms eventually disappear spontaneously. It is not uncommon for lactose intolerance to persist after eradication of the organisms. This condition may be confused with an ongoing infection, and the patient may be subjected to unnecessary treatment.
Subacute and chronic infections with weight loss in adults
Lactose intolerance may persist
DIAGNOSIS
The diagnosis of giardiasis is made by finding the cyst in formed stool or the trophozoite in diarrheal stools, duodenal secretions, or jejunal biopsy specimens. In acutely symptomatic patients, the parasite can usually be demonstrated by examining one to three stool specimens after appropriate concentration and staining. In chronic cases, excretion of the organism is often intermittent, making parasitologic confirmation more difficult. Many of these patients can be diagnosed by examining specimens taken at weekly intervals over 4 to 5 weeks. Another approach is to perform an enterotest, in which a bead encapsulated in a gelatinous capsule and attached to a thread is swallowed and then retrieved. The recovered bead is washed onto a slide and examined for active trophozoites. Alternatively, duodenal secretions can be collected and examined for trophozoites in trichrome or Giemsa-stained preparations. There are now a number of reliable, commercially available, enzyme immunoassays (EIAs) for the direct detection of parasite antigen in stool. They appear to be as sensitive and specific as microscopic examinations. Immunofluorescent assays for the detection of cysts are also available. The organism can be grown in culture, but the methods are not currently adaptable to routine diagnostic work. NAA assays are highly sensitive and can distinguish infecting genotypes.
Demonstration of trophozoites and cysts in stool or duodenal aspirates diagnostic
EIAs detect Giardia antigen in stool
TREATMENT
Five drugs are currently available for the treatment of giardiasis in the United States: quinacrine hydrochloride, metronidazole, tinidazole, furazolidone, and paromomycin. Quinacrine and metronidazole are effective (70%-95%) and are preferred for patients capable of ingesting tablets. Furazolidone is used by pediatricians because of its availability as a liquid suspension, but it has the lowest cure rate. These three agents require 5 to 7 days of therapy. Tinidazole, an oral agent that has been widely used in many countries for more than 25 years outside the United States, is safe and effective as a single-dose treatment. This drug has been shown to be the most effective. It has been available in the United States since 2004. Because of the potential for person-to-person spread, it is important to examine and, if necessary, treat close physical contacts of the infected patient, including playmates at nursery school, household members, and sexual contacts. None of the aforementioned agents should be used in pregnant women because of their potential teratogenicity. Paromomycin, a nonabsorbed but somewhat less effective agent, may be used in this circumstance.
Several drugs available
Close contacts should be examined
PREVENTION
Hikers should avoid ingestion of untreated surface water, even in remote areas, because of the possibility of contamination by feces of other people and potentially by feces of infected animals. Adequate disinfection can be accomplished with halogen tablets yielding concentrations higher than that generally achieved in municipal water systems. The safety of the latter results from additional flocculation and filtration procedures. Use of portable filtration units having a nominal pore size of 1 μm is even more effective. Boiling of water, if possible, is even better.
Avoid drinking untreated surface water
 BLOOD AND TISSUE FLAGELLATES
BLOOD AND TISSUE FLAGELLATES
Two of the many genera of hemoflagellates, Leishmania and Trypanosoma, are pathogenic to humans. They reside and reproduce within the gut of specific insect hosts. When these vectors feed on a susceptible mammal, the parasite penetrates the feeding site, invades the blood and/or tissue of the new host, and multiplies to produce disease. American trypanosomes differ somewhat in that the infective parasite is passed in the feces of the specific vector during the act of feeding on its host and later rubbed into the feeding site wound. The life cycle is completed when a second insect ingests the infected mammalian blood or tissue fluid. During the course of their passage through insect and vertebrate hosts, flagellates undergo developmental change. Within the gut of the insect (and in culture media), the organism assumes the promastigote (Leishmania) or epimastigote (Trypanosoma) form (Figure 53–4). These protozoa are motile and fusiform and have a blunt posterior end and a pointed anterior end from which a single flagellum projects. They measure 15 to 30 μm in length and 1.5 to 4.0 μm in width. In the promastigote form, the kinetoplast complex is located in the anterior extremity, and the flagellum exits from the cell immediately. The kinetoplast complex of the epimastigote form, in contrast, is located centrally, just in front of the vesicular nucleus. The flagellum runs anteriorly in the free edge of an undulating membrane before passing out of the cell. In the mammalian host, hemoflagellates appear as trypomastigotes (Trypanosoma) or amastigotes (Leishmania, T cruzi). The former circulate in the bloodstream and closely resemble the epimastigote form, except that the kinetoplast complex is in the posterior end of the parasite. The amastigote stage is found intracellularly. It is round or oval, measures 1.5 to 5.0 μm in diameter, and contains a clear nucleus with a central karyosome. Although it has a kinetoplast complex and an axoneme, there is no free flagellum.
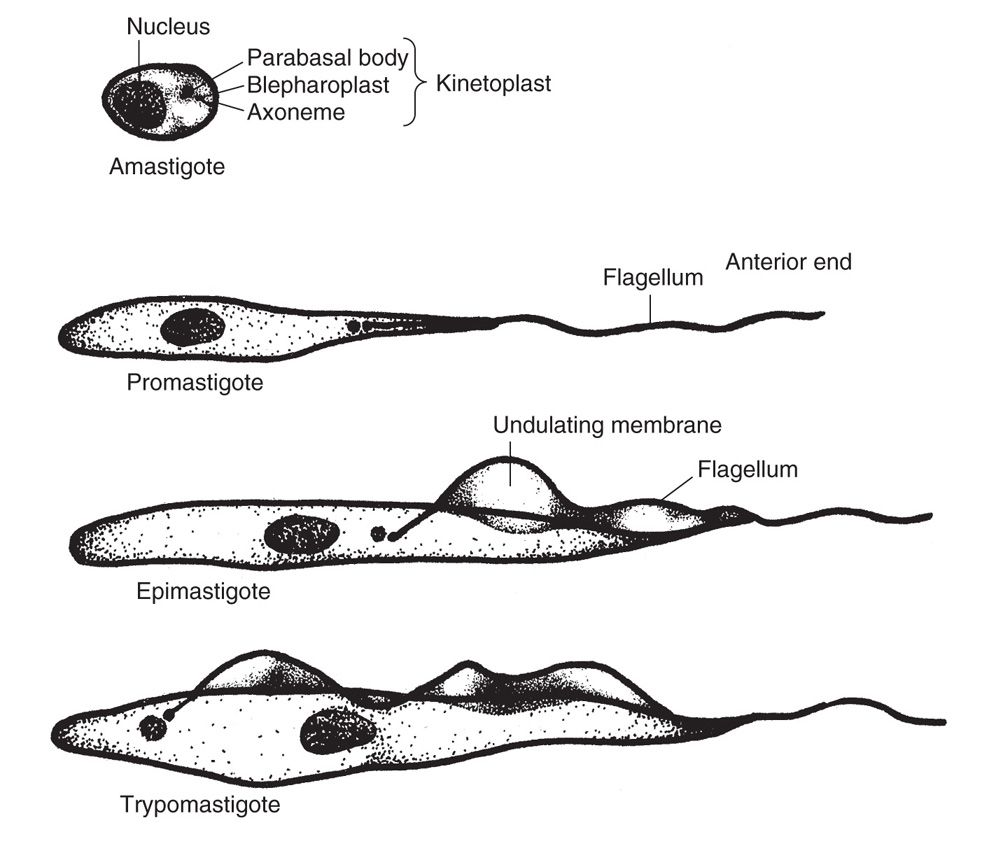
FIGURE 53–4. Stages in the life cycle of the hemoflagellates (trypanosomidae).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


