PARASITOLOGY
Entamoeba histolytica is found throughout the world and the causative agent of diarrhea and amebic dysentery. Infections may spread to extraintestinal sites and become life-threatening. Close to 500 million people are thought to be infected at any one time, but the majority of these are likely due to the morphologically identical E dispar. Because methods are now available to distinguish E histolytica from E dispar, the figure of 500 million infected with E histolytica may actually be closer to 50 million. Transmission is fecal–oral, either directly, or through contaminated water.
LIFE CYCLE, MORPHOLOGY, AND PHYSIOLOGY
Humans are the principal hosts and reservoirs of E histolytica. Transmission from person to person occurs when a cyst passed in the stool of one host is ingested directly or indirectly by another. Human hosts may pass up to 45 million cysts daily. Although the average infective dose exceeds 1000 organisms, ingestion of a single cyst has been known to produce infection. After passage through the stomach, the cyst eventually reaches the distal small bowel. Here, the cyst wall disintegrates, releasing the quadrinucleate parasite, which divides to form eight small trophozoites that are carried to the colon. Colonization is most intense in areas of fecal stasis such as the cecum and rectosigmoid, but may be found throughout the large bowel.
Humans are the hosts and reservoir; fecal–oral transmission
Entamoeba histolytica possesses both trophozoite and cyst forms (Figure 52–1). The trophozoites are microaerophilic, dwell in the lumen or wall of the colon, feed on bacteria and tissue cells, and multiply rapidly in the anaerobic environment of the gut. Even though they are called amitochondriate, they do possess nuclear-encoded mitochondrial genes and a remnant organelle. They do have unusual features including polyploid chromosomes, repetitive DNA, multiple origins of DNA replication, genes lacking introns, and unique endocytic pathways. Trophozoites are passed unchanged in the liquid diarrheic stool. Here they can be recognized by their size (12-20 μm in diameter); directional motility; granular, vacuolated endoplasm; and sharply demarcated, clear ectoplasm with finger-like pseudopods. Invasive strains tend to be larger and may contain ingested erythrocytes within their cytoplasm (Figure 52–2). Appropriate stains reveal a 3 to 5 μm nucleus with a small central karyo-some or nucleolus and fine regular granules evenly distributed around the nuclear membrane (peripheral chromatin). Electron microscopic studies demonstrate microfilaments, an external glycocalyx, and cytoplasmic projections thought to be important for attachment.
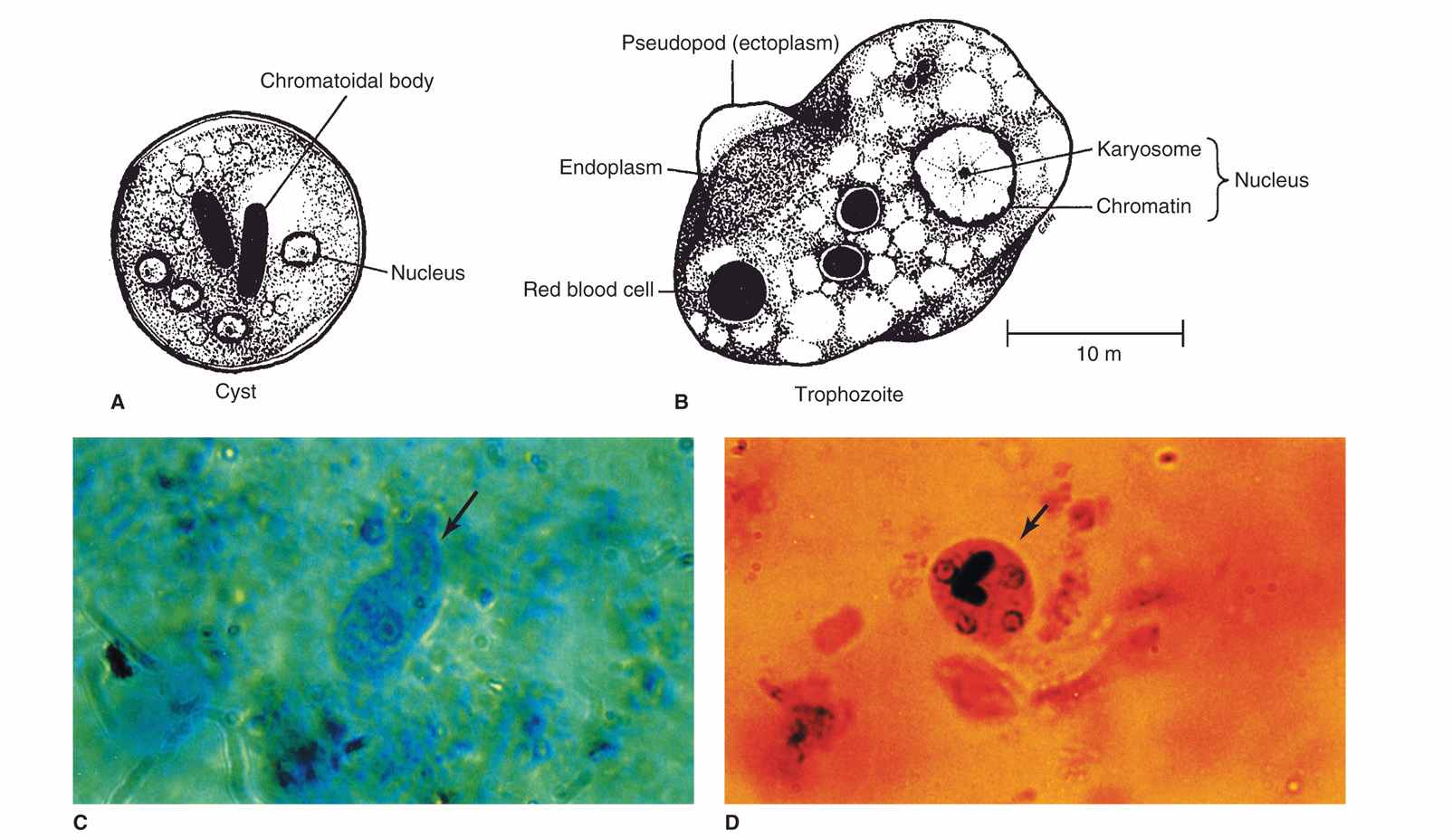
FIGURE 52–1. Entamoeba histolytica. A. Cyst structures. B. Trophozoite structures. C. Trophozoite in stool (arrow). D. Cyst (arrow) in stool iodine preparation and cysts in stool iodine preparation.
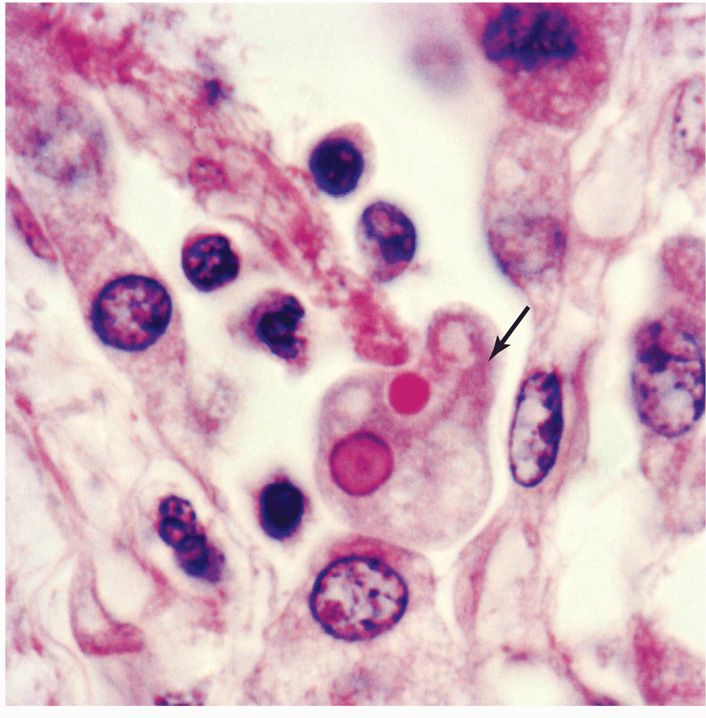
FIGURE 52–2. Amebiasis. An E histolytica trophozoite (arrow) is invading tissue. Note the extending pseudopod and engulfed erythrocyte. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford Ct: appleton & Lange, 1997.)
Trophozoites multiply rapidly in the gut
Trophozoites are facultative anaerobes that require complex media for growth. Sterile culture techniques (axenic) have been developed and are essential for the preparation of the purified antigens required for serologic testing, zymodeme typing, and characterization of virulence factors. Such techniques are generally available only in research laboratories.
Facultative anaerobes
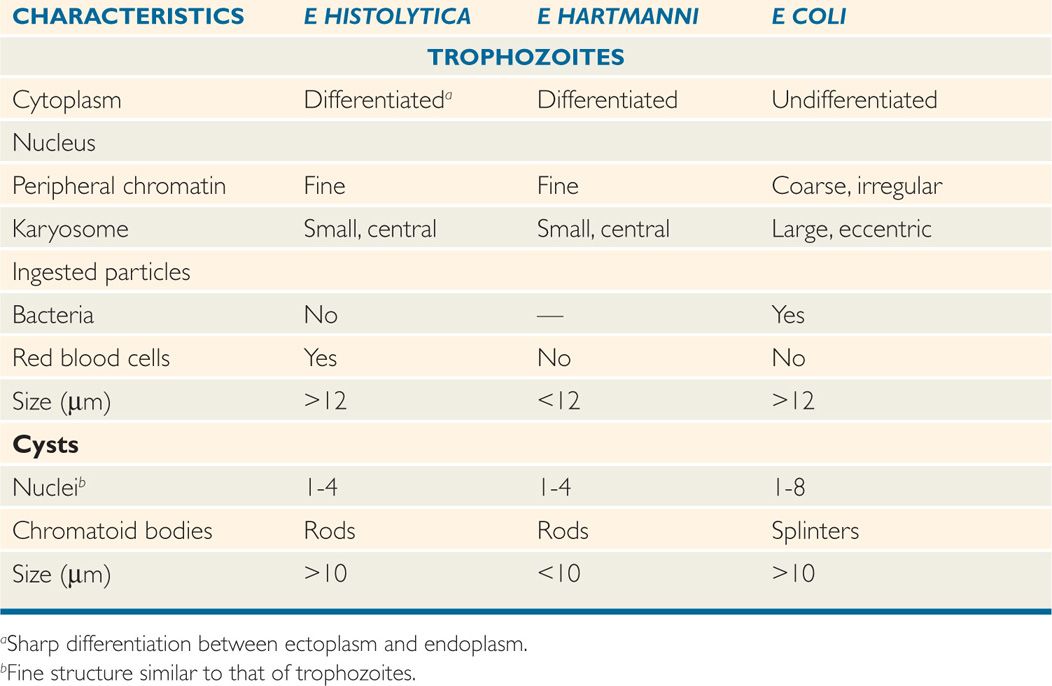
With normal stool transit time, trophozoites usually encyst before leaving the gut. Initially, a cyst contains a single nucleus, a glycogen vacuole, and one or more large, cigar-shaped ribosomal clusters known as chromatoid bodies. With maturation, the cyst becomes quadrinucleate, and the cytoplasmic inclusions are absorbed. In contrast to the fragile trophozoite, mature cysts can survive environmental temperatures up to 55°C, chlorine concentrations normally found in municipal water supplies, and normal levels of gastric acid. Entamoeba histolytica can be differentiated from the other amebas of the gut by its size, nuclear detail, and cytoplasmic inclusions (Table 52–1).
TABLE 52–1 Some Differential Characteristics of Entamoeba Species
Cysts are hardy; can survive in chlorinated water supply
EPIDEMIOLOGY
Entamoeba histolytica infection rates are higher in warm climates, particularly in areas where the level of sanitation is low. Worldwide, this organism is thought to produce more deaths than any other parasite, except those that cause malaria and schistosomiasis. Reports of amebic liver abscess, for instance, emanate primarily from Mexico, western South America, South Asia, and West and South Africa. For reasons apparently unrelated to exposure, symptomatic illness is much less common in women and children than in men.
Worldwide infection; highest rates in warmer climates
Although stool surveys in the United States indicate that 1% to 5% of the population harbors Entamoeba, the majority of these are now known to be colonized with the nonpathogenic E dispar. The incidence of invasive amebiasis in the United States decreased sharply over several decades, reaching a nadir in 1974. Since then, the numbers have increased steadily. It is now seen particularly in institutionalized individuals, Indian reservations, migrant labor camps, victims of acquired immunodeficiency syndrome (AIDS), and travelers to endemic areas.
Invasive disease rare in United States
Symptomatic amebiasis is usually sporadic, the result of direct person-to-person fecal–oral spread under conditions of poor personal hygiene. Venereal transmission is seen in male homosexuals, presumably the result of oral–anal sexual contact. Food- and water-borne spread occurs, occasionally in epidemic form. Such outbreaks, however, are seldom as explosive as those produced by pathogenic intestinal bacteria. One outbreak of intestinal amebiasis was due to colonic irrigation at a chiropractic clinic.
Fecal–oral spread linked to poor hygiene
Food and water are other modes of transmission
PATHOGENESIS
A number of virulence factors have been identified in E histolytica. In an experimental setting, invasiveness correlates well with endocytic capacity, the production of extracellular proteinases capable of activating complement and degrading collagen, the presence of a galactose-specific lectin (Gal/GalNAc) capable of mediating attachment of the organism to colonic mucosa, and—perhaps most important—the capacity to lyse host cells on contact. This has been termed parasite-mediated or contact-dependent cytotoxicity. The latter phenomenon is initiated by the galactose-specific, lectin-mediated adherence of the trophozoite to a target cell. After adherence, the ameba releases a pore-forming protein that polymerizes in the target cell membrane, forming large tubular lesions. Cytolysis rapidly follows. Cysteine proteinases, secreted by the amebas, have also been identified as a major virulence factor. They can degrade portions of the extracellular matrix, including fibronectin, laminin, and type I collagen, and they can interfere with the complement pathway and humoral IgA and IgG responses. Ultimately, this may lead to extraintestinal spread of the trophozoites which may occur in approximately 1% of established infections. Cyst formation does not take place at extraintestinal sites.
Virulence determinants include lectin-mediated adherence to mucosa and capacity to lyse host cells
In most cases of E histolytica infections, however, tissue damage is minimal, and the host remains symptom free, suggesting that host factors may modulate the invasiveness of virulent strains. These factors are still poorly understood, but changes in host resistance, the colonic milieu, or the parasite itself may amplify tissue damage and clinical manifestations. Protein malnutrition, high-carbohydrate diets, corticosteroid administration, childhood, and pregnancy all appear to render the host more susceptible to invasion. Certain colonic bacteria appear to enhance invasiveness, possibly by providing a more favorable redox potential for survival and multiplication or by facilitating the adherence of the parasite to colonic mucosa. Finally, it is known that the pathogenic strains in the tropics are more invasive than those isolated in temperate areas, possibly because poor sanitation results in more frequent passage through humans.
Most infected individuals are symptom free
Colonic microflora may influence invasiveness
PATHOLOGY
The interaction of amebas with the intestinal epithelial barrier results in an inflammatory response marked by the secretion of cytokines. This, in turn, results in neutrophil activation which can be protective or result in enhanced tissue destruction. This type of response is characteristic of early invasive amebiasis and is in contrast to what is seen in well-established infections manifest by colonic ulcers. In the latter instance, there is little inflammatory response other than edema and hyperemia, and the mucosa between ulcers appears normal. Trophozoites are present in large numbers at the junction between necrotic and viable tissue. Once the lesion penetrates below the superficial epithelium, it meets the resistance of the colonic musculature and spreads laterally in the submucosa, producing a flask-like lesion with a narrow mucosal neck and a large submucosal body. It eventually compromises the blood supply of the overlying mucosa, resulting in sloughing and a large necrotic ulcer. Extensive ulceration leads to secondary bacterial infection, formation of granulation tissue, and fibrotic thickening of the colon. In approximately 1% of patients, the granulation tissue is organized into large, tumor-like masses known as amebomas. The major sites of involvement, in order of frequency, are the cecum, ascending colon, rectum, sigmoid, appendix, and terminal ileum. Amebas may also enter the portal circulation and be carried to the liver or, more rarely, to the lung, brain, or spleen. In these organs, liquefaction necrosis leads to the formation of abscess cavities in which only trophozoites are encountered.
Mucosal ulceration with little inflammatory response
Flask-like ulcers extend to submucosa
Amebomas and metastatic amebic abscesses in a few cases
IMMUNITY
Although E histolytica elicits both humoral and cellular immune responses in humans, it is still not clear which, and to what degree, these responses are capable of modulating initial infection or thwarting reinfection. In endemic areas, the prevalence of gastrointestinal colonization increases with age, suggesting that the host is incapable of clearing E histolytica from the gut. However, the relative infrequency with which populations living in these areas suffer repeated bouts of severe amebic colitis or liver abscess indicates that those who experience such infections have protection against recurrent disease.
Innate defense against E histolytica begins with the mucous lining of the intestinal epithelium. Ironically, although this may restrict amebic contact with epithelial cells, it also provides a milieu for colonization because of the mucins present. What is clear is that infected hosts produce a rather strong mucosal IgA response and much of this is directed against the carbohydrate domain of the Gal/GalNAc lectin present on the ameba’s surface. Children with this type of response in Bangladesh had 86% fewer new infections than children without it.
As stated previously, interaction of amebas with the intestinal epithelium results in an inflammatory response causing activation of cytokines. Neutrophils become involved, which may help promote further damage because of their destruction by cysteine proteases released by the amebas and resulting in release of superoxide radicals, or they may help mediate protection following activation via TNF-α.
Patients with invasive disease are known to produce high levels of circulating antibodies. Nevertheless, no correlation exists between the presence or concentration of such antibodies and protective immunity, possibly because pathogenic E histolytica trophozoites have the capacity to aggregate and shed attached antibodies and are resistant to the lytic action of complement. Cell-mediated responses have been described in patients with amebic liver abscess and are associated with lymphocyte proliferation and cytokine secretion. Activated macrophages also have the capacity to kill amebas, presumably through nitric oxide or peroxidase production. The susceptibility to invasive amebiasis of malnourished populations, pregnant women, and steroid-treated individuals or patients indicates that cell-mediated immune mechanisms may be directly involved in the control of tissue invasion. The picture is less clear in patients with AIDS and requires further study.
Immunity is incomplete and does not correlate with antibody response
Trophozoites shed antibody and resist complement lysis
Pathogenic E histolytica strains produce a lectin-like substance that is mitogenic for lymphocytes. It has been suggested that this substance could stimulate viral replication of human immunodeficiency virus-infected lymphocytes as does another mitogen, phytohemagglutinin.
 AMEBIASIS: CLINICAL ASPECTS
AMEBIASIS: CLINICAL ASPECTS
MANIFESTATIONS
Individuals who harbor E histolytica are usually clinically well. In most cases, particularly in the temperate zones, the organism is avirulent, living in the bowel as a normal commensal inhabitant. Spontaneous disappearance of amebas, over a period of weeks to months, among such patients is common. Serologic data, however, suggest that some asymptomatic carriers possess virulent strains and incur minimal tissue invasion. In this population, the infection may eventually progress to produce overt disease.
Relationship usually commensal
Diarrhea, flatulence, and cramping abdominal pain are the most common complaints of symptomatic patients. The diarrhea is intermittent, alternating with episodes of normality or constipation over a period of months to years. Typically, the stool consists of one to four loose to watery, foul-smelling passages that contain mucus and blood. Physical findings are limited to abdominal tenderness localized to the hepatic, ascending colonic, and cecal areas. Sigmoidoscopy reveals the typical ulcerations with normal intertwining mucosa.
Diarrhea, flatulence, and abdominal pain most common
Ulcerations with mucus and blood in stool occur in fulminant disease
Fulminating amebic dysentery is less common. It may occur spontaneously in debilitated or pregnant individuals or maybe precipitated by corticosteroid therapy. Its onset is often abrupt, with high fever, severe abdominal cramps, and profuse diarrhea. Most commonly, abscesses occur singly and are localized to the upper outer quadrant of the right lobe of the liver. This localization results in the development of point tenderness overlying the cavity and elevation of the right diaphragm. Liver function is usually well preserved. Isotopic or ultrasound scanning confirms the presence of the lesion. Needle aspiration results in the withdrawal of reddish-brown, odorless fluid free of bacteria and polymorphonuclear leukocytes; trophozoites may be demonstrated in the terminal portion of the aspirate since they are likely colonizing the intact tissue at the periphery of the abscess.
Hepatic abscess may have acute or insidious onset
Approximately 5% of all patients with symptomatic amebiasis present with a liver abscess. Ironically, fewer than one-half can recall significant diarrheal illness. Although E histolytica can be demonstrated in the stools of 72% of patients with amebic liver abscess when a combination of serial microscopic examinations and culture is used, routine microscopic examination of the stool detects less than half of these. Complications relate to the extension of the abscess into surrounding tissue, producing pneumonia, empyema, or peritonitis. Extension of an abscess from the left lobe of the liver to the pericardium is the single most dangerous complication. It may produce rapid cardiac compression (tamponade) and death or, more commonly, a chronic pericardial disease that may be confused with congestive cardiomyopathy or tuberculous pericarditis.
Hepatic abscess may extend to other tissues
DIAGNOSIS
The microscopic diagnosis of intestinal amebiasis depends on the identification of the organism in stool or sigmoidoscopic aspirates. Because trophozoites appear predominantly in liquid stools or aspirates, a portion of such specimens should be fixed immediately to ensure preservation of these fragile organisms for stained preparations. The specimen may then be examined in wet mount for typical motility, concentrated to detect cysts, and stained for definitive identification. If trophozoites or cysts are seen, they must be carefully differentiated from those of the commensal parasites, particularly E hartmanni and E coli (Table 52–1). Entamoeba histolytica trophozoites can be differentiated from those of E dispar only by the presence of ingested erythrocytes in the former and by molecular methods; the cysts appear identical.
Stools examined for trophozoites and cysts in stained or wet preparations
Entamoeba histolytica trophozoites ingest erythrocytes; E dispar trophozoites do not
Recently, sensitive and specific stool antigen tests for E histolytica have become commercially available; their value in the clinical diagnosis of amebiasis, when compared with microscopic examination, is now clear. Although cultural and polymerase chain reaction techniques are somewhat more sensitive, they are not widely available in many clinical laboratories in developing countries where amebiasis is endemic.
Enzyme immunoassay and other methods can detect antigen in stool
The diagnosis of extraintestinal amebiasis is more difficult, because the parasite usually cannot be recovered from stool or tissue. Serologic tests are therefore of paramount importance. Typically, results are negative in asymptomatic patients, suggesting that tissue invasion is required for antibody production. Most patients with symptomatic intestinal disease and more than 90% with hepatic abscess have high levels of antiamebic antibodies. Unfortunately, these titers may persist for months to years after an acute infection, making the interpretation of a positive test difficult in endemic areas. At present, the indirect hemag-glutination test and enzyme immunoassays using antigens derived from axenically grown organisms appear to be the most sensitive. Several rapid tests, including latex agglutination, agar diffusion, and counterimmunoelectrophoresis, are available to smaller laboratories.
Extraintestinal amebiasis usually demonstrates high antibody levels
TREATMENT
Treatment for noninvasive infection differs from treatment for invasive infection. Paromomycin is useful for noninvasive infection and should probably be used if it is certain that it is truly E histolytica and not E dispar. Treatment is directed toward relief of symptoms, blood and fluid replacement, and eradication of the organism. The drug of choice for eradication in the case of invasive amebiasis is metronidazole. That and its derivatives are effective against many forms of amebiasis, but should be combined with a second agent, such as diloxanide, to improve cure rates in intestinal disease and diminish the chance of recrudescent disease in hepatic amebiasis. It may be prudent to also administer a broad-spectrum antibiotic in severe cases of intestinal amebiasis to treat intestinal bacteria that have the potential to spill into the peritoneum. In severe extraintestinal infections, parenteral dehydroemetine treatment may be considered.
Metronidazole combined with other agents
PREVENTION
Because the disease is transmitted by the fecal–oral route, efforts should be directed toward sanitary disposal of human feces, improvement in personal hygienic practices and the provision of safe drinking water. In the United States, this applies particularly to institutionalized patients and to camps for migrant farm workers. Male homosexuals should be made aware that certain sexual practices substantially increase their risk of amebiasis and other infections.
PATHOGENIC AND OPPORTUNISTIC FREE-LIVING AMEBAS
Pathogenic and opportunistic free-living amebas belong to the genera Acanthamoeba, Balamuthia, Naegleria, and Sappinia. These organisms are widespread in nature and have been found in soil, drinking water, swimming pools, sewage, draining ditches, thermal pools, eyewash solutions, and even dialysis units. Acanthamoeba and Balamuthia are considered opportunistic because they occur primarily in immunocompromised patients. Naegleria and Sappinia infections, on the other hand, have been described from healthy patients, and are therefore considered nonopportunistic.
PRIMARY AMEBIC MENINGOENCEPHALITIS
Primary amebic meningoencephalitis is caused by the free-living ameba Naegleria fowleri. This parasite largely affects children and young adults through full-body contact with warm fresh water, and is almost always fatal. Naegleria species are found in large numbers in shallow fresh water, particularly during warm weather. The organism exists in trophozoite, flagellate, and cyst forms. The trophozoite is an active feeding form that feeds on bacteria and organic matter. It transforms into a bi-flagellate form when deprived of nutrients, but may revert to a trophozoite if conditions become favorable. Under adverse environmental conditions it will encyst.
Meningoencephalitis due to free-living amebas
Warm weather and brackish water favor Naegleria
Approximately 300 cases of Naegleria meningoencephalitis have been reported, mostly in the United States, Australia, and Europe. Serologic studies suggest that inapparent infections are much more common. Most cases in the United States have occurred in the southern states. Characteristically, patients have fallen ill during the summer after swimming or in small, shallow, warm freshwater lakes. A Czechoslovakian case followed swimming in a chlorinated indoor pool, and several cases worldwide have occurred after bathing in hot mineral water.
Naegleria infections associated with freshwater swimming
Infection results from full-body contact with water containing the bi-flagellate parasite form. The parasite enters the body through the nasal passages and traverses the nasal mucosa and the cribriform plate as an ameboid form to the olfactory nerves of the central nervous system. Here, the amebas, which are the only form found in tissue, produce a severe purulent, hemorrhagic inflammatory reaction, which extends perivascularly from the olfactory bulbs to other regions of the brain. The infection is characterized by the rapid onset of severe bifrontal headache, seizures, and at times abnormalities in taste or smell. The disease runs an inexorably downhill course to coma, ending fatally within a few days.
Passage to central nervous system across cribriform plate
A striking feature of this infection is the rapid onset of symptoms following exposure. Because there are no distinctive clinical features to differentiate this infection from acute pyogenic bacterial meningoencephalitis or viral meningoencephalitis, it is imperative for the physician to obtain information regarding the patient’s contact with water within the past few days. A careful examination of the cerebrospinal fluid may often provide a presumptive diagnosis of Naegleria infection. The fluid is usually bloody and demonstrates an intense neutrophilic response. The protein level is elevated and the glucose level decreased. No bacteria can be demonstrated on stain or culture. Early examination of a wet mount preparation of unspun spinal fluid reveals typical trophozoites. Staining with specific fluorescent antibody confirms the identification. The organism can usually be isolated on agar plates seeded with a Gram-negative bacillus (to feed the amebas) or grown axenically in tissue culture. To date, there are reports of only four patients who have survived a Naegleria infection. All were diagnosed early and treated with high-dose amphotericin B along with rifampin.
Purulent bloody cerebrospinal fluid containing Naegleria trophozoites
GRANULOMATOUS AMEBIC ENCEPHALITIS
Granulomatous amebic encephalitis (GAE) is caused by one of seven species of free-living amebas belonging to the genus Acanthamoeba. These amebas are ubiquitous worldwide and have been described from soil, fresh and brackish waters, cooling towers of electric and nuclear power plants, heating, ventilating and air conditioning units, humidifiers, Jacuzzis, hydrotherapy pools, dental irrigation units, dialysis machines, dust, cell cultures, and various clinical samples. They have also received attention because they may serve as hosts for a wide variety of bacterial pathogens.
Acanthamoeba spp. exist in two forms, trophozoite and cyst. The trophozoite feeds on bacteria and detritus in the environment and divides by binary fission. If environmental conditions become unfavorable, the trophozoite encysts. Cysts have been known to survive for up to 20 years in vitro.
The epidemiology of Acanthamoeba encephalitis has not been clearly defined. Infections usually involve older, immunocompromised persons, and a history of freshwater swimming is generally absent. The ameba probably reaches the brain by hematogenous dissemination from an unknown primary site, possibly the respiratory tract, skin, or eye. Metastatic lesions have been reported. Histologically, Acanthamoeba infections produce a diffuse, necrotizing, granulomatous encephalitis (Figure 52–3), with frequent involvement of the mid-brain. Both cysts and trophozoites can be found in the lesions. Cutaneous ulcers and hard nodules containing amebas have been detected in patients with AIDS.
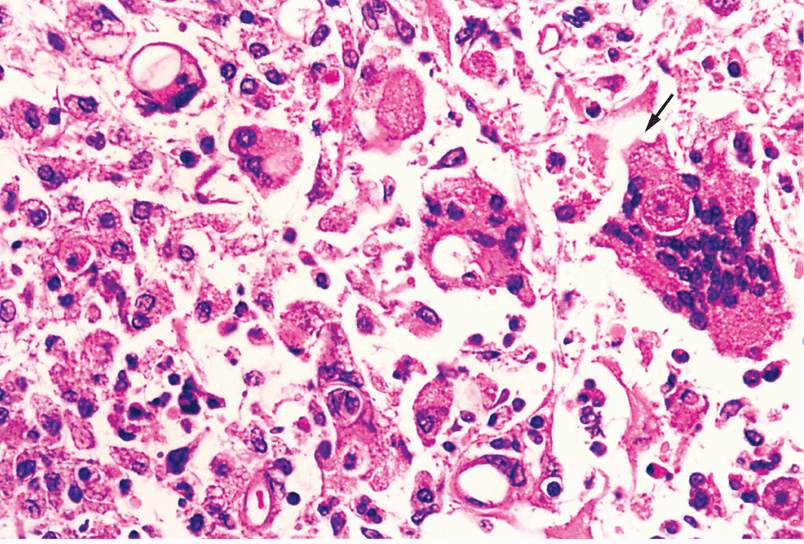
FIGURE 52–3. Acanthamoebic granulomatous encephalitis. A trophozoite (arrow) entering an epithelioid cell is seen at the right. The empty ovals in other cells are collapsed cysts. (reproduced with permission from Connor Dh, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford Ct: appleton & Lange, 1997.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


