CRYPTOCOCCUS
 CRYPTOCOCCUS NEOFORMANS and CRYPTOCOCCUS GATTII
CRYPTOCOCCUS NEOFORMANS and CRYPTOCOCCUS GATTII
Cryptococcus is a genus of yeast 4 to 6 μm in diameter that produces a characteristic capsule (Figure 47–1), extending the overall diameter to 25 μm or more. It is a basidiomycete, and has two species, C neoformans and the more recently recognized C gattii. Each has multiple serotypes or varieties. Here, unless specified otherwise, the use of Cryptococcus or simply the cryptococcus refers to the classic Cryptococcus neoformans.

FIGURE 47–1. Cryptococcus neoformans. This India ink preparation was made by mixing cerebrospinal fluid containing cryptococci with India ink. The yeast cells can be seen within the clear space caused by the large polysaccharide capsule excluding the ink particles. Note that the one on the right is budding. (Reproduced with permission from Nester EW: Microbiology: A Human Perspective, 6th edition. 2009.)
Two species and multiple varieties
The capsule is unique among pathogenic fungi and is a complex polysaccharide polymer, the major components of which are glucuronoxylomannan and glucuronoxylomannogalactan. Together these will be referred to as GXM. Capsule production is repressed under environmental conditions and stimulated in the physiologic conditions found in tissues and in culture on some laboratory media. The cryptococcal cell wall is made up of glucan, chitin, and proteins anchored to mannan or other cell wall elements. At either 25°C or 37°C, yeast colonies are produced in 2 to 3 days. The teleomorph (sexual) forms with hyphae and basidiospores have been produced in the laboratory under specialized conditions. It is suspected but not observed that this is the environmental growth form. In addition to the capsule, extracellular products include urease and laccase enzymes. A melanin pigment is the product of laccase activity.
GXM capsule in tissues
Urease, laccase, and melanin produced
EPIDEMIOLOGY
Cryptococcus neoformans is ubiquitous throughout the world, particularly in soil contaminated with avian droppings and decaying vegetable matter. One environmental niche is the hollowed-out areas of trees, where laccase is involved in the degradation of wood. The infectious form is felt to be either desiccated yeast cells or basidiospores stirred up from these sites and inhaled. Cryptococcus gattii once felt to be restricted to tropical and subtropical area has recently been isolated from cases in the Pacific Northwest (British Columbia, Washington, Oregon). Cases appear sporadically, with no particular occupational predisposition, including bird fanciers and those who work with the cryptococcus in the laboratory. Cryptococcosis in immunocompromised patients occurs primarily in those with defects in T-lymphocyte function, particularly in those with AIDS, in whom it is the most common fungal infection. Cases also occur in immunocompetent persons, particularly with C gattii. In countries with well-developed antiretroviral therapy programs, cryptococcal disease has declined in AIDS patients, but remains persistent in other immunocompromised persons. Disease can occur in persons with no known immune defect and is said to be more likely with certain variants. Case-to-case transmission has not been documented.
Associated with soil and bird droppings
Yeasts or basidiospores inhaled
PATHOGENESIS
After being inhaled, cryptococci reach the alveoli, where production of the polysaccha-ride capsule is the prime determinant of virulence. Pulmonary infection is associated with the appearance of a subpopulation of very large (up to 100 μm) thick-walled forms called Titan cells, which are too large to be phagocytosed. Production of the GXM capsule is induced in the tissue milieu through sensing of multiple environmental signals (iron, pH, CO2, glucose, nitrogen). The capsule is antiphagocytic through complement depletion and has various other immunomodulating effects, such as downregulation of cytokines, interference with antigen presentation, leukocyte migration, specific antibody responses, and the development of TH1 immune responses. These immune-suppressing effects may act at both a local and systemic level because cryptococci produce sufficient capsule that the GXM is readily detected in the blood and other body fluids. If phagocytosed by macrophages the cryptococcus is able to survive and multiply by altering metabolic pathways and by melanin production, which interferes with oxidative killing mechanisms. This muting of the first lines of defense may be what allows the organisms to spread outside the lung. The affinity of C neoformans for the central nervous system (CNS) is striking. Proposed explanations include crossing the blood–brain barrier inside macrophages (Trojan horse) and the ability of laccase to convert the abundant catecholamines in the CNS to melanin.
Antiphagocytic capsule is prime factor
Circulating GXM interferes with immune function
Melanin provides oxidative protection in macrophage
Tissue reaction to C neoformans varies from little or none to purulent or granulomatous. Many cases of pulmonary, cutaneous, and even meningeal cryptococcal infection show a remarkable paucity of inflammatory cells. This certainly fits for a fungus that not only blocks its own phagocytosis but is able to downregulate multiple aspects of the immune response.
Tissue reaction is often minimal
IMMUNITY
The capsule is not particularly antigenic, and anticryptococcal antibodies are not usually detected in the course of infection. Some antibodies are formed but their role in immunity is unknown. Animal studies and the strong clinical association of cryptococcosis with T-cell defects indicate that TH1-type immune responses are most important in the outcome of infection. Cryptococci phagocytosed by macrophages may not be killed, and cytokine activation is needed to complete the clearing of the organisms. Immunodominant manno-proteins have been identified which use dendritic cells as the primary presenter to CD4+ T cells. In patients with cryptococcosis who have no known immune defects, it is often possible to detect subnormal TH1 immune functions in laboratory testing. Clinical recovery in such cases is associated with return of these immune functions.
Antibody role unknown
TH1 responses are dominant
Dendritic cells present mannoproteins
 CRYPTOCOCCOSIS: CLINICAL ASPECTS
CRYPTOCOCCOSIS: CLINICAL ASPECTS
MANIFESTATIONS
Meningitis is the most commonly recognized form of cryptococcal disease; it usually has a slow, insidious onset with relatively nonspecific findings until late in its course. Intermittent headache, irritability, dizziness, and difficulty with complex cerebral functions appear over weeks or months with no consistent pattern. Behavioral changes have been mistaken for psychoses. Fever is usually, but not invariably, present. Seizures, cranial nerve signs, and papilledema may appear later in the clinical course, as may dementia and decreased levels of consciousness. A more rapid course may be seen in AIDS patients, 5% to 15% of whom become infected with C neoformans.
Meningitis is insidious and chronic
Course is more rapid with AIDS
Cryptococcal pneumonia is often asymptomatic or mild. Sputum production is minimal, and no findings are sufficiently specific to suggest the etiology. Skin and bone are the sites most frequently involved in disseminated disease; skin lesions are sometimes the presenting sign and are often remarkable for their lack of inflammation. The diagnosis is sometimes made when lesions are biopsied as suspected neoplasms. There is evidence of differences in the disease spectrum of the two species. Cryptococcus gattii is more likely to produce pulmonary infection and less likely to invade the CNS. In the CNS, C gattii may cause localized lesions as opposed to the diffuse meningoencephalitis typical of C neoformans.
Cryptococcal pneumonia often asymptomatic
CNS involvement varies with species
DIAGNOSIS
Typical CSF findings in cryptococcal meningitis are increased pressure, pleocytosis (usually 100 cells or more) with predominance of lymphocytes, and depression of glucose levels. In some cases, one or all of these findings may be absent, yet cryptococci are isolated on culture. Cryptococcal capsules are demonstrable in CSF in approximately 50% of cases by mixing centrifuged sediment with India ink and examining the mixture under the microscope (Figure 47–1). Experience is necessary to avoid confusion of lymphocytes with cryptococci. Cryptococcus neoformans stains poorly or not at all with routine histologic stains; thus, it is easily missed unless special fungal stains are used (Figure 47–2).
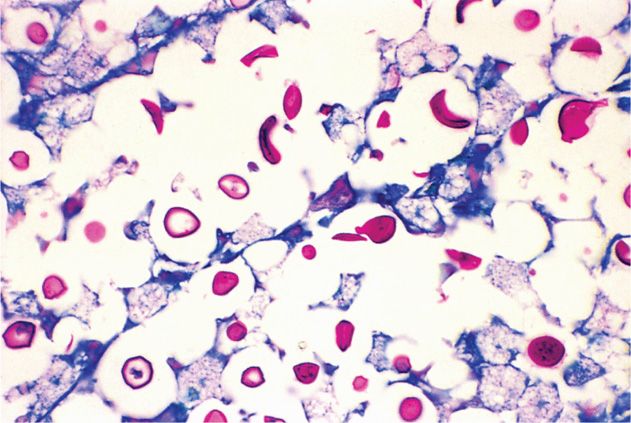
FIGURE 47–2. Cryptococcal meningitis. The C neoformans cells are stained red by this PAS (periodic acid-Schiff) stain. The capsule is not stained but is creating the halo around the organisms. Note the lack of inflammatory cells. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford CT: Appleton & Lange, 1997.)
Cells and glucose depression in CSF may be minimal
India ink preparation is positive in 50% of cases
In the isolation of C neoformans, the volume of CSF sampled is important. The number of organisms present may be small enough to require a substantial volume of fluid (>30 mL) to yield a positive culture. If cryptococcosis is suspected and cultures are negative, detection of the GXM polysaccharide antigen in the CSF or serum by latex agglutination or enzyme immunoassay methods is recommended. These tests are very sensitive and specific, and their quantitation has prognostic significance. A rising antigen level indicates progression and a declining titer is a favorable sign.
Few cryptococci may be present in CSF
GXM is detectable in CSF and serum
TREATMENT
Amphotericin B plus flucytosine followed by an extended course of fluconazole is the primary treatment for systemic cryptococcal disease. Although 75% of persons with meningitis respond to treatment, a significant percentage suffer relapses after antifungal therapy is stopped; many become chronic and require repeated courses of therapy. One half of those cured have some kind of residual neurologic damage.
Amphotericin, flucytosine, and fluconazole used in combination
HISTOPLASMA
 HISTOPLASMA CAPSULATUM
HISTOPLASMA CAPSULATUM
Histoplasma capsulatum is a dimorphic fungus (Figure 47–3B) that grows in the yeast phase in tissue and in cultures incubated at 37°C. The mold phase grows in cultures incubated at 22°C to 25°C and as a saprophyte in soil. There are three varieties of Histoplasma (capsulatum, duboisii, farciminosum), which vary in their geographic distribution. The yeast forms are small for fungi (2-4 μm) and reproduce by budding (blastoconidia). The mycelia are septate and produce microconidia and the diagnostic structure called the tuberculate macroconidium because of its thick wall and radial, finger-like projections (Figure 47–3A). Growth is obtained on blood agar, chocolate agar, and Sabouraud’s agar, but may take many weeks. The designation H capsulatum is actually a misnomer, because no capsules are formed. It comes from the halos seen around the yeasts in tissue sections, which are caused by a shrinkage artifact of routine histologic methods.
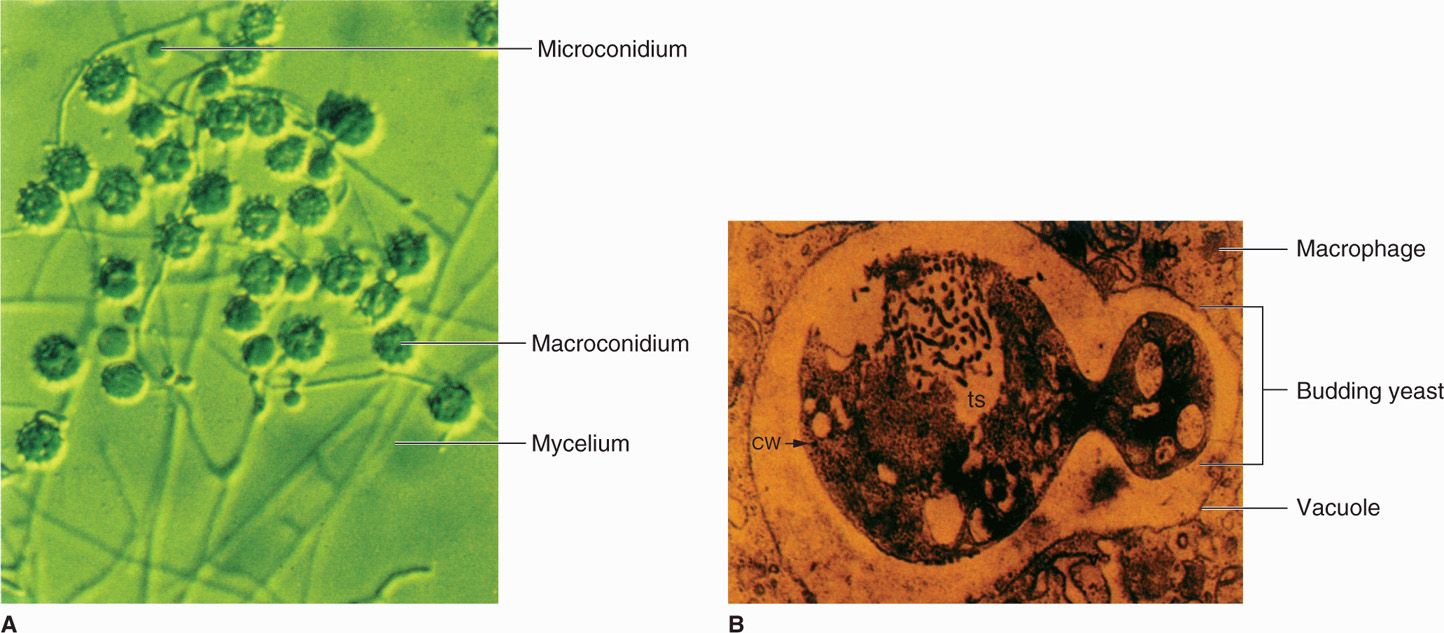
FIGURE 47–3. Histoplasma capsulatum. A. Mold phase with hyphae, microconidia, and tuberculate macroconidia. B. A yeast cell is multiplying (note budding) within a macrophage phagocytic vacuole. (Reproduced with permission from Willey JM: Prescott, Harley, & Klein’s Microbiology, 7th edition. McGraw-Hill, 2008.)
Small dimorphic fungus producing tuberculate macroconidia
Growth may take weeks
![]() HISTOPLASMOSIS
HISTOPLASMOSIS
EPIDEMIOLOGY
Histoplasma capsulatum grows in soil under humid climatic conditions, particularly soil containing bird or bat droppings. Inhalation of the mold microconidia, which are small enough (2-5 μm) to reach the terminal bronchioles and alveoli, is believed to be the mode of infection. The organism is particularly prevalent in certain temperate, subtropical, and tropical zones, and endemic areas are present in all continents of the world except Antarctica. The largest and best defined is the US region drained by the Ohio and Mississippi rivers (Figure 47–4). More than 50% of the residents of states in this area show radiologic evidence of previous infection, and in some locales, up to 90% demonstrate delayed-type hypersensitivity to Histoplasma antigens. Disturbances of bird roosts, bat caves, and soil have been associated with point source outbreaks. Persons in endemic areas whose employment (agriculture, construction) or avocation (spelunkers) brings them in contact with these sites are at increased risk. The infection is not transmitted from person to person. Disease is more common in men, but there are no racial or ethnic differences in susceptibility.
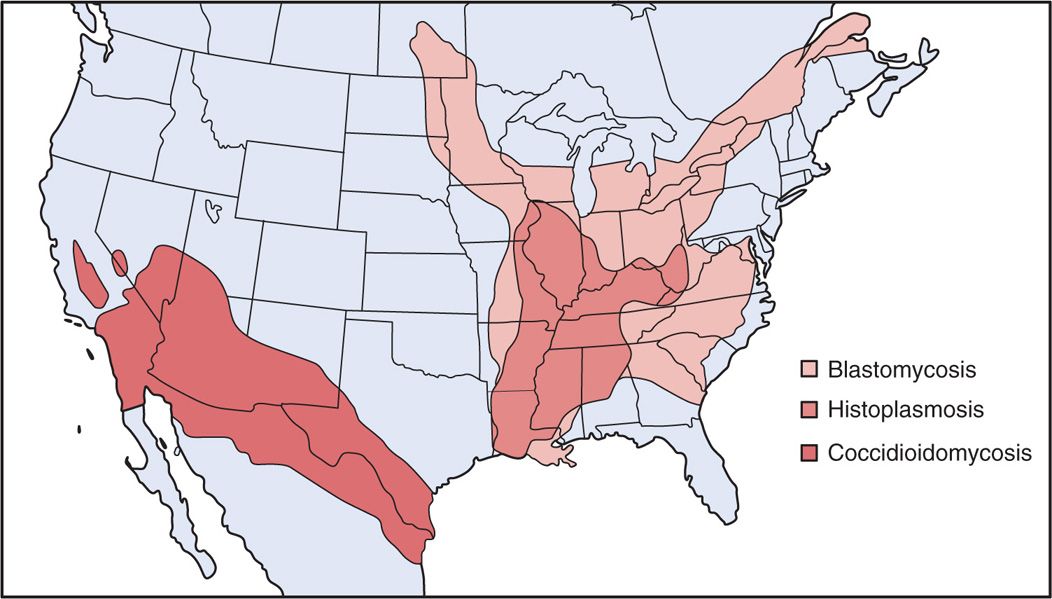
FIGURE 47–4. Geographic distribution of systemic fungal infections in the United States.
Microconidia are infectious
Mold grows in humid soil with bird droppings
High prevalence in central United States
PATHOGENESIS
The hallmark of histoplasmosis is infection of the lymph nodes, spleen, bone marrow, and other elements of the reticuloendothelial system with intracellular growth in phagocytic macrophages. The initial infection is pulmonary, through inhalation of infectious conidia, which convert to the yeast form in the host. They attach to integrin and fibronectin receptors and are readily taken up by professional phagocytes. Dendritic cells kill the invading yeast cells, but inside neutrophils and macrophages they survive the effects of the oxidative burst and inhibit phagosome–lysosome fusion. Key features in this survival and multiplication are the ability of H capsulatum to capture iron and calcium from the macrophage and to modulate phagolysosomal pH. The acidic pH required for optimal killing effect in the lysosome is elevated by H capsulatum toward the less effective neutral range (pH 6.0-6.5).
Reticuloendothelial system is focus of infection
Grows in macrophages by controlling lysosomal pH
With continued growth, there is lymphatic spread and development of a primary lesion similar to that seen in tuberculosis. The extent of spread to the reticuloendothelial system within macrophages during primary infection is unknown, but such spread is presumed to occur. Most cases never advance beyond the primary stage, leaving only a calcified node as evidence of infection. As in tuberculosis, viable cells may remain in these old lesions and reactivate later, particularly if the person becomes immunocompromised.
Lymphatic spread and reactivation are similar to tuberculosis
Pathologically, granulomatous inflammation with necrosis is prominent in pulmonary lesions, but H capsulatum may be difficult to detect, even with special fungal stains. Extra-pulmonary spread involves the reticuloendothelial system, with enlargement of the liver and spleen. Numerous organisms within macrophages may be found in these organs, in lymph nodes, bone marrow, or even peripheral blood (Figure 47–5).
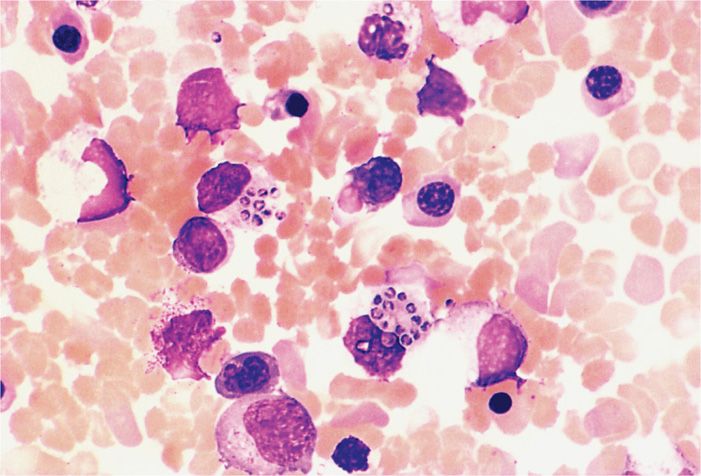
FIGURE 47–5. Histoplasma capsulatum. This peripheral blood smear shows two monocytes with multiple organisms are stuffed within their cytoplasm. Note the size of the yeast cells, which is very small for fungi. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford CT: Appleton & Lange, 1997.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


