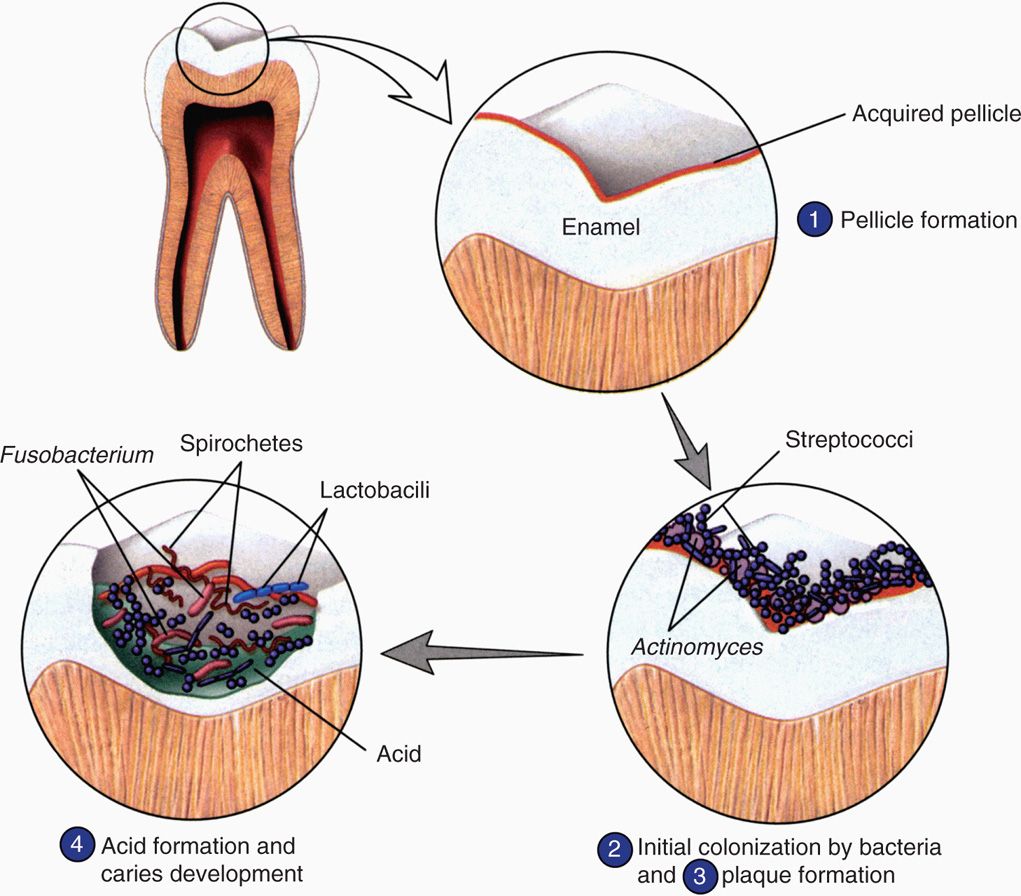
FIGURE 41–1. Dental plaque biofilm. The stages of formation of the bacterial biofilm called dental plaque are shown. Early colonizers bind to the enamel pellicle and late colonizers bind to the other bacteria. (Reproduced with permission from Willey JM: Prescott, Harley, & Klein’s Microbiology, 7th edition. McGraw-Hill, 2008.)
Dental plaque is a bacterial biofilm
Plaque forms in stages
The initial supragingival plaque primarily involves Gram-positive bacteria using specific ionic and hydrophobic interactions as well as lectin-like (carbohydrate binding) surface structures to adhere to the pellicle and to each other. The prototype early colonizer is Streptococcus sanguis, but other streptococci (S mutans, S mitis, S salivarius, S oralis, Sgordonii), lactobacilli, and Actinomyces species are usually present. If the early colonizers are undisturbed, the late colonizers appear in the biofilm in as little as 2 to 4 days. These are primarily Gram-negative anaerobes including anaerobic spirochetes. These include Fusobacterium, Porphyromonas, Prevotella, Veillonella, Treponema denticola, and more Actinomyces species. These bacteria use similar mechanisms to bind to the early colonizers and to each other. This sets up a highly complex biofilm in which coaggregation involves structures that the bacteria brought with them (lectins), quorum sensing, and new metabolic activity. An example of the latter is the formation of extracellular glucan polymers, which act like a cement binding the plaque biofilm together. The biofilm also fastens nutrient and growth regulatory relationships between its members and provides a shield from the outside. In all, there are thought to be 300 to 400 bacterial species present in mature dental plaque. The structure of the involved bacteria is shown in Figure 41-1 and its gross and microscopic appearance in Figure 41-2.
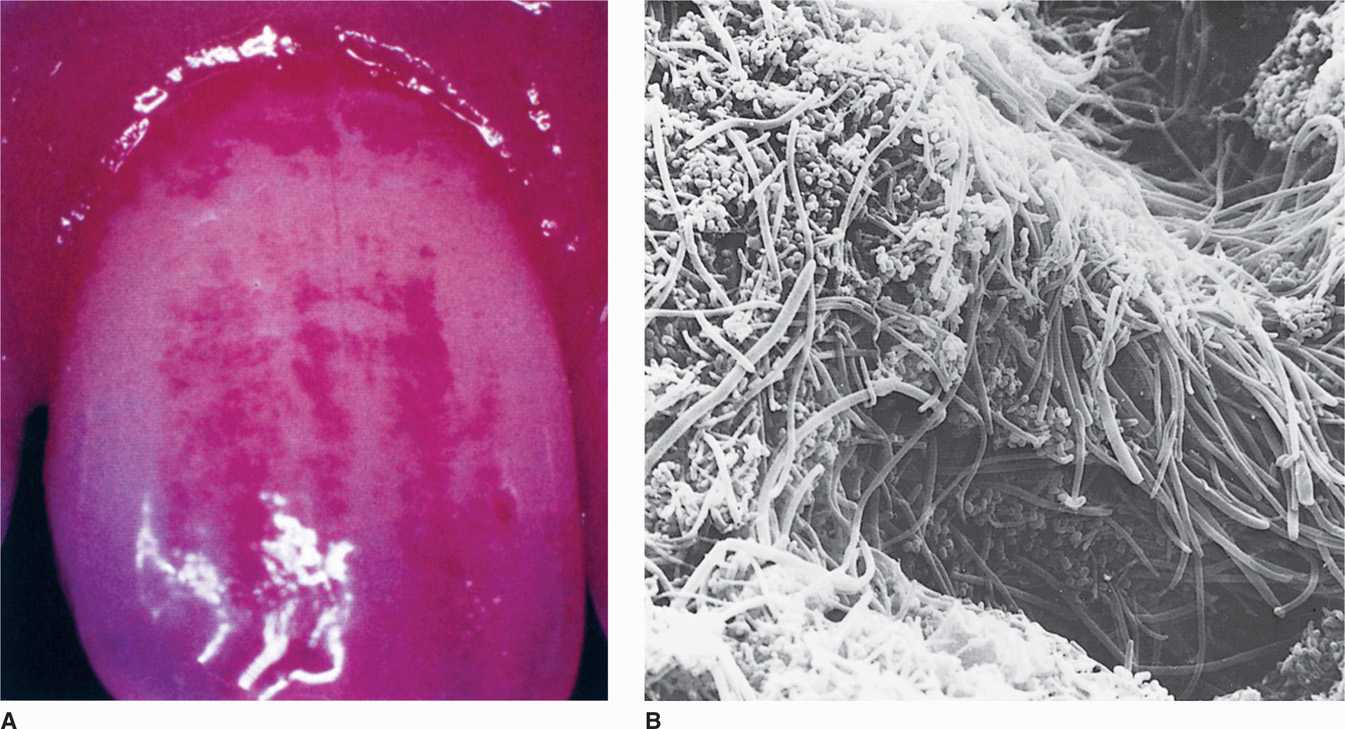
FIGURE 41–2. Dental plaque. A. Disclosing tablets containing vegetable dye stain heavy plaque accumulation at the junction of the tooth and gingival. (Reproduced with permission from Willey JM: Prescott, Harley, & Klein’s Microbiology, 7th edition. McGraw-Hill, 2008.) B. Scanning electron micrograph of supragingival plaque.
Attachment of bacteria to dental pellicle begins colonization
Early and late colonizers differ
Adhesion mechanisms create biofilm
Dental plaque would coat the tooth surfaces uniformly but for its physical removal during chewing and other oral activities. Characteristically, plaque remains in the non-self-cleansing areas of the teeth such as pits and fissures, along the margins of the gingiva, and between the teeth. For this reason, the plaque-related diseases—caries, gingivitis, and periodontitis—occur most frequently and most severely at these locations. Subgingival plaque extends below the gum line to the sulcus around the tooth and periodontal pockets, which are pathologic extensions of the sulcus. This plaque has a thin adherent layer attached to the tooth surface and a nonadherent bacterial zone between that and the epithelial cells lining the sulcus. Supragingival plaque lacks such a distinct nonadherent zone. The bacterial composition of subgingival plaque is shifted toward the Gram-negative anaerobic bacteria and spirochetes. In addition to the late colonizers cited above, it may also include members of the Campylobacter, Capnocytophagia, and Eikenella genera.
Plaque accumulates in non-self-cleansing areas
Subgingival plaque differs in bacterial composition
Because the causative organisms of both dental caries and chronic periodontitis are believed to be in the dental plaque, a prime method for maintaining oral health is regular home care practices for plaque removal. Dental plaque cannot be effectively removed from the teeth solely by chemical or enzymatic means, and the use of antibiotics for prophylactic inhibition of plaque formation cannot be clinically justified, although patients undergoing long-term antibiotic treatment for other medical reasons demonstrate a lower incidence of caries and periodontal disease. Antiseptic substances that bind to tooth surfaces and inhibit plaque formation, such as the bis-biguanides, chlorhexidine, and alexidine, have been shown to be effective in reducing plaque, caries, and gingival inflammation. A commercial preparation containing 0.12% chlorhexidine can be used in controlling dental plaque and associated disease. Toothpaste and mouth rinse additives such as phenolic compounds, essential oils, triclosan, fluorides, herbal extracts, and quaternary ammonium compounds have been shown to have some plaque-reducing ability as well. The use of these substances must be accompanied by proper tooth brushing, flossing, and periodic professional cleaning to ensure effective disease prevention.
Removal of plaque prime element of oral hygiene
Chemicals may be used along with brushing and flossing
DENTAL CARIES
Dental caries are the result of progressive destruction of the mineralized tissues of the tooth. They are primarily caused by the acid products of glycolytic metabolic activity when the plaque bacteria are fed the right substrate. The basic characteristic of the carious lesion is that it progresses inward from the tooth surface, either the enamel-coated crown, or the cementum of the exposed root surface, involving the dentin and finally the pulp of the tooth (Figures 41–3 and 41–4). From there, infection can extend into the periodontal tissues at the root apex or apices.
FIGURE 41–3. Cariogenesis. A microscopic view of pellicle and plaque formation, acidification, and destruction of tooth enamel. (Reproduced with permission from Willey JM: Prescott, Harley, & Klein’s Microbiology, 7th edition. McGraw-Hill, 2008.)
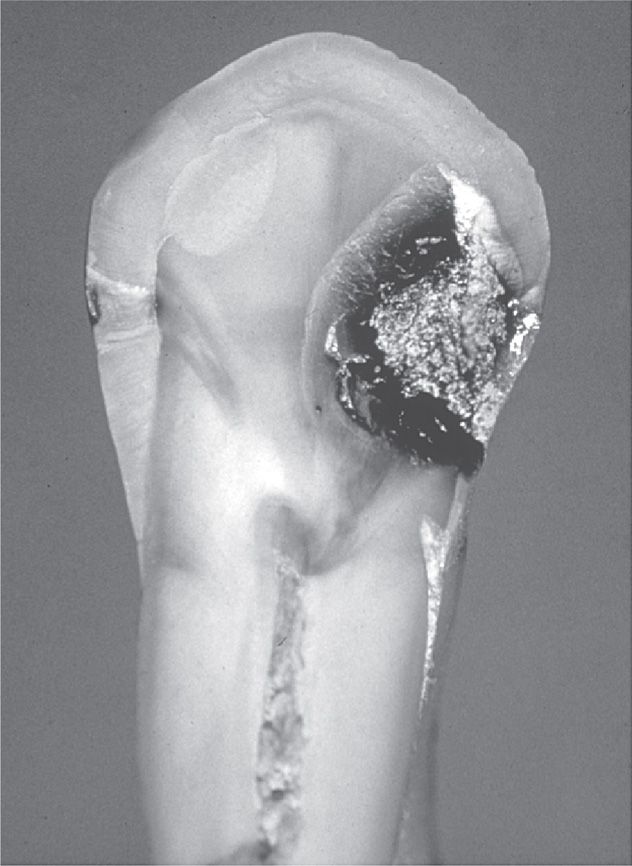
FIGURE 41–4. Hemisected human tooth showing an advanced carious lesion on the right side of the crown and a much smaller lesion on the left side. Note the progression of the lesion through the enamel and dentin, pointing toward the pulp chamber in the center of the tooth.
Caries produced by plaque bacteria
Members of biofilm produce acid
S mutans is most cariogenic
The microbial basis of dental caries has been long established based on work first with Lactobacillus acidophilus and then Streptococcus mutans. Although S mutans is now regarded as the dominant organism for the initiation of caries, multiple members of the plaque biofilm participate in the evolution of the lesions. These include other streptococci (S salivarius, S sanguis, S sobrinus), lactobacilli (L acidophilus, L casei), and actinomycetes (A viscosus and A naeslundii). The acid products produced by the interaction of S mutans with multiple species in the biofilm are the underlying cause of dental caries.
Dietary monosaccharides and disaccharides such as glucose, fructose, sucrose, lactose, and maltose provide an appropriate substrate for bacterial glycolysis and acid production to cause tooth demineralization. A possible edge for S mutans is its ability to metabolize sucrose more efficiently than other oral bacteria. It also has regulatory systems which stimulate the conversion of dietary carbohydrates to acid and intracellular storage polymers. Ingested carbohydrates permeating the dental plaque are absorbed by the bacteria, and are metabolized so rapidly that organic acid products accumulate and cause the pH of the plaque to drop to levels sufficient to react with the hydroxyapatite of the enamel, demineralizing it to soluble calcium and phosphate ions. Production of acid and the decreased pH are maintained until the substrate supply is exhausted. Upon exhaustion of the immediate source S mutans is able to survive long periods of sugar starvation. Obviously, foods with high sugar content, particularly sucrose, which adhere to the teeth and have long oral clearance times are more car-iogenic than less retentive foodstuffs such as sugar-containing liquids. Once the substrate is exhausted, the plaque pH returns slowly to its more neutral pH resting level and some recovery can take place. This sets up a demineralization-remineralization cycle, which depends on carbohydrate refueling from the diet. With repeated snacking between meals, the plaque pH may never return to normal and demineralization dominates.
Demineralization is by acid production from dietary carbohydrate
Acid production facilitated by sticky carbohydrates
Demineralization-remineralization related to snacking
An additional factor with sucrose is that it is also used in the synthesis of extracellular polyglycans such as dextrans and levans by transferase enzymes on the bacterial cell surfaces. This polyglycan production by S mutans contributes to aggregation and accumulation of the organism on the tooth surface. Extracellular polyglycan may also increase cario-genicity by serving as an extracellular storage form of substrate. Certain microorganisms synthesize extracellular polyglycan when sucrose is available but then break it down into monosaccharide units to be used for glycolysis when dietary carbohydrate is exhausted. Some oral bacteria also use dietary monosaccharides and disaccharides internally to form glycogen, which is stored intracellularly and used for glycolysis after the dietary substrate has been exhausted; thus, the period of acidogenesis is again prolonged and the carioge-nicity of the microorganism increased. These microorganisms can prolong acidogenesis beyond the oral clearance time of the substrate.
Extracellular polyglycans from sucrose important in adherence and carbohydrate storage
Acidogenesis prolonged by intracellular glycogen stores
The most common complications of dental caries are extension of the infection into the pulp chamber of the tooth (pulpitis), necrosis of the pulp, and extension of the infection through the root canals into the periapical area of the periodontal ligament. Periapical involvement may take the form of an acute inflammation (periapical abscess), a chronic nonsuppurating inflammation (periapical granuloma), or a chronic suppurating lesion that may drain into the mouth or onto the face via a sinus tract. A cyst may form within the chronic nonsuppurating lesion as a result of inflammatory stimulation of the epithelial rests normally found in the periodontal ligament. If the infectious agent is sufficiently virulent or host resistance is low, the infection may spread into the alveolar bone (osteomyelitis) or the fascial planes of the head and neck (cellulitis). Alternatively, it may ascend along the venous channels to cause septic thrombophlebitis. Because most carious lesions represent a mixed infection by the time cavities have developed, it is not surprising that most oral infections resulting from the extension of carious lesions are mixed and frequently include anaerobic organisms.
Extension to pulp and periapical locations complicate infections
Severe complications spread to bone or local fascia
Dental caries is the single greatest cause of tooth loss in the child and young adult. Its onset can occur very soon after the eruption of the teeth. The first carious lesions usually develop in pits or fissures on the chewing surfaces of the deciduous molars and result from the metabolic activity of the dental plaque that forms in these sites. Later in childhood, the incidence of carious lesions on smooth surfaces increases; these lesions are usually found between the teeth. The factors involved in the formation of a carious lesion are (1) a susceptible host or tooth, (2) the proper microflora on the tooth, and (3) a substrate from which the plaque bacteria can produce the organic acids that result in tooth demineralization.
Greatest cause of tooth loss in children and young adults
Require microflora and suitable substrates for organic acid production
The newly erupted tooth is most susceptible to the carious process. It gains protection against this disease during the first year or so by a process of posteruptive maturation believed to be attributable to improvement in the quality of surface mineral on the tooth. Saliva provides protection against caries, and patients with dry mouth (xerostomia) suffer from high caries attack rates unless suitable measures are taken. In addition to the mechanical flushing and diluting action of saliva and its buffering capacity, the salivary glands also secrete several antibacterial products. Thus, saliva is known to contain lysozyme, a thiocy-anate-dependent sialoperoxidase, and immunoglobulins, principally those of the secretory IgA class. The individual importance of these antibacterial factors is unknown, but they clearly play some role in determining the ecology of the oral microflora.
Saliva protects by mechanical flushing and multiple chemical actions
Proper levels of fluoride, either systemically or topically administered, result in dramatic decreases in the incidence of caries (50% to 60% reduction by water fluoridation, 35% to 40% reduction by topical application). In the case of systemic fluoridation, the protective effect is thought to result from the incorporation of fluoride ions in place of hydroxyl ions of the hydroxyapatite during tooth formation, producing a more perfect and acid-resistant mineral phase of tooth structure. Topical application of fluoride is believed to achieve the same result on the surface of the tooth by initial dissolution of some of the hydroxyapa-tite, followed by recrystallization of apatite, which incorporates fluoride ions into its lattice structure. Another important mode of action, namely, the inhibition of demineralization, and the promotion of remineralization of incipient carious lesions by fluoride ions in the oral fluid, has more recently been proposed as an important anticaries mechanism of fluoride, perhaps more important than the other proposed mechanisms. In any event, fluoridation represents the most effective means known for rendering the tooth more resistant to the carious process.
Fluoride produces more acid-resistant mineral phase of tooth
CHRONIC PERIODONTITIS
Plaque-induced periodontal disease encompasses two separate disease entities: gingivitis and chronic periodontitis. These diseases are believed to be related, in that gingivitis, although a reversible condition, is thought to be an early stage leading ultimately to chronic periodontitis in the susceptible subject. The term gingivitis is used when the inflammatory condition is limited to the marginal gingiva and bone resorption around the necks of teeth has not yet begun. Gingivitis develops within 2 weeks in individuals who fail to practice effective tooth cleansing. Chronic periodontitis is used to connote the stage of chronic periodontal disease in which there is progressive loss of tooth support owing to resorption of the alveolar bone and periodontal ligament. Periodontitis can also lead to periodontal abscess when the chronic inflammatory state around the necks of the teeth becomes acute at a specific location.
Causes destruction of supporting tissues
Both gingivitis and chronic periodontitis are caused by bacteria in the dental plaque that lie in close proximity to the necks of the teeth and marginal gingival tissues. Thus, subgingival plaque found within the gingival crevice or the sulcus around the necks of the teeth is thought to house the etiologic agent(s). The characteristic histopathologic picture of gingivitis is of a marked inflammatory infiltrate of polymorphonuclear leukocytes, lymphocytes, and plasma cells in the connective tissue that lies immediately adjacent to the epithelium lining the gingival crevice and attached to the tooth. Collagen is lost from the inflamed connective tissue. There does not seem to be any direct invasion of the gingival tissues by large numbers of intact bacteria, at least in the early stages of the disease.
Subgingival plaque causes collagen loss
All forms of periodontitis are polymicrobial infections primarily involving anaerobic bacteria in much the same way described for other anaerobes in Chapter 29. The agents involved are derived from the predominantly Gram-negative anaerobic flora of the subgingival plaque (see previous text) led by Porphyromonas gingivalis and Treponema denticola. Just as bacteria-bacteria interactions determine the plaque, cross-feeding and growth stimulation have been observed between these two organisms when grown together. This kind of synergism between P gingivalis, T denticola, and other plaque members is felt to foster progression of gingivitis to chronic periodontitis. Some of these organisms have also been shown to produce virulence factors similar to those associated with other invasive bacterial pathogens. Treponema denticola is able to bind serum factors that interfere with complement deposition, and P gingivalis is a potent producer of extracellular proteases. The former facilitates survival in tissues and the latter injury to those tissues.
Polymicrobial anaerobic infection from subgingival plaque
Synergistic interaction facilitate growth
Virulence factors cause disease
Chronic periodontitis is responsible for most tooth loss in people older than 35 to 40 years. The disease progresses slowly and results in the progressive destruction of the supporting tissues of the tooth (periodontal ligament and alveolar bone) from the margins of the gingiva toward the apices of the roots of the teeth. Progression may occur as a series of acute episodes separated by quiescent periods of indeterminate duration. More aggressive forms of periodontitis result in more rapid loss of tooth support. Aggressive types of disease called localized aggressive periodontitis occur in adolescents, and generalized aggressive periodontitis occurs in young adults. There is some evidence that the causative agents may differ in this form of periodontitis. A small capnophilic (carbon dioxide-requiring) Gram-negative rod (Actinobacillus actinomycetemcomitans) has been indicted based on studies of the flora of disease sites. A virulence factor found in those strains of A actinomy-cetemcomitans that are associated with this disease is the production of a leukotoxin by the bacteria.
Chronic periodontitis causes tooth loss
Acute juvenile periodontitis associated with Actinobacillus
As the disease progresses, a point may be reached at which the alveolar bone around the necks of the teeth is resorbed; the condition is then no longer termed gingivitis, but peri-odontitis. With resorption of the bone, the attachment of the periodontal ligament is lost and the gingival sulcus deepens into a periodontal pocket. Periodontitis is not considered to be a reversible disease in that the lost alveolar bone and periodontal ligament do not regenerate with cessation of the inflammation, even though further progression may be halted. If unchecked, bone resorption progresses to loosening of the tooth, which may ultimately be exfoliated. Figure 41-5 shows a case of advanced chronic periodontitis. Occasionally, the neck of a periodontal pocket becomes constricted, the bacteria proliferate causing an acute inflammatory response in the occluded pocket, and a periodontal abscess results. This acute exacerbation requires drainage in the same way as abscesses elsewhere for the patient to obtain symptomatic relief.
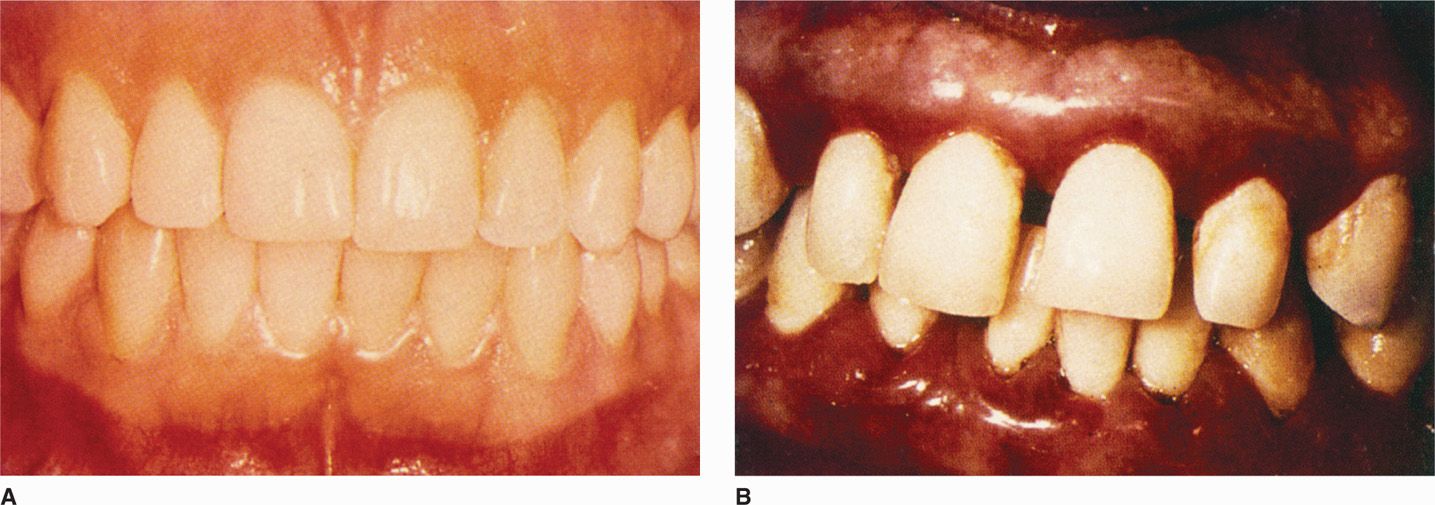
FIGURE 41–5. Periodontitis. A. Normal gingival. B. Periodontal disease, with plaque, inflammatory changes, bleeding, and shortening of the gingival between the teeth. (Reproduced with permission from Nester EW: Microbiology: A Human Perspective, 6th edition. 2009.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree