BACTERIOLOGY
STRUCTURE
Rickettsiae are small coccobacilli (Figure 40–1) which measure no more than 0.3 to 0.5 μm. Although the Gram reaction is negative, rickettsiae take the usual bacterial stains poorly and are better demonstrated by specific immunofluorescence. The ultrastructural morphology, which is similar to that of other Gram-negative bacteria, includes a Gram-negative type of cell envelope, ribosomes, and a nuclear body. Chemically, the cell wall contains lipopolysaccharide and at least two large proteins in the outer membrane, as well as peptidoglycan. The outer membrane proteins extend to the cell surface, where they are the most abundant protein present. They are discussed here as members of either the spotted fever group (SFG) or typhus group (TG). Due to differences in protein composition and its lack of lipopolysaccharide Orientia tsutsugamushi (formerly R tsutsugamushi) has been placed in a separate genus.
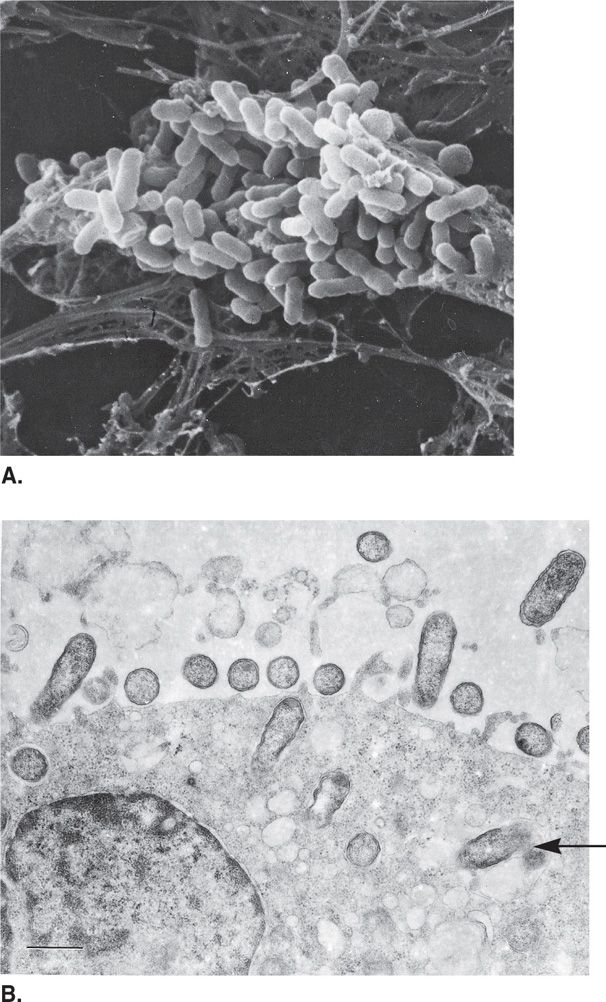
FIGURE 40–1 Rickettsia. Rickettsial morphology and reproduction. A. A human fibroblast filled with Rickettsia prowazekii. B. Rickettsiae attachment to endothelial cell and subsequent phagocytosis. Rickettsiae leave a disrupted phagosome (arrow) and enter the cytoplasmic matrix.
Small, Gram-negative coccobacilli stained best by immunofluorescence
Abundant outer membrane proteins at surface
METABOLISM
Rickettsia grow freely in the cytoplasm of eukaryotic cells to which they are highly adapted, in contrast to Ehrlichia and Bartonella, that replicate in cytoplasmic vacuoles. Rickettsiae can be grown only in the living eukaryotic cells found in cell cultures, organ cultures, and embryonated eggs. Rickettsia are able to adhere to a wide variety of cell types through the binding of outer membrane proteins. They enter cells by induced phagocytosis and escape to the cytoplasms by elaboration of a phospholipase. In the cytoplasm the SFG rickettsiae move about utilizing an actin-based motility similar to that already described for Listeria and Shigella (Chapters 26, 33). Intracytoplasmic growth eventually produces lysis of the cell.
Grow in cytoplasm following induced endocytosis
Growth slow compared with most bacteria
The obligate intracellular parasitism of Rickettsiae has several interesting features. Failure to survive outside the cell is related to requirements for nucleotide cofactors (coenzyme A, NAD), amino acids, phosphorylated sugars, and ATP. Outside the host cell, Rickettsiae not only cease metabolic activity, but leak protein, nucleic acids, and essential small molecules. This instability leads to rapid loss of infectivity because the penetration of another cell requires energy. Over time, Rickettsiae have lost some of their core metabolic capabilities by reductive evolution and instead use transport systems which extract these essential elements from their host cells.
Exogenous cofactors and ATP required for survival
Rapidly loses infectivity outside of host cell
![]() RICKETTSIAL DISEASE
RICKETTSIAL DISEASE
EPIDEMIOLOGY
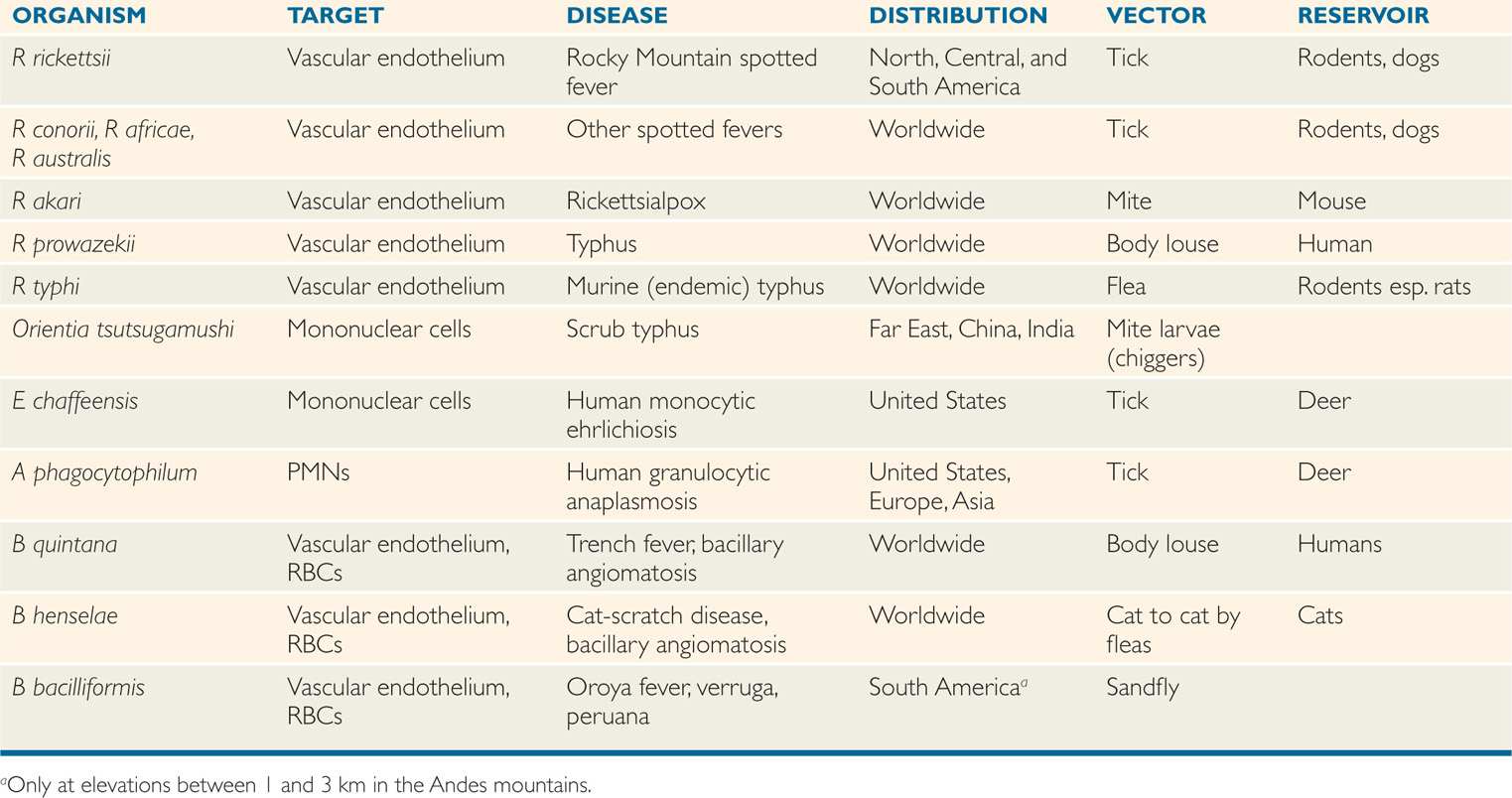
Most rickettsiae have animal reservoirs and are spread by ticks, fleas, mites, or lice, which are prominent components of their life cycles (Table 40–1). The global distribution of specific rickettsial infections is determined by climate, reservoir, vector, and human interactions as detailed under the clinical aspects of each entity. These epidemiologic differences of rickettsial infections are important despite their shared features in pathogenesis. Rickettsial infections of humans usually result in clinical illness.
TABLE 40–1 Features of Rickettsia, Ehrlichia, Anaplasma, and Bartonella
PATHOGENESIS
Following transmission from the salivary gland of infected ticks the bacteria spread locally creating a necrotic eschar. The major rickettsial species have a tropism for vascular endothelium (Figure 40–1B). The primary pathologic lesion is a vasculitis in which they multiply in the endothelial cells lining the small blood vessels (Figure 40–2). Pathophysiologically this leads to increased vascular permeability, hypovolemia, and hypotension. Focal areas of endothelial proliferation and perivascular infiltration leading to thrombosis and leakage of red blood cells into the surrounding tissues account for the rash and petechial lesions. Vascular lesions occur throughout the body and produce the systemic manifestations of the disease. They are obviously most apparent in skin but most serious in the adrenal glands. Orientia tsutsugamushi infects mononuclear cells but still produces fever and rash.
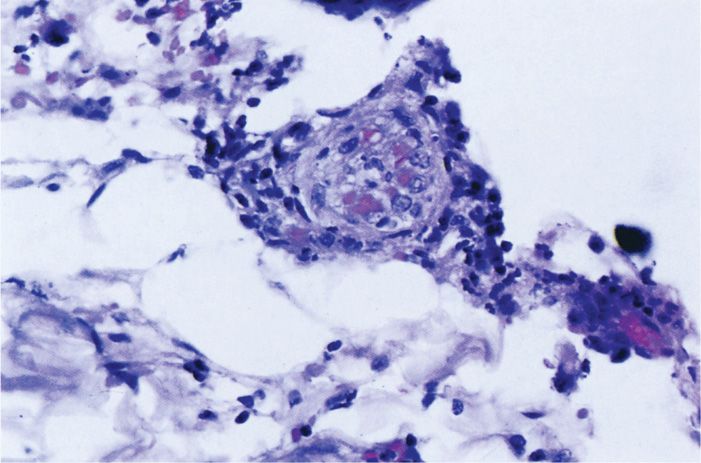
FIGURE 40–2. Rickettsia vasculitis. The endothelial cells of this small vessel in the skin are swollen and injured by infection with Rickettsia typhi. Note also the perivascular lymphocytic infiltrate. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford CT: Appleton & Lange, 1997.)
Infect vascular endothelium with resultant vasculitis and thrombosis
Increased vascular permeability leads to hypotension
 RICKETTSIAL DISEASE: CLINICAL ASPECTS
RICKETTSIAL DISEASE: CLINICAL ASPECTS
SPOTTED FEVER GROUP
The most important rickettsial disease in North America is Rocky Mountain spotted fever (RMSF), which is caused by Rickettsia rickettsii. A number of other spotted fever rickett-sioses are found in other parts of the world (Table 40–1); the name often reveals the locale (eg, Mediterranean spotted fever, Marseilles fever). They are caused by Rickettsia species, serologically related to, but distinct from, R rickettsii (eg, R conorii, R africae, etc). Another less severe spotted fever, rickettsialpox, also occurs in North America.
Many tick-borne rickettsioses occur throughout the world
 Rocky Mountain Spotted Fever
Rocky Mountain Spotted Fever
Rocky Mountain spotted fever is an acute febrile illness that occurs in association with residential and recreational exposure to wooded areas where infected ticks exist. The disease has a significant mortality rate (25%) if untreated.
Epidemiology
Rickettsia rickettsii is primarily a parasite of ticks. In the western United States, the wood tick (Dermacentor andersoni) is the primary vector. In the East, the dog tick (Dermacentor variabilis) is the natural carrier and vector of the disease; and in the Southwest and Midwest, the vector is the Lone Star tick (Amblyomma americanum). Recently, another dog tick, Rhipicephalus sanguineus, has been implicated in cases occurring in rural eastern Arizona. Rickettsia rickettsii does not kill its arthropod host, so the parasite is passed through unending generations of ticks by transovarial spread. Adult females require a blood meal to lay eggs and thus may transmit the disease. Infected adult ticks have been shown to survive as long as 4 years without feeding.
Ticks naturally infected
Transovarial spread perpetuates tick infection
Rickettsia rickettsii is found in North, Central, and South America. The United States has over 500 cases per year, and the highest attack rate is in the mid-Atlantic states with North Carolina being the epicenter (Figure 40–2). More than two-thirds of cases are in children younger than 15 years. The illness is generally seen between April and September because of increased exposure to ticks. A history of tick bite can be elicited in approximately 70% of cases.
Most cases in children
Manifestations
The incubation period between the tick bite and the onset of illness is usually 6 to7 days, but it may be from 2 days to 2 weeks. Fever, headache, rash, toxicity, mental confusion, and myalgia are the major clinical features. The rash is the most characteristic feature of the illness, but may not occur in up to one-third of cases. Rash usually develops on the second or third day of illness as small erythematous macules that rapidly become petechial (Figure 40-3). The lesions appear initially on the wrists and ankles and then spread up the extremities to the trunk in a few hours. A diagnostic feature of RMSF is the frequent appearance of the rash on the palms and soles, a finding not usually seen in maculopapular eruptions associated with other infections, including typhus. Muscle tenderness, especially in the gastrocnemius, is characteristic and maybe extreme. If untreated, or occasionally in patients despite therapy, complications such as disseminated intravascular coagulation, thrombocytopenia, encephalitis, vascular collapse, and renal and heart failure may ensue.
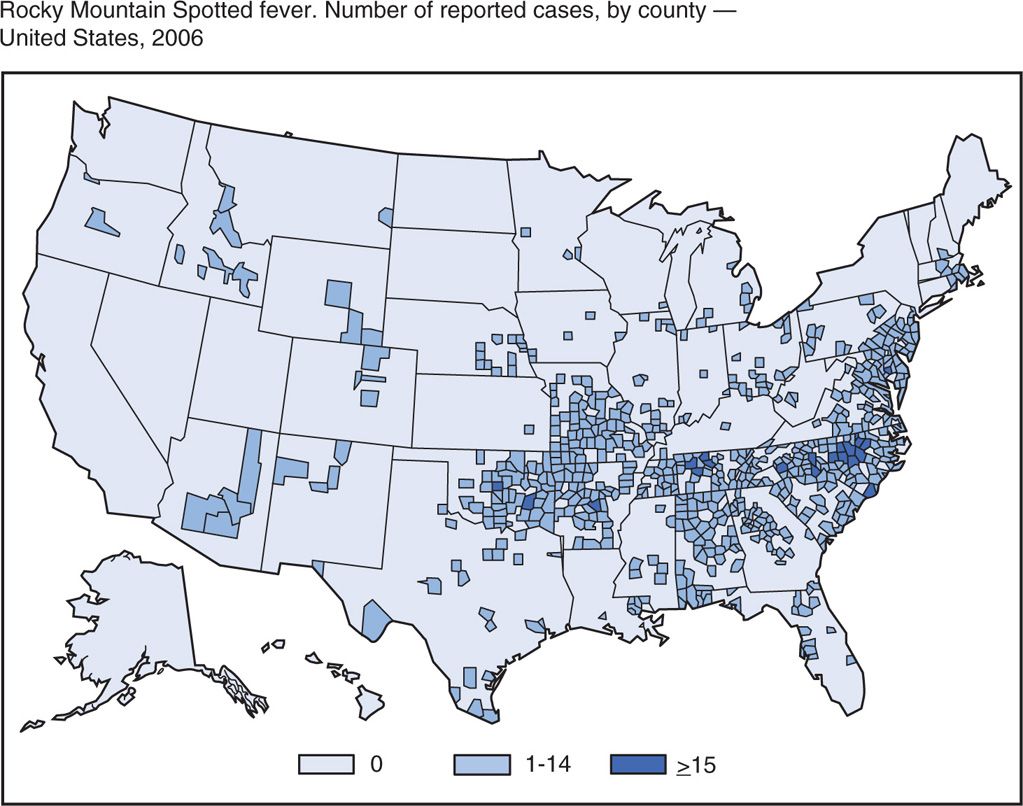
FIGURE 40–3. Distribution of Rocky Mountain spotted fever. Number and distribution of cases in the United States in 2006. (Reprinted with permission from Centers for Disease Control and Prevention. Summary of Notifiable Diseases, MMWR 2008;55(53):63.)
Incubation period 2 to 14 days after tick bite
Rash spreads from extremities to trunk and often involves palms and soles
Diagnosis
Culture of rickettsiae is both difficult and hazardous. Their isolation in fertile eggs or cell cultures is generally attempted only in reference centers with special facilities and personnel experienced in handling the organisms. For this reason, serologic tests are the primary means of specific diagnosis. A number of test systems using specific rickettsial antigens have been developed, of which the indirect fluorescent antibody (IFA) method is generally the most sensitive and specific. This test is usually available only in reference laboratories. For rapid diagnosis, examination of biopsies such as skin lesions by immunofluorescence or immunoenzyme methods to detect antigens can be used.
In-vitro cultivation is hazardous
IFA method usually employed for serologic diagnosis
It is often difficult to establish the diagnosis of RMSF early in the course of illness. However, antibodies may appear by the sixth or seventh day of illness, and a fourfold rise in antibody titer between acute serum and convalescent serum establishes the diagnosis. A skin lesion biopsy may be stained with specific immunofluorescent antibody to provide rapid confirmation of the diagnosis. Most often specific therapy must be started solely on the basis of clinical signs, symptoms, and epidemiologic considerations and can be life-saving.
Rising antibody titers or DFA of skin biopsy confirm diagnosis
Prompt initiation of therapy based on clinical and epidemiologic features
Treatment
Appropriate antibiotic therapy is highly effective if given during the first week of illness. If delayed into the second week or when pathologic processes such as disseminated intravascular coagulation are present, therapy may be futile. The antibiotic of choice is doxycycline. Sulfonamides may worsen the disease process and are thus contraindicated. Before specific therapy became available, the mortality rate associated with RMSF was approximately 25%. Treatment has reduced this figure to between 5% and 7%. Death results primarily in patients in whom diagnosis and therapy are delayed into the second week of illness.
Early empirical treatment crucial
Doxycycline is the treatment of choice
Prevention
The major means of preventing RMSF is avoidance or reduction of tick contact. Frequent deticking in tick-infested areas is important, because ticks generally must feed for 6 hours or longer before they can transmit the disease. Tick surveys in the Carolinas have shown infection in about 5% of samples. Killed vaccines prepared from infected ticks or rickettsiae grown in embryonated eggs and cell cultures have been developed. None is licensed for clinical use at present.
Frequent deticking, avoidance, and protective clothing is important in prevention
 Rickettsialpox
Rickettsialpox
Rickettsialpox was first recognized in 1946 in New York City, where an average of 5 cases per year continue to occur. It has been reported in other US cities and in eastern Europe, Korea, and South Africa. It is a benign rickettsial illness caused by Rickettsia akari and transmitted by a rodent mite. Distinguishing features of the disease include an eschar at the site of the bite and a vesicular rash. The house mouse and other semidomestic rodents are the primary reservoirs. Humans acquire infection when the mite seeks an alternative host.
Benign disease transmitted by rodent mites
Rickettsialpox is a biphasic illness. The first phase is the local lesion at the bite, which starts as a papulovesicle and develops into a black eschar in 3 to 5 days. Fever and constitutional symptoms appear as the organism disseminates. The second phase of the disease is a diffuse rash distributed randomly in the body, which, like the local lesion, becomes vesicular and develops into eschars. However, the rash does not occur in palms or soles. Rickettsialpox is self-limiting after 1 week, and no deaths have been reported. Doxycycline therapy shortens the course to 1 to 2 days.
Local eschar followed by fever and vesicular rash
Doxycycline therapy
TYPHUS GROUP
 Epidemic Louse-Borne Typhus Fever
Epidemic Louse-Borne Typhus Fever
Primary louse-borne typhus fever is caused by Rickettsia prowazekii, which is transmitted to humans by the body louse. Historically, it has appeared during times of misery (war, famine) that create conditions favorable to human body lice (crowding, infrequent bathing). This is the only rickettsial disease that can occur as an epidemic. Foci of typhus persist in parts of Africa, Latin America, and Asia. After the Civil War in Burundi in 1993, upwards of 100 000 cases of epidemic typhus occurred in refugees with case-fatality rates exceeding 5%. In disrupted countries, the homeless population is a focus. Epidemic typhus has not been seen in the United States for more than half a century. Rickettsia prowazekii has been recovered from flying squirrels and their ectoparasites in the southeastern United States, and a few human cases of sylvatic typhus have occurred in these areas.
Severe louse-borne disease due to R prowazekii
Endemic foci in the homeless population
The chain of epidemic typhus infection starts with R prowazekii circulating in a patient’s blood during an acute febrile infection. The human body louse becomes infected during one of its frequent blood meals, and after 5 to 10 days of incubation, large numbers of rickettsiae appear in its feces. Since the louse defecates while it feeds, the organisms can be rubbed into the louse bite wounds when the host scratches the site. Dried louse feces are also infectious through the mucous membranes of the eye or respiratory tract. The louse dies of its infection in 1 to 3 weeks, and the rickettsiae are not transmitted transovarially.
Infection involves feeding and defecation by louse
Fever, headache, and rash begin 1 to 2 weeks after the bite. A maculopapular rash occurs in 20% to 80% of patients and appears first on the trunk and then spreads centrifugally to the extremities, a pattern opposite to that of RMSF. Headache, malaise, and myalgia are prominent components of the illness. Complications include myocarditis and central nervous system dysfunction. In untreated disease, the fatality rate increases with age from 10% to as high as 60%. The diagnostic test of choice is serology, but therapy must be initiated immediately on clinical suspicion. Treatment with doxycycline is effective. Louse control is the best means of prevention and is particularly important in controlling epidemics. No effective vaccine is available.
Fever, headache, and rash with high mortality rate
Louse control is primary prevention
Endemic (Murine) Typhus
Endemic or murine typhus is caused by Rickettsia typhi and transmitted to humans by the rat flea (Xenopsylla cheopis). Human illness is incidental to the natural transmission of the disease among urban rodents, which serve as the reservoir. The disease occurs worldwide but only 50 to 100 cases of murine typhus are reported in the United States each year. These typically occur along the Gulf Coast of Texas and in Southern California.
Transmitted by rat fleas
The pathogenesis is similar to that of louse-borne typhus, but the history includes exposure to rats, rat fleas, or both. The flea defecates when it takes a blood meal, and the infected feces gain access through the bite wound. After an incubation period of 1 to 2 weeks, illness begins with headache, myalgia, and fever. The rash is maculopapular, not petechial; it starts on the trunk and then spreads to the extremities in a manner similar to typhus. Because of antigens shared by R typhi and R prowazekii, serologic tests may not separate the two diseases. In the untreated patient, fever may last 12 to 14 days. With doxycycline therapy, the course is reduced to 2 to 3 days. Mortality and complications are rare, even if the disease is untreated.
Resembles typhus but less severe
R typhi shares antigens with R prowazekii
Scrub Typhus
Scrub typhus is found predominantly in South Asia, China, and Indonesia (the scrub typhus triangle). The causative organism is Orientia tsutsugamushi, a rickettsial organism. Mites that infest rodents are the reservoir and vectors and transmit the rickettsiae to their own progeny via infected ova. Humans pick up the mites as they pass by low trees or brush. The mite larvae (chiggers) deposit rickettsiae as they feed.
Scrub typhus transmitted by rodent mite larvae (chiggers)
The typical initial lesion, a necrotic eschar at the site of the bite on the extremities, develops in only 50% to 80% of cases. Fever increases slowly over the first week, sometimes reaching 40.5°C. Headache, rash, and generalized lymphadenopathy follow later.
Local eschar followed by fever, headache, rash, and lymphadenopathy
The maculopapular rash, which appears after about 5 days, is more evanescent than that seen with louse-borne or murine typhus. Hepatosplenomegaly and conjunctivitis may also appear. Specific diagnosis requires demonstration of a serologic response with the IFA test or polymerase chain reaction (PCR) on blood or biopsy. The prognosis is good with doxycycline therapy, but the mortality rate of untreated patients is as high as 30%.
Serologic diagnosis by IFA
EHRLICHIA AND ANAPLASMA
Ehrlichia and Anaplasma include several species of tick-borne Gram-negative bacteria that cause animal and human disease. The principal diseases are human monocytic ehrlichiosis (HME), which is due to Ehrlichia chaffeensis, and human granulocytic anaplasmosis (HGA), which is due to Anaplasma phagocytophilum. The structure of these species does not include lipopolysaccharide or peptidoglycan, but they can independently carry out basic metabolic tasks such as the Krebs cycle and generation of ATP. All are obligate intracellular pathogens that infect WBCs. The preferred bone marrow derived lineage of WBC varies with the animal species infected. In humans, Echaffeensis primarily infects mononuclear cells and A phagocytophilum polymorphonuclear cells (PMNs). They enter their preferred cell type by receptor-induced endocytosis and multiply in the endocytotic vacuole. The replicative cycle includes replicative forms and denser infectious forms in inclusions (morulae) similar to those seen in Chlamydia. Replication and survival is enhanced by blocking lysosomal fusion with their vacuole and resistance to killing by reactive oxygen species. No toxins or other virulence factors have been described. Injury in human disease is primarily related to inflammatory host responses and can be especially severe in HIV-positive patients.
No LPS or peptidoglycan
Obligate intracellular parasites of mononuclear cells or PMNs
Endocytotic vacuole resists lysosomal fusion
Ehrlichia chaffeensis infections tend to occur in the southeastern and lower midwestern United States, whereas HGA tends to cluster in the northern states with a distribution similar to Lyme disease (Chapter 37). It has also been reported from other areas of the world, including Asia and Europe. Human granulocytic anaplasmosis is the predominant form of ehrlichiosis and is second only to Lyme disease as a tick-borne infection in the United States. Human monocytic ehrlichiosis is transmitted by deer ticks, and the white-tailed deer is the animal reservoir. HGA is transmitted by Ixodes ticks, as is Lyme disease, and the animal reservoir is small mammals (eg, mice, rats, voles). The findings are clinically similar to RMSF, but rashes are less commonly seen. Other ehrlichieae are shown in Table 40–1.
Tick-borne and WBC-associated
On occasion, the diagnosis of ehrlichiosis may be suggested by observation of characteristic ehrlichial intracytoplasmic inclusions (morulae) in granulocytes (HGA) or mononuclear cells (HME) (Figure 40–4). The diagnosis is usually made serologically by a fourfold or greater rise in IFA antibody or a titer greater than or equal to 1:64 to the specific antigen. These tests require the assistance of specialized laboratories. Another diagnostic test for detection of ehrlichia DNA is PCR. Laboratory clues to human ehrlichiosis include a falling leukocyte count, thrombocytopenia, anemia, and impaired liver and renal function. Doxycycline is the drug of choice for ehrlichiosis. The risk of infection can be reduced by avoiding wooded areas and tick bites.
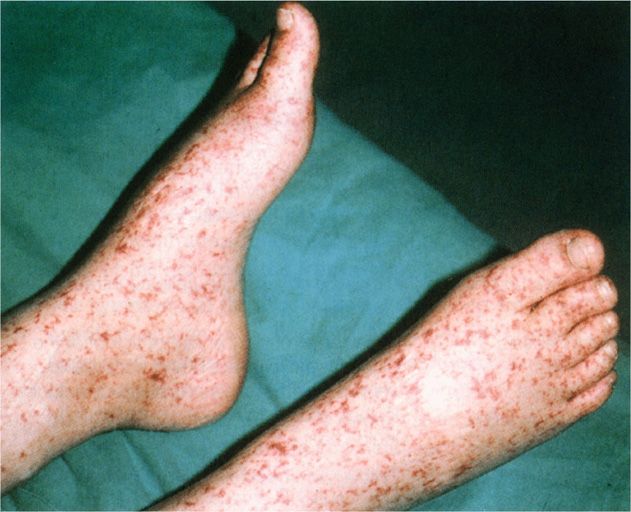
FIGURE 40–4. Rocky Mountain spotted fever. The rash begins on the arms and legs and spreads centrally. (Reproduced with permission from Nester EW: Microbiology: A Human Perspective, 6th edition. 2009.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


