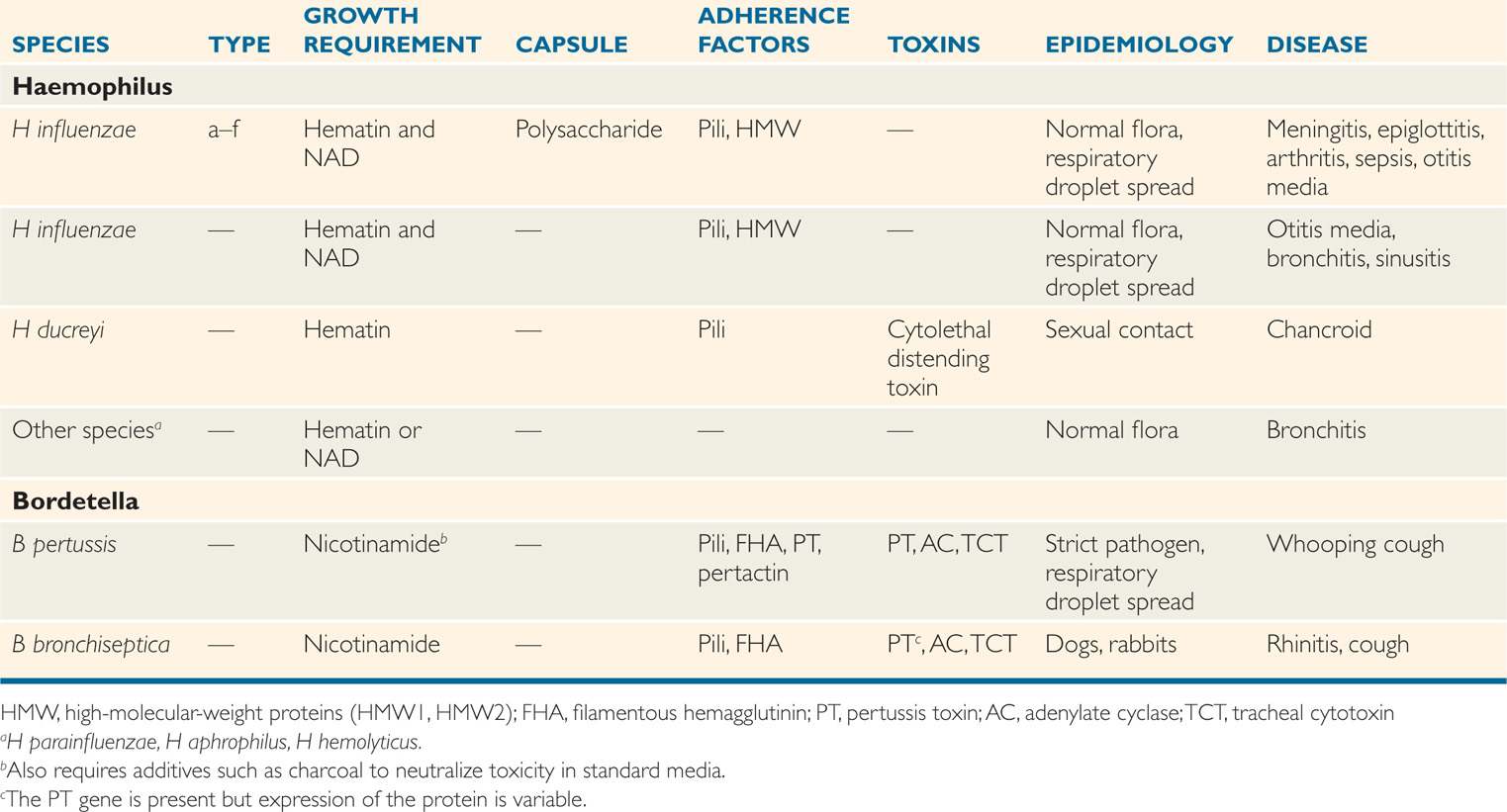
FIGURE 31–1. Haemophilus influenzae Gram stain. The Gram-negative bacilli are small and so short that some appear almost round. This is the basis of the term coccobacilli. The morphology of Bordetella pertussis is the same. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford CT: Appleton & Lange, 1997.)
Tiny Gram-negative coccobacilli
The cultivation of Haemophilus species requires the use of culture media enriched with blood or blood products (Greek haema, blood, and philos, loving) for optimal growth. This requirement can be attributed to the need for exogenous hematin and/or nicotinamide adenine dinucleotide (NAD). These growth factors, also termed X factor (hematin) and V factor (NAD), are present in erythrocytes. In culture media, optimal concentrations are not available unless the red blood cells are lysed by gentle heat (chocolate agar) or added separately as a supplement. Although erythrocytes are the only convenient source of hematin, sufficient amounts of NAD may be provided by certain other bacteria and yeasts. This is responsible for the “satellite phenomenon,” in which colonies of Haemophilus have been observed to grow only in the vicinity of a colony of Staphylococcus aureus. The several species of Haemophilus are defined by their requirement for hematin and/or NAD, CO2 dependence, and other cultural characteristics (Table 31–1). Species of Haemophilus other than H influenzae have the same biology described below for the nonencapsulated strains of H influenzae.
Require hematin and/or NAD
Staphylococcus aureus may provide NAD
Species other than H influenzae are similar
Haemophilus Influenzae
 BACTERIOLOGY
BACTERIOLOGY
Haemophilus that meets the species requirements for H influenzae may or may not have a capsule. Those that do are divided into six serotypes (a–f) based on the capsular polysaccharide antigen. The type b capsule comprises a polymer of ribose, ribitol, and phosphate, called polyribitol phosphate (PRP). These surface polysaccharides are strongly associated with virulence, particularly H influenzae type b (Hib). The surface of H influenzae includes pili and an outer membrane similar to the structure of other Gram-negative bacteria. The outer membrane includes proteins (HMW1, HMW2), lipopolysaccharide (LPS), and lipooligosaccharides (LOS). The nonencapsulated, and thus nontypable, H influenzae (NTHi) can be classified by various typing schemes based on outer membrane proteins and other factors. H influenzae produces no known exotoxins.
Six serotypes are based on capsular polysaccharide
Hib capsule is PRP
![]() HAEMOPHILUS INFLUENZAE DISEASE
HAEMOPHILUS INFLUENZAE DISEASE
EPIDEMIOLOGY
Haemophilus influenzae is a strictly human pathogen and has no known animal or environmental sources. It can be found in the nasopharyngeal flora of 20% to 80% of healthy persons, depending on age, season, and other factors. Most of these are NTHi, but capsulated strains, including Hib, are not rare. Spread is by respiratory droplets, as with streptococci. Before the introduction of effective vaccines, approximately 1 in every 200 children developed invasive disease by the age of 5 years. Meningitis is the most common form and most often attacks those under 2 years of age. Cases of epiglottitis and pneumonia tend to peak in the 2 to 5 year age group. More than 90% of these cases are due to a single serotype, Hib.
Nasopharyngeal colonization is common
Meningitis develops in children under 2 years of age
The introduction of universal immunization with the Hib protein conjugate vaccine (see Prevention) has reduced invasive disease rates by 99%. Most of the cases in immunized populations are now caused by serotypes other than b but there is no evidence of an increase in the non-b serotypes. Unfortunately, Hib disease continues as before in countries and populations unable to afford the vaccine.
Immunization (where implemented) has dramatically reduced disease
At one point in time H influenzae that caused meningitis was believed to be an isolated endogenous infection, but reports of outbreaks in closed populations and careful epidemiologic studies of secondary spread in families have changed this view. The risk of serious infection for unimmunized children younger than 4 years of age living with an index case is more than 500-fold than for nonexposed children. This risk indicates a need for protection of susceptible contacts (see Prevention).
Person-to-person spread requires prophylaxis
PATHOGENESIS
 Invasive Disease
Invasive Disease
For unknown reasons, H influenzae strains commonly found in the flora of the nasopharynx occasionally invade deeper tissues. Bacteremia then leads to spread to the central nervous system and metastatic infections at distant sites, such as bones and joints (Figure 31–2). These events seem to take place within a short period (<3 days) after an encounter with a new virulent strain. Systemic spread is typical only for capsulated H influenzae strains, and more than 90% of invasive strains are type b. Even among Hib strains there are distinct clones, which account for approximately 80% of all invasive disease worldwide.
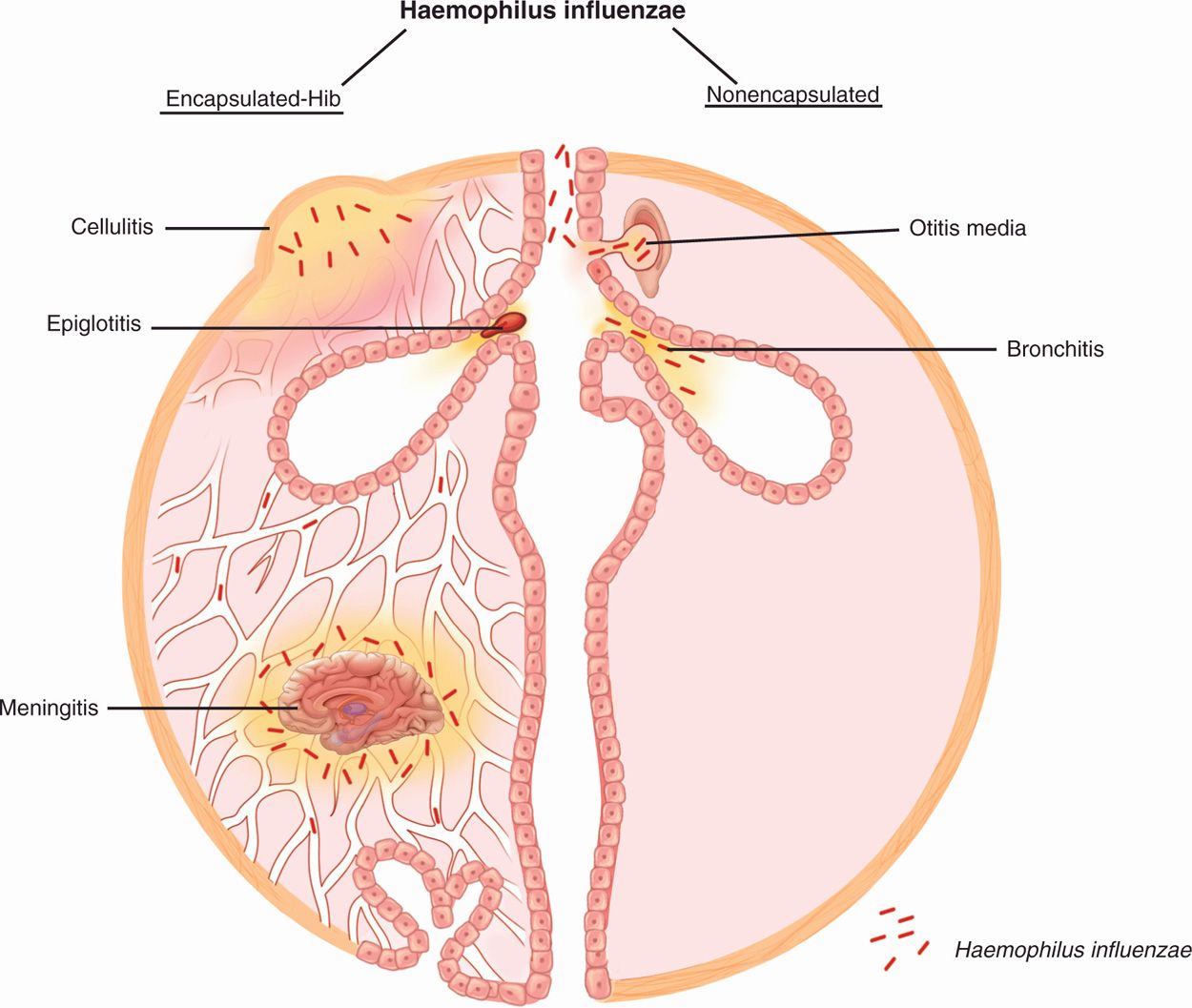
FIGURE 31–2. Haemophilus disease overview. (Left) Invasive disease is caused by encapsulated strains, mostly type b (Hib). From a nasopharyngeal colonization site, the organisms invade locally to produce cellulitis or epiglottitis. Invasion of the blood occurs in all Hib forms and most frequently leads to meningitis. (Right) Localized disease is produced when nonencapsulated strains from the nasopharynx are trapped in the middle ear paranasal sinuses or compromised bronchi.
Only capsulated strains are invasive
Certain clones account for most disease
Attachment to respiratory epithelial cells is mediated by pili and outer membrane proteins. Evidence suggests that this is a complex regulatory cascade, coordinating capsular biosynthesis and adherence factors that act cooperatively in establishing the microbe within susceptible hosts. Haemophilus influenzae can be seen to invade between the cells of the respiratory epithelium (Figure 31–3), and for a time resides between and below them. Once past the mucosal barrier, the antiphagocytic capsule confers resistance to C3b deposition in the same manner as it does with other encapsulated bacteria. As with the pathogenic Neisseria, there is evidence that H influenzae LOS may provide an antiphagocytic effect by binding host components such as sialic acid. Outer membrane LOS is toxic to ciliated respiratory cells, and when circulating in the bloodstream produces all the features of endotoxemia.
Capsule prevents phagocytosis
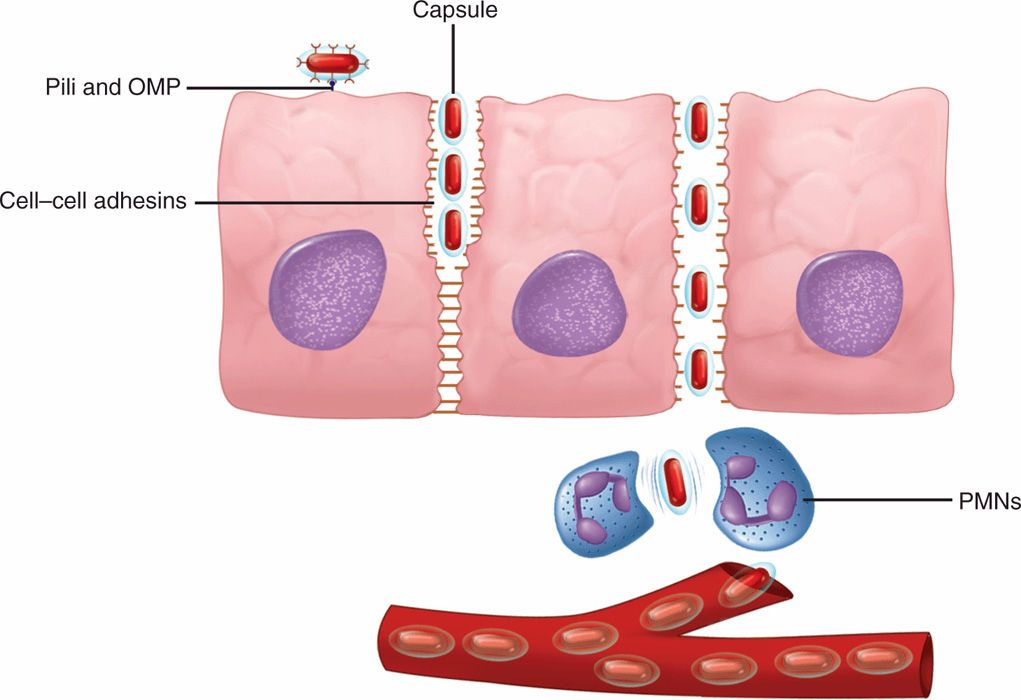
FIGURE 31–3. Haemophilus influenzae disease, cellular view. Organisms attach to epithelial cells using pili and outer membrane proteins (OMP). Invasion takes place between cells by disruption of cell–cell adhesion molecules. In the submucosa, the capsule allows the bacteria to evade phagocytosis and enter the bloodstream. PMNs, polymorphonuclear neutrophils.
Pili and other adhesins bind to epithelial cells
 Localized Disease
Localized Disease
The NTHi produces disease under circumstances in which they are entrapped at a luminal site adjacent to the normal respiratory flora, such as the middle ear, sinuses, or bronchi (Figure 31–2). This is usually associated with some compromise of normal clearing mechanisms, which is caused by a viral infection or structural damage. Consistent with their relative prevalence in the respiratory tract, NTHi account for more than 90% of localized H influenzae disease, particularly otitis media, sinusitis, and exacerbations of chronic bronchitis. NTHi attaches to bronchial epithelial cells and laminin using pili, OMPs, and other proteins.
Bacterial trapped in middle ear, sinuses, and bronchi produce localized infections
Most are NTHi
Adherence is by pili, OMPs, and other proteins
IMMUNITY
Immunity to Hib infections has long been associated with the presence of anticapsular (PRP) antibodies, which are bactericidal in the presence of complement. The infant is usually protected by passively acquired maternal antibody for the first few months of life. Thereafter, actively acquired antibody increases with age; it is present in the serum of most children by 10 years of age. The peak incidence of Hib infections in unimmunized populations occurs at 6 to 18 months of age, when serum antibody is least likely to be present. This inverse relationship between infection and serum antibody is similar to that for Neisseria meningitidis (see Figure 30–4). The major difference is that substantial immune protection is provided by antibody directed against a single type (Hib) rather than the multiple immunotypes of other encapsulated bacteria, such as N meningitidis and S pneumoniae. Thus, systemic H influenzae infections (meningitis, epiglottitis, cellulitis) are rare in adults. When such infections develop, the immunologic deficit is the same as that with meningococci—lack of type-specific circulating antibody.
Anticapsular antibody is bactericidal and protective
Hib infections occur at ages when antibody is absent
Like other polysaccharides, Hib PRP behaves as a T-cell–independent antigen, and antibody responses to immunization are poor in children younger than 18 months of age. Significant secondary responses from boosters are not elicited. Conjugation of PRP to protein dramatically improves the immunogenicity by eliciting the T-cell–dependent responses typical while preserving the specificity for PRP.
T-cell–independent response to PRP is poor at less than 18 months of age
Protein conjugate vaccine elicits T-cell response in infants
 HAEMOPHILUS INFLUENZAE DISEASE: CLINICAL ASPECTS
HAEMOPHILUS INFLUENZAE DISEASE: CLINICAL ASPECTS
MANIFESTATIONS
Of the major acute Hib infections, meningitis accounts for just over 50% of cases. The remaining cases are distributed among pneumonia, epiglottitis, septicemia, cellulitis, and septic arthritis. Localized infections can be caused by capsulated strains including Hib, but most are NTHi.
 Meningitis
Meningitis
Hib meningitis follows the same pattern as other causes of acute purulent bacterial meningitis. Meningitis is often preceded by signs and symptoms of an upper respiratory infection, such as pharyngitis, sinusitis, or otitis media. Whether these represent a predisposing viral infection or early invasion by the organism is not known. Just as often, meningitis is preceded by vague malaise, lethargy, irritability, and fever. Mortality is 3% to 6% despite appropriate therapy, and roughly one-third of all survivors have significant neurologic sequelae.
Acute purulent meningitis may follow sinusitis or otitis media
Mortality and neurologic sequelae are significant
 Acute Epiglottitis
Acute Epiglottitis
Acute epiglottitis is a dramatic infection in which the inflamed epiglottis and surrounding tissues obstruct the airway. Hib is one of several other causes. The onset is sudden, with fever, sore throat, hoarseness, an often muffled cough, and rapid progression to severe prostration within 24 hours. Affected children have air hunger, inspiratory stridor, and retraction of the soft parts of the chest with each inspiration. The hallmark of the disease is an inflamed, swollen, cherry-red epiglottis that protrudes into the airway (Figure 31–4) and can be visualized on lateral X-rays. As with meningitis, this infection is treated as a medical emergency, with prime emphasis on antimicrobial therapy and maintenance of an airway (tracheostomy or endotracheal intubation). Manipulations, including direct examination or attempting to take a throat swab, can trigger a fatal laryngospasm and acute obstruction.
Cherry-red, swollen epiglottitis, and stridor are hallmarks
Airway maintenance is needed
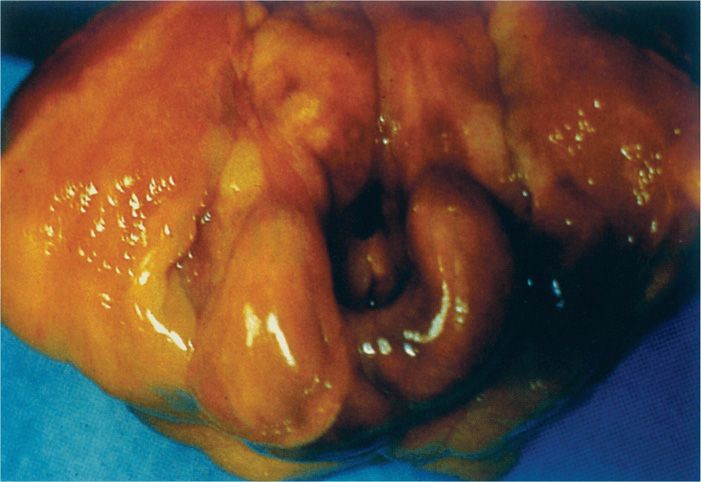
FIGURE 31–4. The swollen epiglottis characteristic of Haemophilus influenzae acute epiglottitis. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford CT: Appleton & Lange, 1997.)
 Cellulitis and Arthritis
Cellulitis and Arthritis
A tender, reddish-blue swelling in the cheek or periorbital areas is the usual presentation of Hib cellulitis. Fever and a moderately toxic state are usually present, and the infection may follow an upper respiratory infection or otitis media. Joint infection begins with fever, irritability, and local signs of inflammation, often in a single large joint. Haemophilus arthritis is occasionally the cause of a more subtle set of findings, in which fever occurs without clear clinical evidence of joint involvement. Bacteremia is often present in both cellulitis and arthritis.
Cellulitis is usually facial
Large joints are involved
 Other Infections
Other Infections
Haemophilus influenzae is an important cause of conjunctivitis, otitis media, and acute and chronic sinusitis. It is also one of several common respiratory organisms that can cause and exacerbate chronic bronchitis. Most of these infections are caused by NTHi strains and remain localized without bacteremia. Disease may be acute or chronic, depending on the anatomic site and underlying pathology. For example, otitis media is acute and painful because of the small, closed space involved, but after antimicrobial therapy and reopening of the eustachian tube, the condition usually clears without sequelae. The association of H influenzae with chronic bronchitis is more complex. There is evidence to suggest that H influenzae and other bacteria play a role in inflammatory exacerbations, but a unique cause-and-effect relationship has been difficult to prove. The underlying cause of the bronchitis is usually related to chronic damage resulting from factors such as smoking. Haemophilus pneumonia may be caused by either encapsulated or nonencapsulated organisms. Encapsulated strains have been observed to produce a disease much like pneumococcal pneumonia; however, NTHi strains may also produce pneumonia, particularly in patients with chronic bronchitis.
Nonencapsulated strains are common in otitis media, sinusitis, and bronchitis
Pneumonia is linked to underlying damage
DIAGNOSIS
The combination of clinical findings and a typical Gram smear is usually sufficient to make a presumptive diagnosis of Haemophilus infection. The tiny cells are usually of uniform shape except in cerebrospinal fluid, in which some may be elongated to several times their usual length (Figure 31–1). The diagnosis must be confirmed by isolation of the organism from the site of infection or from the blood. Blood cultures are particularly useful in systemic H influenzae infections because it is often difficult to obtain an adequate specimen directly from the site of infection. Bacteriologically, small coccobacillary Gram-negative rods that grow on chocolate agar but not blood agar strongly suggest Haemophilus. Confirmation and speciation depend on demonstration of the requirement for hematin (X factor) and/or NAD (V factor) and/or biochemical tests. Serotyping is unnecessary for clinical purposes, but important in epidemiologic and vaccine studies.
Blood cultures are useful in systemic infections
Demonstrating X and V requirement defines species
TREATMENT
All forms of H influenzae disease were effectively treated with ampicillin until the 1970s, when resistance in a pattern similar to that of Neisseria gonorrhoeae emerged. The major mechanism was production of a β-lactamase identical with that found in Escherichia coli. The frequency of β-lactamase–producing strains varies between 5% and 50% in different geographic areas. Ampicillin-resistant strains due to alterations in the transpeptidase-binding site also occur, but are less common. Current practice is to start empiric therapy with a third-generation cephalosporin (eg, ceftriaxone, cefotaxime), which can be changed to ampicillin if susceptibility tests indicate that the infecting strain is susceptible.
Ampicillin-resistant strains produce β-lactamase
Third-generation cephalosporin is initial treatment
PREVENTION
Purified PRP vaccines became available in 1985; however, owing to the typically poor immune response of infants to polysaccharide antigens, their use was limited to children 24 months of age and older. Because immunization at this age misses the group most susceptible to Hib invasive disease, a new vaccine strategy was needed to include improved stimulation of T-cell–dependent immune responses in infants. To achieve this, the first protein conjugate vaccines were developed by linking PRP to proteins derived from bacteria (diphtheria toxoid, N meningitidis outer membrane protein). The first PRP–protein conjugate vaccines were licensed in 1989; by late 1990, they were recommended for universal immunization in children beginning at 2 months of age. As illustrated in Figure 31–5, the impact has been dramatic. This 99% reduction in what was once one of the most feared diseases of childhood is one of the greatest achievements in medical history. Fortunately, the decline in Hib has not been accompanied by compensatory rise in the numbers of non-b cases or in the other causes of acute purulent meningitis. An unexpected concomitant finding has been a dramatic drop in H influenzae colonization rates in immunized populations. Under the direction of the World Health Organization, government and philanthropic efforts like those of the Bill and Melinda Gates Foundation are underway to implement Hib immunization of children throughout the world.
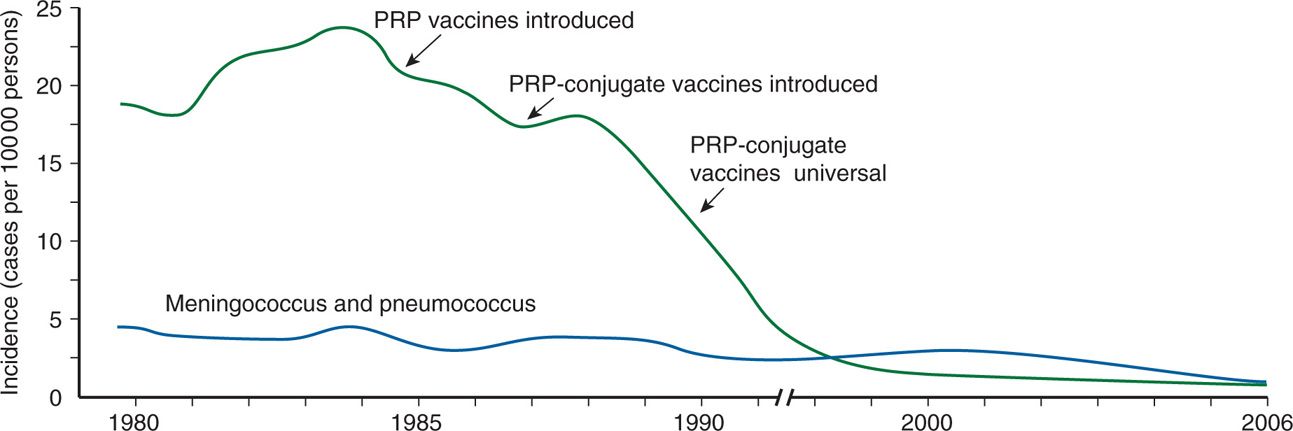
FIGURE 31–5. The decline in Haemophilus influenzae type b (Hib) meningitis in association with the introduction of new vaccines is shown. Note also the steady state of the other major causes of childhood meningitis. They did not increase to “fill in the gap” nor did H influenzae invasive disease caused by other serotypes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree