FIGURE 30–1. Neisseria gonorrhoeae. Gram stain of urethral exudate. Note the many pairs of Gram-negative bean-shaped diplococci (arrow) collected in polymorphonuclear neutrophils (PMNs) and free in the purulent material. The morphology of N meningitidis and other Neisseria is identical. (Image contributed by Professor Shirley Lowe, University of California, San Francisco School of Medicine, with permission.)
Gram-negative diplococci are bean shaped
Gonococci and meningococci require an aerobic atmosphere with added carbon dioxide and enriched medium for optimal growth. Gonococci grow more slowly and are more fastidious than meningococci, which can grow on routine blood agar. All Neisseria are oxidase-positive. Species are defined by growth characteristics and patterns of carbohydrate fermentation. Reagents are also available to distinguish N gonorrhoeae and N meningitidis from the other Neisseria by immunologic methods such as slide agglutination and immunofluorescence.
Gonococci are more fastidious than meningococci
All Neisseria are oxidase-positive
Both pathogenic species possess pili and outer membrane proteins (OMPs), which vary in their function and antigenic composition. In the study of these meningococcal and gonococcal proteins, investigators have assigned names for molecules which appear to have similar functions in pathogenesis. Table 30–1 is an attempt to show similarities and differences. It should be understood that the assignment of the same name (eg, PorA) to a protein found in both species does not mean they are identical. It does suggest that they have similar structure and function.
TABLE 30–1 Bacteriologic and Pathogenic Features of Neisseria
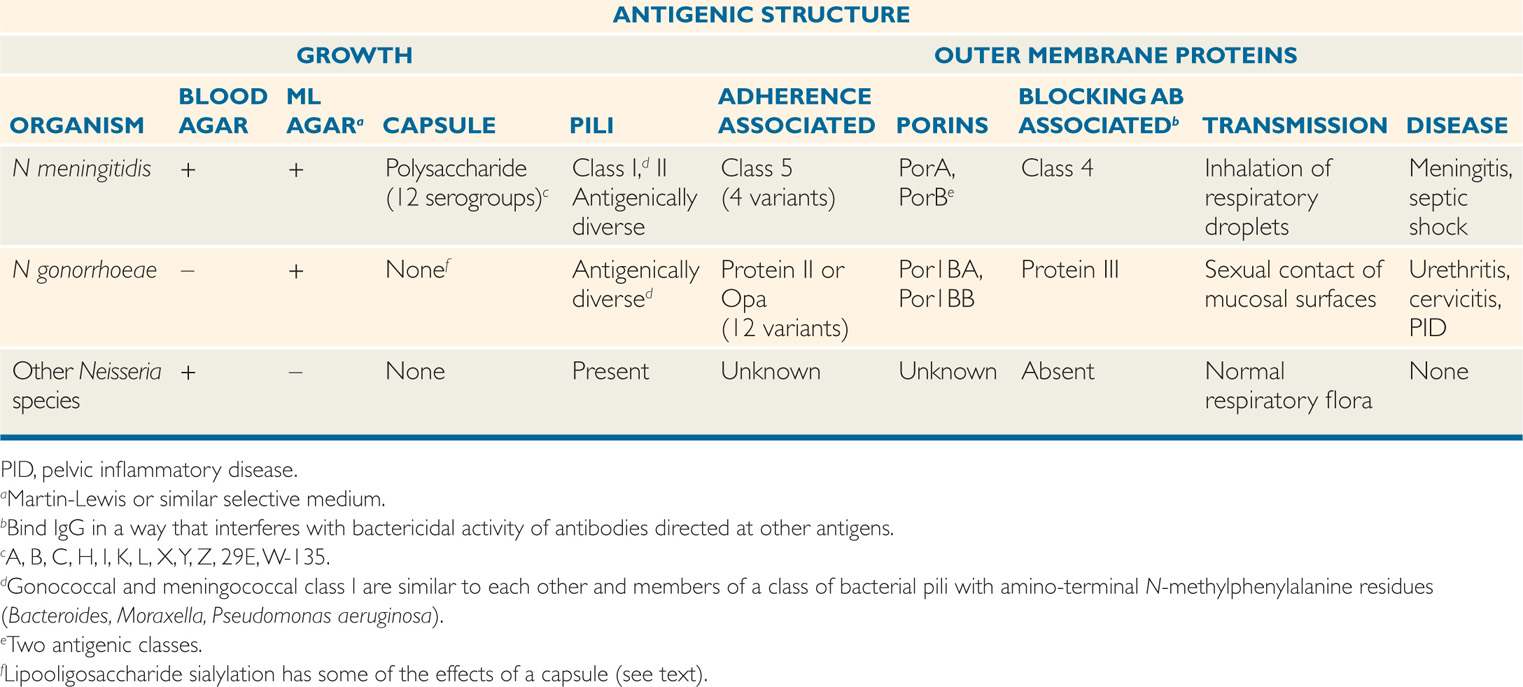
Similar pili and OMPs are present in both species
In addition to lipopolysaccharide (LPS) the outer membrane of the two pathogenic Neisseria contains a variant which differs from that of other Gram-negative bacteria. The major difference is that the polysaccharide side chains are shorter lacking the variable O-antigen units of most other Gram-negative bacteria. This short-chain neisserial polymer is called lipooligosaccharide (LOS). The lipid A and core oligosaccharide are structurally and functionally similar to the LPS of other Gram-negative bacteria and LOS has the same endotoxic power of LPS. The pili, OMPs, and LOS are antigenic and have been used in typing schemes.
Outer membrane LOS has short side chains
NEISSERIA MENINGITIDIS
 BACTERIOLOGY
BACTERIOLOGY
Meningococci produce medium-sized smooth colonies on blood agar plates after overnight incubation. Carbon dioxide enhances growth, but is not required. Thirteen serogroups have been defined on the basis of the antigenic specificity of their polysaccharide capsule. The most important disease-producing serogroups are A, B, C, W-135, and Y. In addition to the group polysaccharides, individual N meningitidis strains may contain two distinct classes of pili and multiple classes of OMPs including porins and adherence proteins, some of which have structural and functional similarities to those found in gonococci. The function of other OMPs is unknown. The N meningitidis genome employs multiple genetic mechanisms to alter its antigenic profile including transformation followed by homologous recombination. When these changes involve the capsular polysaccharide it is called capsule switching and could be a mechanism for emergence of serogroups not included in a vaccine.
Serogroups are based on the polysaccharide capsule
Some OMPs are similar to gonococci
EPIDEMIOLOGY
The combination of rapidly progressive disease and obvious person-to-person spread has long made meningococcal disease one of the most feared of all infections. In fact, meningococci are found in the nasopharyngeal flora of approximately 10% of healthy individuals. Transmission occurs by inhalation of aerosolized respiratory droplets. Close, prolonged contact such as occurs in families and closed populations promotes transmission. The estimated attack rate among family members residing with an index case is 1000 times higher than in the general population; this fact is evidence of the contagious nature of meningococcal infection. Other factors that foster transmission are contact with a virulent strain and host susceptibility (lack of protective antibody). Typical settings of larger outbreaks are schools, dormitories, and camps for military recruits. In these close living circumstances, N meningitidis spreads readily among newly exposed individuals, but disease develops only in those who lack group-specific antibody.
Nasopharyngeal carrier rate is 10%
Spread is by respiratory droplets
The incidence of invasive meningococcal infection varies widely depending on age, geographic locale, and serogroup. In the United States, attack rates vary between 0.5 and 1.5 cases per 100 000 population, but in some countries rates as high as 25 per 100 000 have been sustained for some time. Most disease occurs in infants with a second peak at 18 years of age. Most cases are sporadic or in small family or closed-population (school, day care center) outbreaks. B, C, Y, and W-135 are the most common serogroups in developed countries. Serogroup A strains tend to emerge every 10 to 15 years in large epidemics, with attack rates as high as 1000 per 100 000. Since the second half of the 20th century, serogroup A epidemics have been largely confined to tropical locales, particularly Africa.
B, C, Y, and W-135 are most common serogroups in developed countries
Group A strains can cause widespread epidemics
PATHOGENESIS
The meningococcus is an exclusively human parasite; it can either exist as an apparently harmless member of the resident flora or produce acute disease. For most individuals, the carrier state is associated with acquisition of protective antibodies, but for some, spread from the nasopharynx to produce bacteremia, endotoxemia, and meningitis takes place too quickly for immunity to develop. Meningococcal pili protruding through the capsule are the primary mediators of initial attachment to surface proteins (CD46) on nonciliated cells in the nasopharyngeal epithelium. This is a prelude to invasion. In this process, the pili aggregate the bacteria into microcolonies which then bind to epithelial microvilli and then enter these cells in membrane-bound vesicles. Once inside, meningococci quickly pass through the cytoplasm, exiting into the submucosa and eventually the bloodstream (Figure 30–2). In the process, they damage the ciliated cells, possibly by direct release of endotoxin.
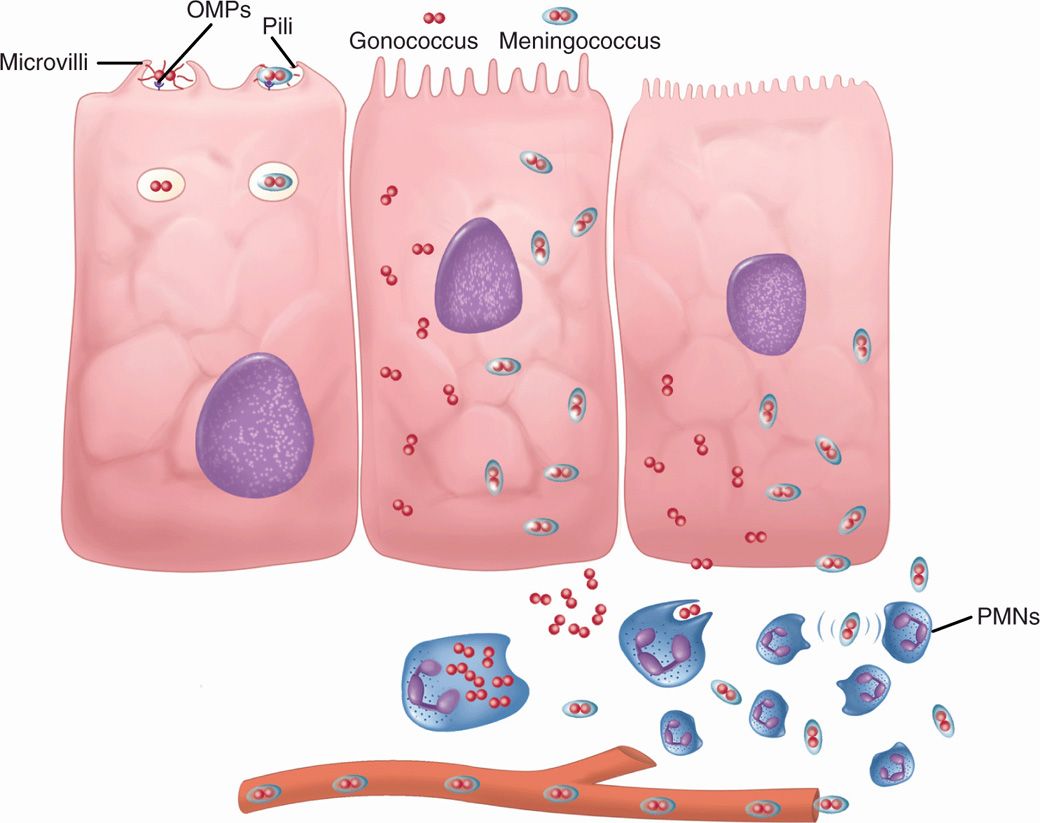
FIGURE 30–2. Gonococcus and meningococcus, cellular view. Neisseria gonorrhoeae and Neisseria meningitidis differ in that Ν meningitidis has a capsule. (Left) Both attach to microvilus cells by outer membrane proteins (OMP) and pili. They are endocytosed in vacuoles. (Middle) Both multiply freely in the cytoplasm. (Right) Both escape to the submucosa, but the gonococcus is actively phagocytosed and remains localized. The meningococcal capsule allows it to evade phagocytosis and it enters the bloodstream. PMNs, polymorphonuclear neutriphils.
Meningococci range from carrier state to bacteremia
Pili attach to microvilli as prelude to invasion
Once meningococci gain access to the submucosa, their ability to produce disease is enhanced by factors that allow them to scavenge essential nutrients like iron and evade the host immune response. As with other encapsulated bacteria, the polysaccharide capsule enables meningococci to resist complement-mediated bactericidal activity by binding serum factor H to their surface (see Chapter 22). Meningococci also have surface proteins which bind this downregulator of C3b deposition. In addition, the LOS side chains are able to incorporate sialic acid, another factor H binder, from host substrates. Like the capsule of group B streptococci (Chapter 25), the capsules of group B and C meningococci also include sialic acid.
Proteins scavenge iron from transferrin
Capsule and proteins bind serum factor H
LOS + sialic acid interferes with C3 deposition
The most serious manifestations of meningococcal disease are related to its spread to the bloodstream and, its namesake, the meninges. The exact mechanism of CNS invasion is unclear but is probably related to the level of the bacteremia. It occurs in the choroid plexus with its exceptionally high rate of blood flow. After CNS invasion, an intense subarachnoid space inflammatory response is induced by the release of cell wall peptidoglycan fragments, LOS, and possibly other virulence factors. This causes the release of inflammatory cytokines. A prominent feature of meningococcal disease with or without CNS invasion is systemic endotoxin activity (see Manifestations). When grown in culture, N meningitidis readily releases endotoxin-containing blebs of its outer membrane from the cell surface as shown in Figure 30–3. It is not known whether this occurs in vivo, but the model of the meningococcus as a hyperproducer of endotoxin certainly fits with its most serious disease manifestations.
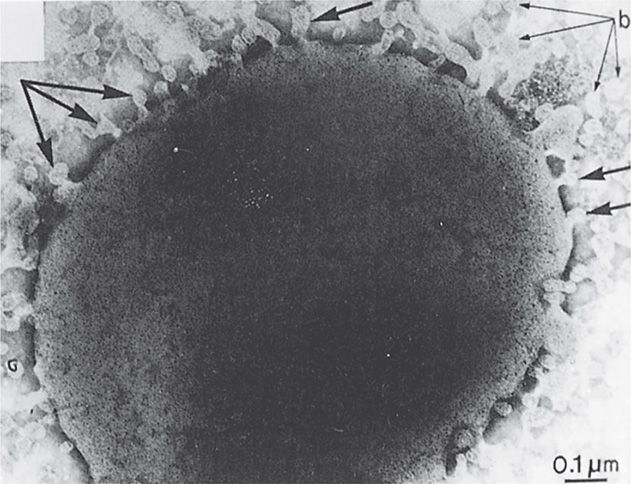
FIGURE 30–3. Neisseria meningitidis. Cell wall is shown shedding multiple “blebs” (arrows) containing lipopolysaccharide–endotoxin. Note the typical trilamellar Gram-negative cell wall structure in the wall and the blebs. (Reprinted with permission from Devoe IW, Gilcrist JE. J Exp Med 1973;138:1160.)
Spread to blood and CNS produces systemic endotoxemia
LOS and peptidoglycan trigger cytokine release
Outer membrane blebs contain endotoxin
IMMUNITY
Immunity to meningococcal infections is related to group-specific antipolysaccharide antibody, which is bactericidal and facilitates phagocytosis. The bactericidal activity is due to complement-mediated cell lysis via the classical complement pathway. Individuals with deficiencies in the terminal complement components have an enhanced risk for meningococcal disease but not for other polysaccharide capsule pathogens, such as Haemophilus influenzae type b.
Group-specific anticapsular antibody is protective
Complement component deficiencies enhance risk
Through the first 12 years of life the incidence of meningococcal meningitis is inversely proportional to the percentage of the population with bactericidal antibody (Figure 30–4). The peak incidence of disease occurs between 6 months and 2 years of age. This corresponds to the nadir in the prevalence of bactericidal antibody in the general population. This is the time gap between loss of maternal transplacental antibody and the appearance of naturally acquired antibody. By adult life, serum antibody to one or more meningococcal serogroups is usually present, but an immune deficit remains for the serogroups not encountered in the local community. Infections appear when populations carrying virulent strains mix (college, summer camp, military barracks) allowing susceptible individuals acquire strains of serogroups to which they have no immunologic experience.
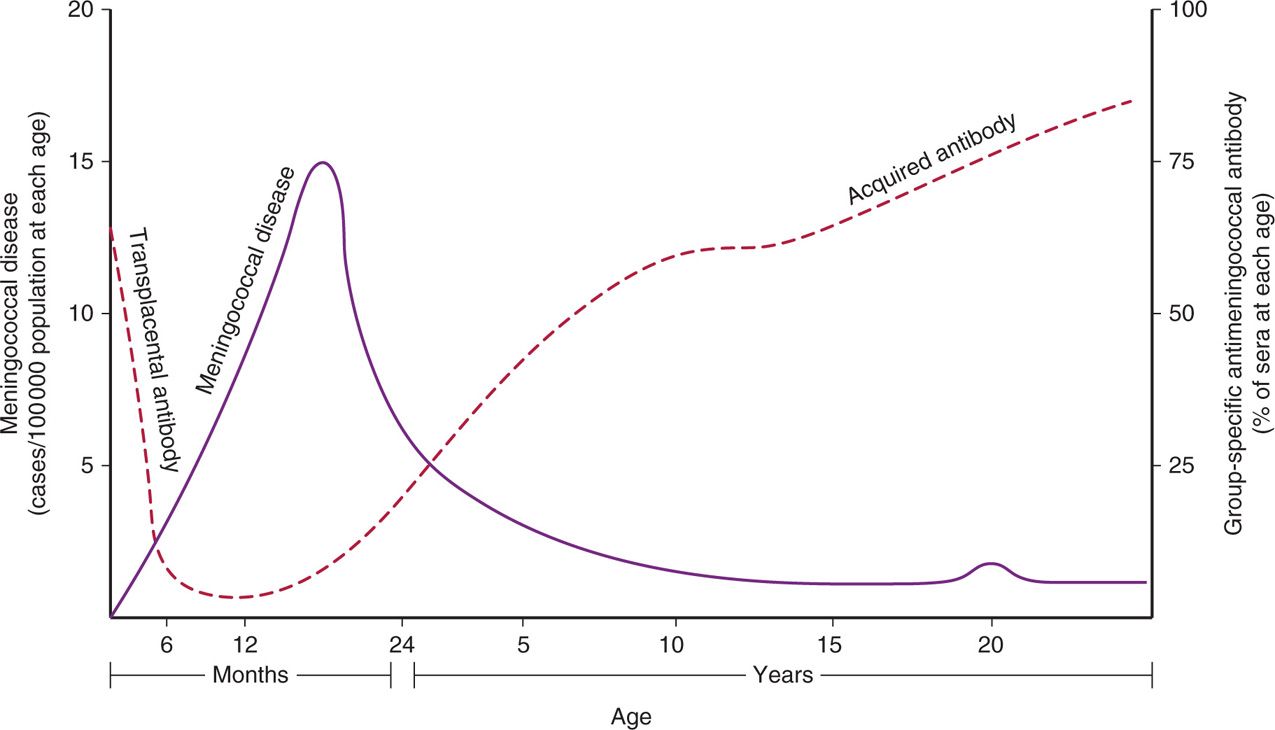
FIGURE 30–4. Immunity to the meningococcus. The inverse relationship between bactericidal meningococcal antibody and meningococcal disease is demonstrated. The “blip” in the disease curve around age 20 is attributable in part to military and other closed-population outbreaks. (Adapted with permission from Goldschneider I, Gotschlich EC, Liu TY, Artenstein MS. Human immunity to the meningococcus I-V. J Exp Med 1969; 129:1307-1395.)
Most common age of infection is 6-24 months
Absence of antibody correlates with susceptibility
Protective antibody is stimulated by infection and through the carrier state, which produces immunity within a few weeks. The natural immunization shown in Figure 30–4 may not require colonization with every serogroup or even with N meningitidis, because antibody may be produced in response to cross-reactive polysaccharides possessed by other Neisseria or even other genera. For example, Escherichia coli strains of a particular serotype (K1) have a polysaccharide capsule identical to that of the group B meningococcus. These E coli also have enhanced potential to produce meningitis in neonates.
Infection, carrier state, or other polysaccharides may stimulate antibody
Purified capsular polysaccharides are immunogenic, generating T-cell–independent immune responses in which IgG2 is the predominant antibody. As with other polysaccharide immunogens, these responses are not strong, particularly in early childhood when there is a relative deficiency of IgG2. The group B polysaccharide differs from that of the other groups in failing to stimulate bactericidal antibody at all. This is believed to be due to the similarity of its sialic acid polymer to human neural cell adhesion molecules. That is, it is recognized as self.
T-cell-independent mechanisms are weak
Group B polysaccharide is not immunogenic
 MENINGOCOCCAL DISEASE: CLINICAL ASPECTS
MENINGOCOCCAL DISEASE: CLINICAL ASPECTS
MANIFESTATIONS
The most common form of meningococcal infection is acute purulent meningitis, with clinical and laboratory features similar to those of meningitis from other causes. A prominent feature of meningococcal meningitis is the appearance of scattered skin petechiae, which may evolve into ecchymoses or a diffuse petechial rash (Figure 30–5). These cutaneous manifestations are signs of the disseminated intravascular coagulation (DIC) syndrome, which is part of the endotoxic shock brought on by meningococcal bacteremia (meningococcemia). Meningococcemia sometimes occurs without meningitis and may progress to fulminant DIC and shock with bilateral hemorrhagic destruction of the adrenal glands (Waterhouse-Friderichsen syndrome). However, the disease is not always fulminant, and some patients have only low-grade fever, arthritis, and skin lesions that develop slowly over a period of days to weeks. Meningococci are a rare cause of other infections such as pneumonia, but it is striking that localized infections are almost never recognized in advance of systemic disease.
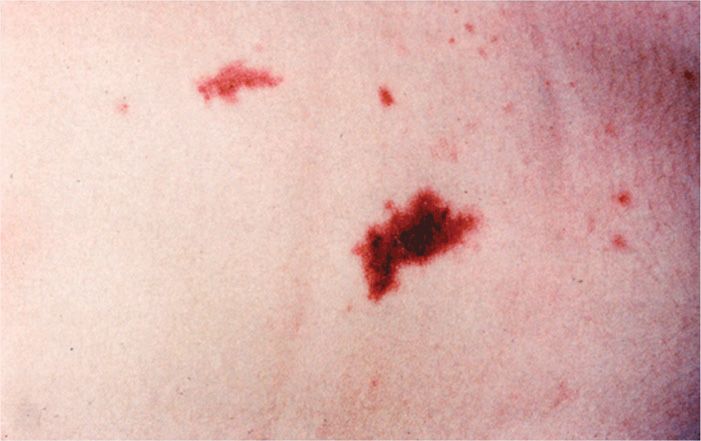
FIGURE 30–5. Meningococcemia. Small and large coalesced petechiae are shown in the skin of a patient with meningococci circulating in the blood. (Reproduced with permission from Nester EW: Microbiology: A Human Perspective, 6th edition. 2009.)
Meningitis is most common infection
Meningococcemia and rash may progress to DIC
Systemic features resemble endotoxic shock
DIAGNOSIS
Direct Gram smears of cerebrospinal fluid (CSF) in meningitis usually demonstrate the typical bean-shaped, Gram-negative diplococci (Figure 30–1). Definitive diagnosis is by culture of CSF, blood, or skin lesions. Although N meningitidis is reputed to be somewhat fragile, it requires no special laboratory handling for isolation from presumptively sterile sites such as blood and CSF. Growth is good on blood or chocolate agar after 18 hours of incubation. Speciation is based on carbohydrate fermentation patterns or immunologic tests. Serogrouping may be performed by slide agglutination methods but has no immediate clinical importance.
Direct CSF Gram smears are diagnostic
Culture requires only blood agar
TREATMENT
Penicillin has long been the treatment of choice for meningococcal infections because of its high antimeningococcal activity and good CSF penetration. Although resistance mediated by both β-lactamase and altered penicillin-binding proteins (PBPs) has been reported, it is still rare. Third-generation cephalosporins such as ceftriaxone and cefotaxime are also effective and are treatments of choice for acute meningitis until the meningococcal etiology is proven. In countries where penicillin resistance is significant, cephalosporins become the first-line treatment.
Penicillin resistance is still rare
PREVENTION
Until the development and spread of sulfonamide resistance in the 1960s, chemoprophylaxis with these agents was the primary means of preventing spread of meningococcal infections. Rifampin or ciprofloxacin are now the primary chemoprophylactic agents. In the absence of resistance, penicillin is still not effective for prophylaxis, probably due to inadequate penetration into the uninflamed nasopharyngeal mucosa. Selection of cases to receive prophylaxis is based on epidemiologic assessment. Risk is highest for siblings of the index case and declines with increasing age. The closeness and duration of contact with the index case are also important. For example, an infant sibling sharing a room with a person with meningococcal disease would be at the highest risk. Typically, family members are given prophylaxis, but other adults are not. Common-sense exceptions, such as playmates and healthcare workers with very close contact (eg, mouth-to-mouth resuscitation), are made at the discretion of the physician or epidemiologist. The presence or absence of nasopharyngeal carriage of N meningitidis plays no role in this decision because it does not accurately predict risk of disease.
Rifampin and ciprofloxacin are primary agents for chemoprophylaxis
Close contact with case is indication for prophylaxis
The first purified polysaccharide vaccines for any bacterial infection were developed at the Walter Reed Army Institute of Research driven by the impact of sulfonamide resistance on recruit camp outbreaks of meningococcal meningitis. These vaccines were shown to stimulate group-specific antibody and to prevent disease in military and adult civilian populations. A vaccine containing A, C, Y, and W-135 polysaccharides was licensed in the United States, but proved poorly immunogenic for infants and children under 2 years of age. This was a huge disappointment because young children are the largest group at risk (Figure 30–4). We now know the reason. Purified polysaccharide vaccines only stimulate T-cell–independent responses and these become fully developed only after 2 years of age. As with pneumococcal and H influenzae polysaccharide vaccines, this problem was overcome by conjugating the polysaccharide to a protein carrier (diphtheria toxoid). This Meningococcal Conjugate Vaccine Quadravalent (MCV4) stimulates T-cell–dependent responses, which are both stronger and present at an earlier age. Its use is now recommended beginning at age 11 with boosters at 16 years. It is also recommended down to the age of 9 months for anyone at high risk for meningococcal disease (complement deficiency, asplenia, HIV infection). Hopefully, further experience and solution of the group B problem (see below) will push universal application of this protection down to infants and toddlers as is done with the highly successful H influenzae Hib vaccine (Chapter 31).
Polysaccharide vaccines only stimulate T-cell–independent immunity
MCV4 vaccine stimulates T-cell–dependent immunity
The protein conjugate approach faces a unique difficulty with the meningococcus—the failure of the group B polysaccharide to be immunogenic at all. If this is due to its similarity to a human neural cell adhesion molecule, as suspected, it may not be overcome simply by protein conjugation. Group B causes up to one-third of all disease, so no vaccine that omits it is likely to be completely successful. For this reason, other approaches such as the use of OMPs (eg, PorA) or serum factor H binding proteins are being pursued. In an approach called reverse vaccinology, genetically engineered vaccines based on the DNA sequence of the entire group B meningococcal genome hold the promise of defining proteins that would immunize against all serogroups of N meningitidis.
Nonimmunogenic serogroup B polysaccharide remains a problem
OMPs are vaccine candidates
NEISSERIA GONORRHOEAE
 BACTERIOLOGY
BACTERIOLOGY
Neisseria gonorrhoeae grows well only on chocolate agar and on specialized medium enriched to ensure its growth. It requires carbon dioxide supplementation. Small, smooth, nonpigmented colonies appear after 18 to 24 hours and are well developed (2-4 mm) after 48 hours. Gonococci possess numerous pili which are structurally similar to those of meningococci and extend beyond the outer membrane (Figure 30–6), (Table 30–1). The gonococcal outer membrane is composed of phospholipids, LPS, LOS, and several distinct OMPs. The OMPs include porins (Por1BA and Por1BB) and adherence proteins known as Opa. Opa proteins are a set of at least 12 proteins that get their name from the opaque appearance they give to colonies as a result of adhesion between gonococcal cells. A variable number of the Opa proteins may be expressed at any one time.
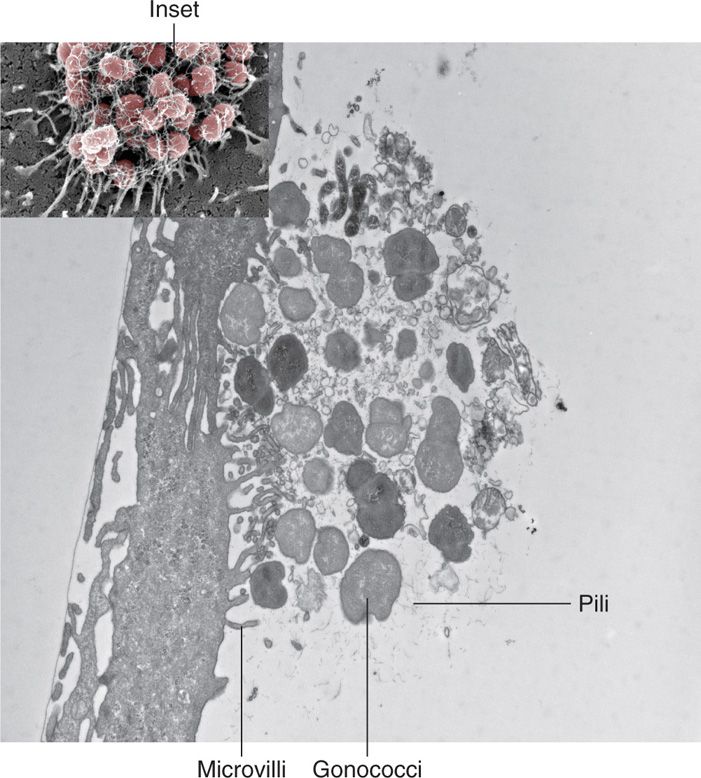
FIGURE 30–6. Neisseria gonorrhoeae pili. This view is a cross-section of the microcolony of gonococci on the surface of an epithelial cell originally shown in Figure 22–2 (inset). Pili are actively attaching to the epithelial cell surface and using a contractile force (twitching motility) to move and modify the surface. (Photomicrographs kindly provided by Dustin L. Higashi and Magdalene So.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


