you can, you had better leave the profession: cast your diploma
into the fire; you are not worthy to hold it.
—Jacob M. Da Costa (1833-1900): College and Clinical Record
The bacteria discussed in this chapter are united by a common requirement for anaerobic conditions for growth. Organisms from multiple genera and all Gram stain categories are included. Most of them produce endogenous infections adjacent to the mucosal surfaces, where they are members of the indigenous flora. The clostridia form spores that allow them to produce diseases such as tetanus and botulism after environmental contamination of tissues or foods. Another anaerobic genus of bacteria, Actinomyces, is discussed in Chapter 28.
ANAEROBES AND ANAEROBIC INFECTION: GROUP CHARACTERISTICS
 BACTERIOLOGY
BACTERIOLOGY
THE NATURE OF ANAEROBIOSIS
Anaerobes not only survive under anaerobic conditions, they require them to initiate and sustain growth. By definition, anaerobes fail to grow in the presence of 10% oxygen, but some are sensitive to oxygen concentrations as low as 0.5% and are killed by even brief exposures to air. However, oxygen tolerance is variable, and many organisms can survive briefly in the presence of 2% to 8% oxygen, including most of the species pathogenic for humans. The mechanisms involved are incompletely understood, but clearly represent a continuum from species described as aerotolerant to those so susceptible to oxidation that growing them in culture requires the use of media prepared and stored under anaerobic conditions.
Anaerobes require low oxygen to initiate growth
Oxygen tolerance is a continuum
Anaerobes lack the cytochromes required to use oxygen as a terminal electron acceptor in energy-yielding reactions and thus to generate energy solely by fermentation (see Chapter 21). Some anaerobes do not grow unless the oxidation-reduction potential is extremely low (-300 mV); because critical enzymes must be in the reduced state to be active, aerobic conditions create a metabolic block.
Low redox potential is required
Another element of anaerobiosis is the direct susceptibility of anaerobic bacteria to oxygen. For most aerobic and facultative bacteria, catalase and/or superoxide dismutase neutralize the toxicity of the oxygen products hydrogen peroxide and superoxide. Most anaerobes lack these enzymes and are injured when these oxygen products are formed in their microenvironment. As discussed in the following text, many of the virulent anaerobic pathogens are able to produce antioxidant enzymes like catalase or superoxide dismutase.
Defense against oxygen products is lacking
Pathogens often have catalase and superoxide dismutase
CLASSIFICATION
The anaerobes indigenous to humans include almost every morphotype and hundreds of species. Typical biochemical and cultural tests are used for classification, although this is difficult because the growth requirements of each anaerobic species must be satisfied. Characterization of cellular fatty acids and metabolic products by chromatography has been useful for many anaerobic groups. Nucleic acid base composition and homology have been used extensively to rename older taxonomy. The genera most commonly associated with disease are shown in Table 29-1 and discussed below.
TABLE 29–1 Usual Locations of Some Opportunistic Anaerobes
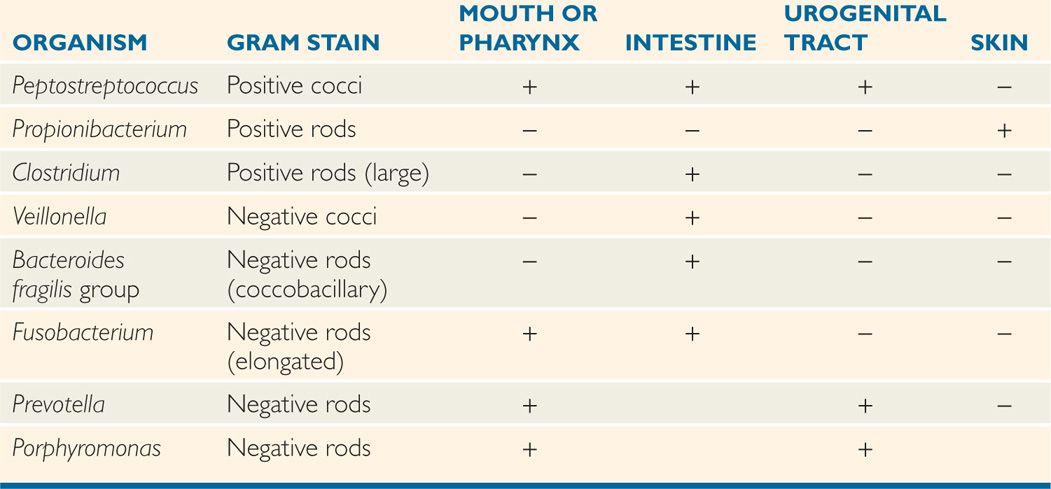
Biochemical, cultural, and molecular criteria define many species
 Anaerobic Cocci
Anaerobic Cocci
The medically important species of anaerobic Gram-positive cocci include species of the genus, Peptostreptococcus, Peptococcus, Peptoniphilus, Aerococcus, and six others. With Gram staining, these bacteria are most often seen as long chains of tiny cocci. On the Gram-negative side Veillonella deserves mention because of its potential for confusion with Neisseria although there are a few others (Acidaminococcus, Megasphera, Anaeroglobus).
Gram(+) in long chains
Veillonella may resemble Neisseria
 Clostridia
Clostridia
The clostridia are large, spore-forming, Gram-positive bacilli. Like their aerobic counterpart, Bacillus, clostridia have spores that are resistant to heat, desiccation, and disinfectants. They are able to survive for years in the environment and return to the vegetative form when placed in a favorable milieu. The shape of the cell and location of the spore vary with the species, but the spores themselves (Figure 29-1) are rarely seen in clinical specimens.
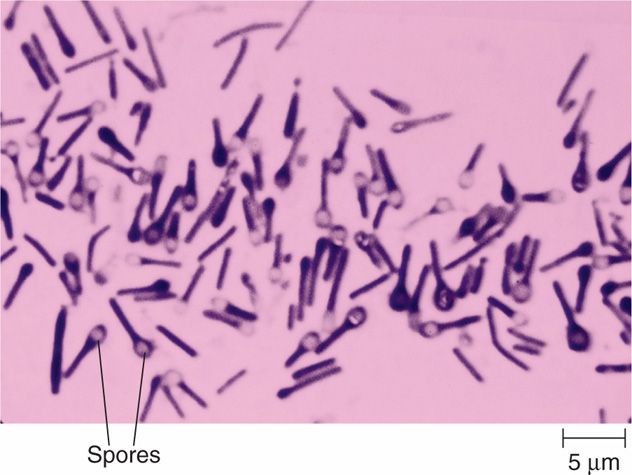
FIGURE 29–1. Clostridium tetani. Many of these bacilli show the typical terminal “tennis racquet” spores typical of this species. (© Arthur Siegelman/Visuals Unlimited)
The medically important clostridia are potent producers of one or more protein exotoxins. The histotoxic group including Clostridium perfringens and five other species (Table 29-2) produces hemolysins at the site of acute infections that have lytic effects on a wide variety of cells. The neurotoxic group including C tetani and C botulinum produces neurotoxins that exert their effect at neural sites remote from the bacteria. Clostridium difficile produces enterotoxins and disease in the intestinal tract. Many of the more than 80 other nontoxigenic clostridial species are also associated with disease.
TABLE 29–2 Features of Pathogenic Anaerobes
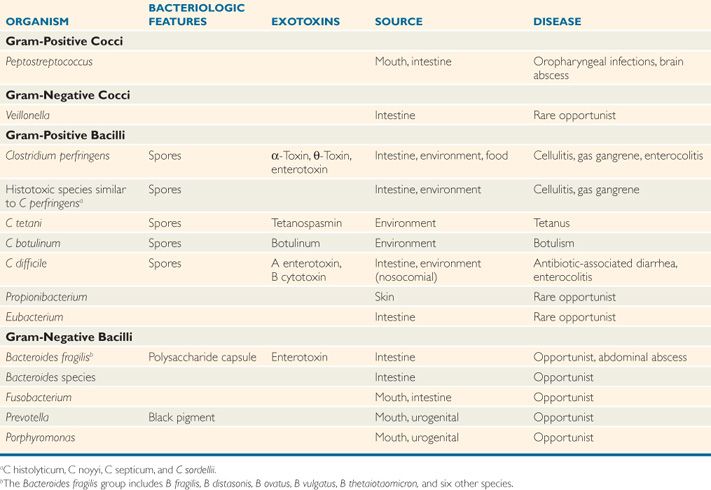
Spores vary in shape and location
Hemolysin, neurotoxin, and enterotoxin production cause disease
 Nonsporulating Gram-positive Bacilli
Nonsporulating Gram-positive Bacilli
Propionibacterium is a genus of small pleomorphic bacilli sometimes called anaerobic diphtheroids because of their morphologic resemblance to corynebacteria. They are among the most common bacteria in the resident flora of the skin. Eubacterium is a genus that includes long slender bacilli commonly found in the colonic flora. These organisms are occasionally isolated from infections in combination with other anaerobes, but they rarely produce disease on their own. Other anaerobic Gram-positive bacilli play roles in dental caries (Chapter 41).
Low-virulence members of skin, oral, and intestinal flora
 Gram-negative Bacilli
Gram-negative Bacilli
Gram-negative, non–spore-forming bacilli are the most common bacteria isolated from anaerobic infections. In the past, most species were lumped into the genus Bacteroides, which still exists along with five other genera. Of these, Fusobacterium, Porphyromonas, and Prevotella are medically the most important. The Bacteroides fragilis group contains B fragilis and 10 similar species noted for their virulence and production of β-lactamases. (Species outside this group generally lack these features and are more similar to the other anaerobic Gram-negative bacilli.) Bacteroides fragilis is a relatively short Gram-negative bacillus with rounded ends sometimes giving a coccobacillary appearance. Almost all B fragilis strains have a polysaccharide capsule and are particularly oxygen-tolerant. Prevotella, Porphyromonas, and Fusobacterium are distinguished by biochemical and other taxonomic features. Prevotella melaninogenica forms a black pigment in culture, and Fusobacterium, as its name suggests, is typically elongated and has tapered ends.
Bacteroides and other genera are medically important
B fragilis group is oxygen tolerant and produces β-lactamase
EPIDEMIOLOGY
Despite our constant immersion in air, anaerobes are able to colonize the many oxygen-deficient or oxygen-free microenvironments of the body. These conditions are created by the presence of resident bacteria whose growth reduces oxygen and decreases the local oxidation–reduction potential. Such sites include the sebaceous glands of the skin, the gingival crevices of the gums, the lymphoid tissue of the throat, and the lumina of the intestinal and urogenital tracts. Except for infections with some environmental clostridia, anaerobic infections are almost always endogenous with the infective agent(s) derived from the patient’s own microbiota. The specific anaerobes involved are linked to their prevalence in the flora of the relevant sites as shown in Table 29-1. In addition to the presence of clostridia in the lower intestinal tract of humans and animals, their spores are widely distributed in the environment, particularly in soil exposed to animal excreta. The spores may contaminate any wound caused by a nonsterile object (eg, splinter, nail) or exposed directly to soil.
Low redox normal flora sites are the origin of most infections
Spore-forming clostridia also come from the environment
PATHOGENESIS
The anaerobic flora normally lives in a harmless commensal relationship with the host. However, when displaced from their niche on the mucosal surface into normally sterile tissues, these organisms may cause life-threatening infections. This can occur as the result of trauma (gunshot, surgery), disease (diverticulosis, cancer), or isolated events (aspiration). Host factors such as malignancy or impaired blood supply increase the probability that the dislodged flora will eventually produce an infection. The anaerobes most often causing infection are those both present in the microbiota at the adjacent mucosal site and which possess other features enhancing their virulence. For example, B fragilis represents a small percent of the normal colonic flora but is the bacterial species most frequently isolated from intraabdominal abscesses.
Anaerobes displaced from normal flora to deeper sites may cause disease
Trauma and host factors create the opportunity for infection
The relation between the microbiota and site of infection may be indirect. For example, aspiration pneumonia, lung abscess, and empyema typically involve anaerobes found in the oropharyngeal flora. The brain is not a particularly anaerobic environment, but brain abscess is most often caused by these same oropharyngeal anaerobes. This presumably occurs by extension across the cribriform plate to the temporal lobe, the typical location of brain abscess. In contaminated open wounds, clostridia can come from the intestinal flora or from spores surviving in the environment.
Flora may be aspirated or displaced at a distance
Brain abscess typically involves anaerobic bacteria
Although gaining access to tissue sites provides the opportunity, additional virulence factors are needed for anaerobes to produce infection. Some anaerobic pathogens produce disease even when present as a minor part of the displaced resident flora, and other common members of the normal flora rarely cause disease. Classic virulence factors such as toxins and capsules are known only for the toxigenic clostridia and B fragilis, but a feature such as the ability to survive brief exposures to oxygenated environments can also be viewed as a virulence factor. Anaerobes found in human infections are far more likely to produce catalase and superoxide dismutase than their more docile counterparts of the microbiota. Exquisitely oxygen-sensitive anaerobes are seldom involved, probably because they are injured by even the small amounts of oxygen dissolved in tissue fluids.
Capsules and toxins are known for some anaerobes
Survival in oxidized conditions can be a virulence factor
A related feature is the ability of the bacteria to create and control a reduced microenvironment, often with the apparent help of other bacteria. Most anaerobic infections are mixed; that is, two or more anaerobes are present, often in combination with facultative bacteria such as Escherichia coli. In some cases, the components of these mixtures are believed to synergize each other’s growth either by providing growth factors or by lowering the oxidation–reduction potential. These conditions may have other advantages such as the inhibition of oxygen-dependent leukocyte bactericidal functions under the anaerobic conditions in the lesion. Anaerobes that produce specific toxins have a pathogenesis on their own, which are discussed in the sections devoted to individual species.
Mixed infections may facilitate an anaerobic microenvironment
 ANAEROBIC INFECTIONS: CLINICAL ASPECTS
ANAEROBIC INFECTIONS: CLINICAL ASPECTS
MANIFESTATIONS
Bacteroides, Fusobacterium, and anaerobic cocci, alone or together with other facultative or obligate anaerobes, are responsible for the overwhelming majority of localized abscesses within the cranium, thorax, peritoneum, liver, and female genital tract. As indicated earlier, the species involved relate to the pathogens present in the microbiota of the adjacent mucosal surface. Those derived from the oral flora also include dental infections and infections of human bites.
Abscesses are usually caused by Bacteroides, Fusobacterium, or anaerobic cocci
In addition, anaerobes play causal roles in chronic sinusitis, chronic otitis media, aspiration pneumonia, bronchiectasis, cholecystitis, septic arthritis, chronic osteomyelitis, decubitus ulcers, and soft tissue infections of patients with diabetes mellitus. Dissection of infection along fascial planes (necrotizing fasciitis) and thrombophlebitis are common complications. Foul-smelling pus and crepitation (gas in tissues) are signs associated with, but by no means exclusive to, anaerobic infections. As with other bacterial infections, they may spread beyond the local site and enter the bloodstream. The mortality rate of anaerobic bacteremias arising from nongenital sources is equivalent to the rates with bacteremias due to staphylococci or Enterobacteriaceae.
Foul-smelling pus suggests anaerobic infection
DIAGNOSIS
The key to detection of anaerobes is a high-quality specimen, preferably pus or fluid taken directly from the infected site. The specimen needs to be taken quickly to the microbiology laboratory and protected from oxygen exposure while on the way. Special anaerobic transport tubes may be used, or by expression of any air from the syringe in which the specimen was collected. A generous collection of pus serves as its own best transport medium unless transport is delayed for hours.
Specimens must be direct and protected from oxygen
A direct Gram-stained smear of clinical material demonstrating Gram-negative and/or Gram-positive bacteria of various morphologies is highly suggestive, often even diagnostic of anaerobic infection. Because of the typically slow and complicated nature of anaerobic culture, the Gram stain often provides the most useful information for clinical decision making. Isolation of the bacteria requires the use of an anaerobic incubation atmosphere and special media protected from oxygen exposure. Although elaborate systems are available for this purpose, the simple anaerobic jar is sufficient for isolation of the clinically significant anaerobes. The use of media that contain reducing agents (cysteine, thioglycollate) and growth factors needed by some species further facilitates isolation of anaerobes. The polymicrobial nature of most anaerobic infections requires the use of selective media to protect the slow-growing anaerobes from being overgrown by hardier facultative bacteria, particularly members of the Enterobacteriaceae. Antibiotics, particularly aminoglycosides to which all anaerobes are resistant, are frequently incorporated in culture media. Once the bacteria are isolated, identification procedures include morphology, biochemical characterization, and metabolic end-product detection by gas chromatography.
Gram staining is particularly useful
Anaerobic incubation jar provides atmosphere
Selective media inhibit facultative bacteria
TREATMENT
As with most abscesses, drainage of the purulent material is the primary treatment, in association with appropriate chemotherapy. Antimicrobial agents alone may be ineffective because of failure to penetrate the site of infection. Their selection is empiric to a large degree because such infections typically involve mixed species. Cultural diagnosis is delayed by the slow growth and the time required to distinguish multiple species. In addition, antimicrobial susceptibility testing methods are slow and less standardized than they are for the rapidly growing bacteria. The usual approach involves selection of antimicrobials based on the expected susceptibility of the anaerobes known to produce infection at the site in question. For example, anaerobic organisms derived from the oral flora are often susceptible to penicillin, but infections below the diaphragm are caused by fecal anaerobes such as B fragilis which is resistant to many β-lactams. These latter infections are most likely to respond to metronidazole, imipenem, aztreonam, or ceftriaxone, a cephalosporin not inactivated by the β-lactamases produced by anaerobes.
Mixed infections and slow growth dictate empiric therapy
Abdominal infections require β-lactamase-resistant antimicrobials
CLOSTRIDIUM PERFRINGENS
 BACTERIOLOGY
BACTERIOLOGY
Clostridium perfringens is a large, Gram-positive, nonmotile rod with square ends. It grows overnight under anaerobic conditions, producing hemolytic colonies on blood agar. In the broth containing fermentable carbohydrate, growth of C perfringens is accompanied by the production of large amounts of hydrogen and carbon dioxide gas, which can also be produced in necrotic tissues; hence the term gas gangrene.
Hemolysis and gas production are characteristic
Clostridium perfringens produces multiple exotoxins that have different pathogenic significance in different animal species and serve as the basis for classification of the five types (A-E). Type A is by far the most important in humans and is found consistently in the colon and often in soil. The most important exotoxin is the α-toxin, a phospholipase that hydrolyzes lecithin and sphingomyelin, thus disrupting the cell membranes of various host cells, including erythrocytes, leukocytes, and muscle cells. The θ-toxin alters capillary permeability and is toxic to heart muscle. This toxin also has pore-forming activity similar to streptolysin O. A minority of strains (<5%) produce an enterotoxin, which inserts into enterocyte membranes to form pores leading to alterations in intracellular calcium and membrane permeability. This leads to loss of cellular fluid and macromolecules.
Typing system is based on toxins
Phospholipase α-toxin, pore-forming θ-toxin, and enterotoxin cause disease
![]() CLOSTRIDIUM PERFRINGENS DISEASE
CLOSTRIDIUM PERFRINGENS DISEASE
EPIDEMIOLOGY
 Gas Gangrene
Gas Gangrene
Gas gangrene develops in traumatic wounds with muscle damage when they are contaminated with dirt, clothing, or other foreign material containing C perfringens or another species of histotoxic clostridia (see Table 29-2). The clostridia can come from the patient’s own intestinal flora or spores in the environment. Compound fractures, bullet wounds, or the kind of trauma seen in wartime are prototypes for this infection. A significant delay (many hours) between the injury and definitive surgical management is required for bacterial multiplication and toxin production to develop. In peacetime these conditions are more likely to be satisfied in a remote hiking accident than in an automobile collision. The difference is the time between injury and medical intervention.
Spores from the host or environment contaminate wounds
Delays allow multiplication
 Clostridial Food Poisoning
Clostridial Food Poisoning
Clostridium perfringens can cause food poisoning if spores of an enterotoxin-producing strain contaminate food. Outbreaks usually involve rich meat dishes such as stews, soups, or gravies that have been kept warm for a number of hours before consumption. This allows time for the infecting dose to be reached by conversion of spores to vegetative bacteria, which then multiply in the food. Clostridial food poisoning is common in developed countries and is second among foodborne illnesses in the United States with over a million cases per year.
Bacteria multiply in meat dishes
PATHOGENESIS
 Gas Gangrene
Gas Gangrene
If the oxidation-reduction potential in a wound is sufficiently low, C perfringens spores can germinate and then multiply, elaborating α-toxin. The process passes along the muscle bundles, producing rapidly spreading edema and necrosis as well as conditions that are favorable for growth of the anaerobes. Very few leukocytes are present in the myonecrotic tissue (Figure 29-2). As the disease progresses, increased vascular permeability and systemic absorption of the toxin leads to shock. α-Toxin is the major cause of both local destruction and shock. α-Toxin and oxygen deprivation due to the metabolic activities of C perfringens are probable contributors. The basis for the profound systemic effects is not known, but α-toxin absorption seems probable because fatal cases occur without bacteremia.
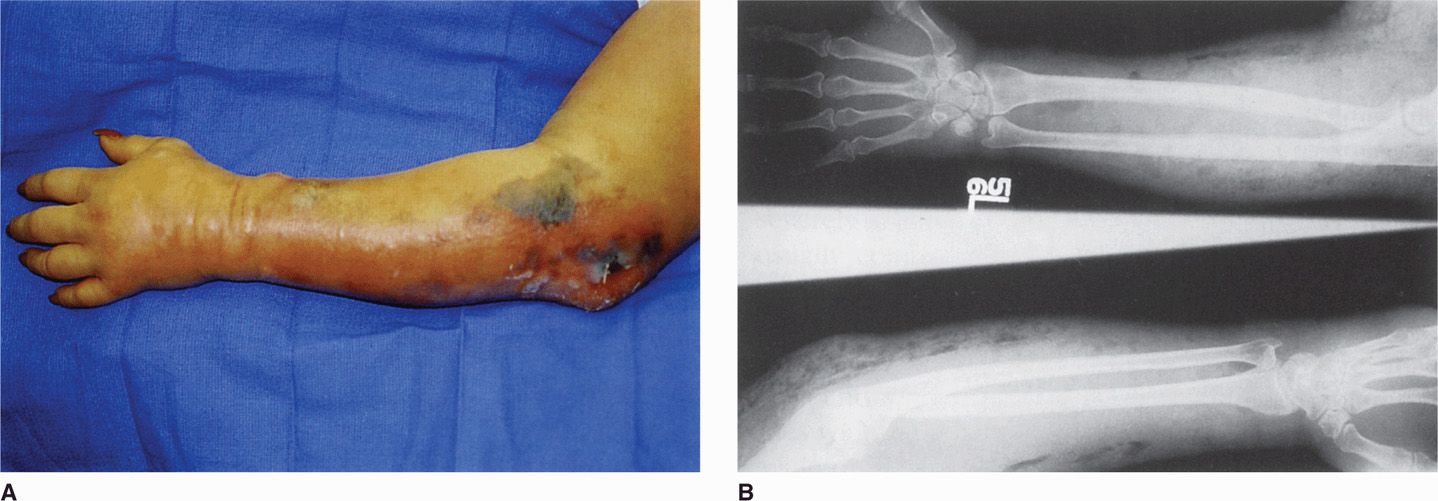
FIGURE 29–2. Gas gangrene. A. arm of a drug abuser with ulcers and swelling traced to needle tracks. B. radiographs from the same patient demonstrating gas (clear spaces) in the tissues. (reproduced with permission from Connor Dh, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford Ct: appleton & Lange, 1997.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


