BACTERIOLOGY
STRUCTURE
The mycobacteria are slim, poorly staining bacilli, which demonstrate the property of acid-fastness. They are nonmotile, obligate aerobes that do not form spores. The cell wall contains peptidoglycan similar to that of other Gram-positive organisms, to which many branched-chain polysaccharides, proteins, and lipids are attached. Porins and other proteins are found throughout the cell wall. Of particular importance is the presence of long-chain fatty acids called mycolic acids (for which the mycobacteria are named) and lipoarabino-mannan (LAM), a lipid polysaccharide complex extending from the plasma membrane to the surface (Figure 27–1). LAM is structurally and functionally analogous to the lipopolysaccharide of Gram-negative bacteria. These elements give the mycobacteria a cell wall with unusually high lipid content (>60% of the total cell wall mass), which accounts for many of their biologic characteristics. It can be thought of as a waxy coat that makes them hardy, impenetrable, and hydrophobic. The staining characteristic of acid-fastness is the most frequently observed of these features. The mycobacterial cell wall can be stained only through the use of extreme measures (prolonged time, heat, penetrating agents) but once in, the stain is fast. Even the strongest of decolorizing agents (acid and alcohol) do not wash it out (Figure 27–2).
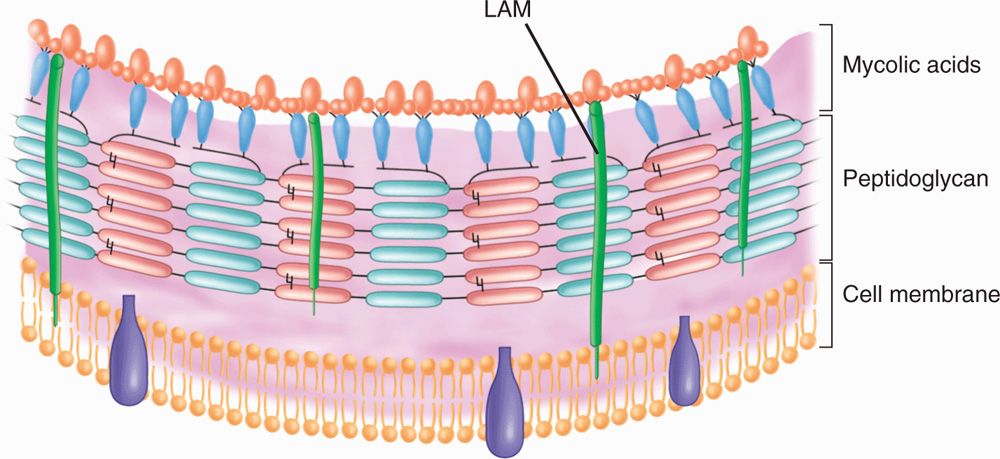
FIGURE 27–1. Mycobacterial cell wall. LaM, lipoarabinomannan. (reproduced with permission from Willey J, Sherwood L, Woolverton C (eds). Prescott’s Principles of Microbiology. New York: McGraw-hill; 2008.)
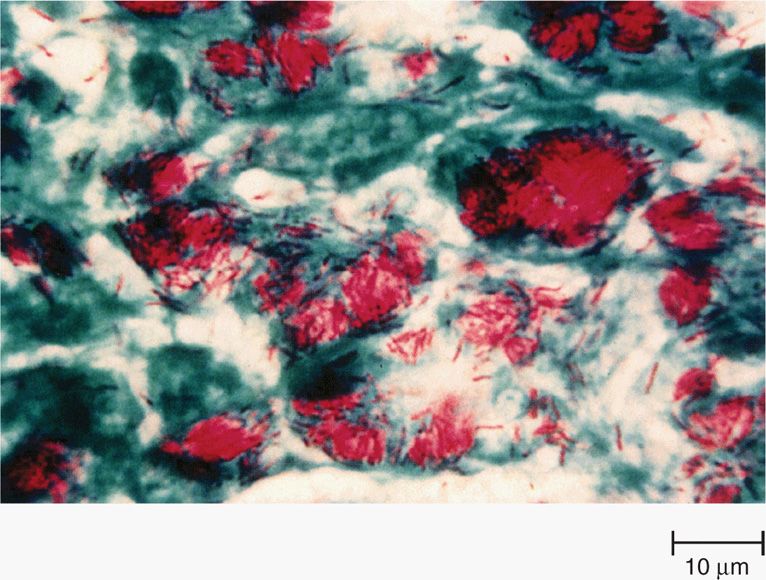
FIGURE 27–2. Mycobacterium tuberculosis in sputum stained by the acid-fast technique. the mycobacteria retain the red carbol fuchsin through the decolorization step. the cells, background, and any other organisms stain with the contrasting methylene blue counterstain. (reproduced with permission from Nester EW: Microbiology: A Human Perspective, 6th edition. 2009.)
Cell wall has high lipid content
Mycolic acids and LAM form waxy coat
Acid fastness: Once stained, difficult to decolorize
GROWTH
The most important pathogen, M tuberculosis, shows enhanced growth in 10% carbon dioxide and at a relatively low pH (6.5-6.8). Nutritional requirements vary among species and range from the ability of some nonpathogens to multiply on the washers of water faucets to the strict intracellular parasitism of M leprae, which does not grow in artificial media or cell culture. Mycobacteria grow more slowly than most human pathogenic bacteria because of their hydrophobic cell surface, which causes them to clump and limits permeability of nutrients into the cell.
Strict aerobes, many species grow slowly
CLASSIFICATION
Classic mycobacterial classification has been based on a constellation of phenotypic characteristics, including nutritional and temperature requirements, growth rates, pigmentation of colonies grown in light or darkness, key biochemical tests, the cellular constellation of free fatty acids, and the range of pathogenicity in experimental animals. There are now over 120 recognized species, the most important of which are summarized in Table 27–1. Increasingly, this classification system is yielding to molecular-based techniques. The identification of species-specific rRNA and DNA sequences has resulted in the revision and expansion of the older phenotype-based classification system and the provision of an increasing array of species-specific DNA probes to clinical mycobacteriology laboratories.
TABLE 27–1 Mycobacteria of Major Clinical Importancea
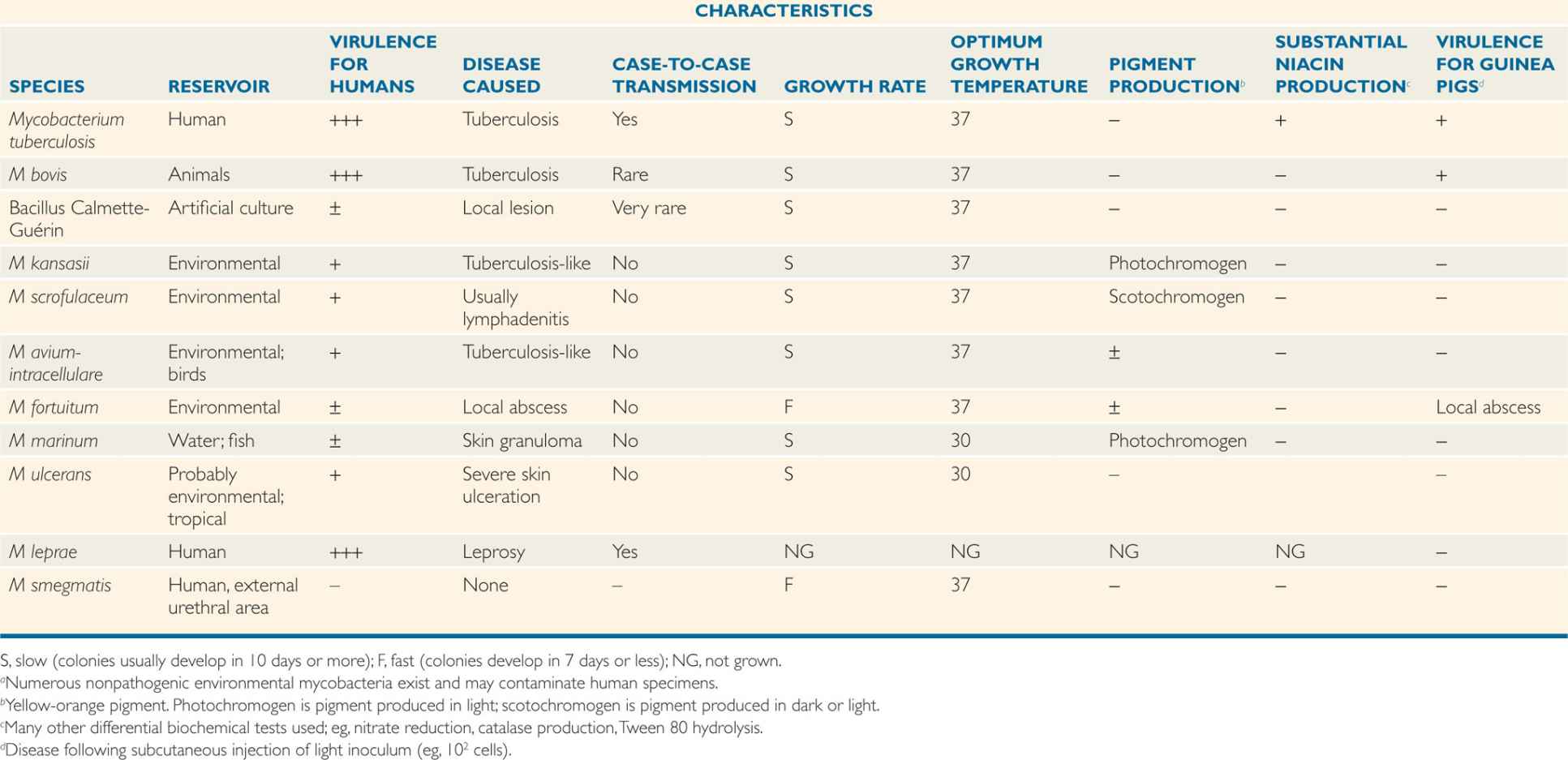
Distinguished by cultural features, biochemical reactions, and pathogenicity
Genomic sequences define classification
![]() MYCOBACTERIAL DISEASE
MYCOBACTERIAL DISEASE
Mycobacteria include a wide range of species pathogenic for humans and animals. Some, such as M tuberculosis, occur exclusively in humans under natural conditions. Others, such as M intracellulare, can infect various hosts, including humans, but also exist in a free-living state. Most nonpathogenic species are widely distributed in the environment. Diseases caused by mycobacteria usually develop slowly, follow a chronic course, and elicit a granulomatous response. Infectivity of pathogenic species is high, but virulence for healthy humans is moderate. Disease following infection with M tuberculosis is the exception rather than the rule.
Includes human and animal pathogens
Slowly progressive diseases
Mycobacteria do not produce classic exotoxins or endotoxins although a protein called early secreted antigenic target (ESAT-6) causes cytolysis and is associated with virulence. Disease processes are thought to be the result of two related host responses. The first, a delayed-type hypersensitivity (DTH) reaction to mycobacterial proteins, results in the destruction of nonactivated macrophages containing multiplying organisms. It is detected by intradermal injections of purified proteins from the mycobacteria. The second, cell-mediated immunity (CMI), activates macrophages, enabling them to destroy mycobacteria contained within their cytoplasm. The balance between these two responses determines the pathology and clinical response to a mycobacterial infection.
Lack exotoxins or endotoxins
MYCOBACTERIUM TUBERCULOSIS
 BACTERIOLOGY
BACTERIOLOGY
Mycobacterium tuberculosis (MTB) is a slim, strongly acid–alcohol–fast rod. It frequently shows irregular beading in its staining, appearing as connected series of acid-fast granules (Figure 27–2). It grows at 37oC, but not at room temperature, and it requires enriched or complex media for primary growth. The classic medium, Lôwenstein-Jensen, contains homogenized egg in nutrient base with dyes to inhibit the growth of nonmycobacterial contaminants. Growth is very slow, with a mean generation time of 12 to 24 hours. The dry, rough, buff-colored colonies usually appear after 3 to 6 weeks of incubation. Growth is more rapid in semisynthetic (oleic acid–albumin) and liquid media. The major phenotypic tests for identification are summarized in Table 27–1. Of particular importance is the ability of MTB to produce large quantities of niacin, which is uncommon in other mycobacteria.
Growth takes weeks
Biochemical tests distinguish from other mycobacteria
Because of its hydrophobic lipid surface, MTB is unusually resistant to drying, to most common disinfectants, and to acids and alkalis. Tubercle bacilli are sensitive to heat, including pasteurization, and individual organisms in droplet nuclei are susceptible to inactivation by ultraviolet light. As with other mycobacteria, the MTB cell wall structure is dominated by mycolic acids and LAM. Its antigenic makeup includes many protein and polysaccharide antigens, of which tuberculin is the most studied. It consists of heat-stable proteins liberated into liquid culture media. A purified protein derivative (PPD) of tuberculin is used for skin testing for hypersensitivity and is standardized in tuberculin units according to skin test activity.
Unusual resistance to drying and disinfectants but not to heat
PPD is mix of tuberculin proteins
EPIDEMIOLOGY
A recognized disease of antiquity, tuberculosis first reached epidemic proportions in the Western world during the Industrial Revolution beginning in the 18th and 19th centuries. Associated with urbanization and crowding, consumption accounted for 20% to 30% of all deaths in cities, winning tuberculosis the appellation of “the captain of all the men of death.” Morbidity rates were many times higher. The disease has had major sociologic components, flourishing with ignorance, poverty, and poor hygiene, particularly during the social disruptions of war and economic depression. Under these conditions, the poor are the major victims, but all sectors of society are at risk. Chopin, Paganini, Rousseau, Goethe, Chekhov, Thoreau, Keats, Elizabeth Barrett Browning, and the Brontës, to name but a few, were all lost to tuberculosis in their prime. With knowledge of the cause and transmission of the disease and the development of effective antimicrobial agents, tuberculosis was increasingly brought under control in developed countries. Unfortunately, morbidity and mortality remain at 19th-century levels in many developing countries. In 2011 the worldwide tally was over 165 000 new cases and 30 000 deaths every week. As shown in Figure 27–3 the global distribution is unequal. Twenty-two high-burden countries account for 80% of active cases.
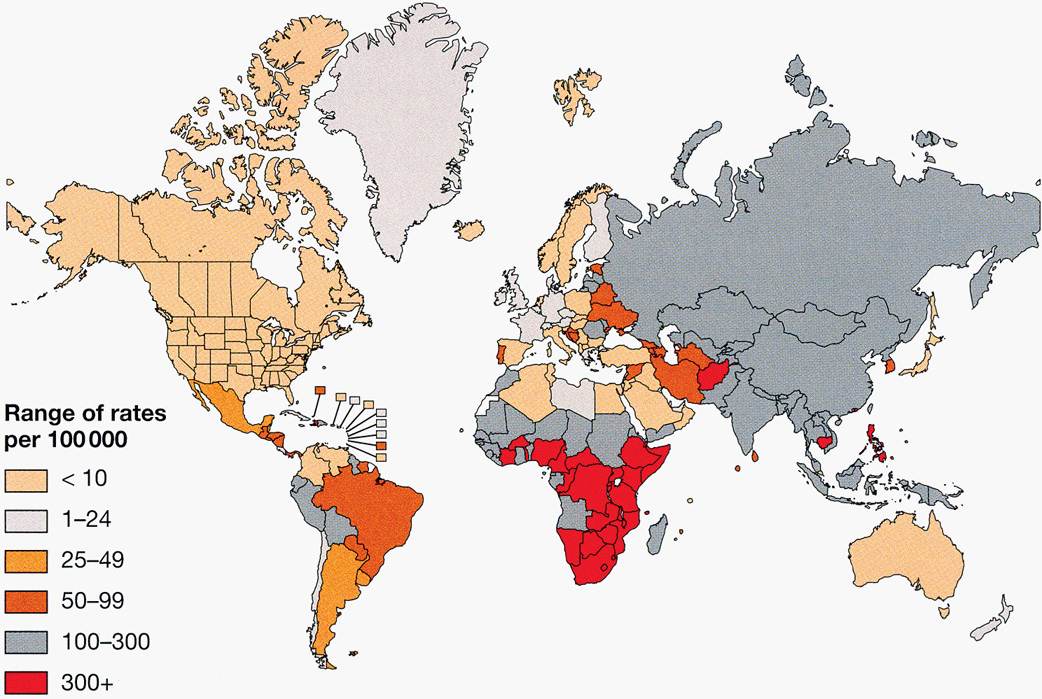
FIGURE 27–3. the worldwide incidence and distribution of tuberculosis. (Reproduced with permission from Willey JM: Prescott, Harley, & Klein’s Microbiology, 7th edition. McGraw-Hill, 2008.)
Infection of the 18th and 19th centuries
Attack rates still high in many developing countries
The majority of tuberculous infections are contracted by inhalation of droplet nuclei carrying the causative organism (Figure 27–4). Humans may also be infected through the gastrointestinal tract after ingestion of milk from tuberculous cows (now uncommon because of pasteurization) or, rarely, through abraded skin. It has been estimated that a single cough can generate as many as 3000 infected droplet nuclei which dry while airborne and remain suspended for long periods. It is estimated that less than 10 bacilli may initiate a pulmonary infection in a susceptible individual. The likelihood of acquiring infection thus relates to the numbers of organisms in the sputum of an open case of the disease, the frequency and efficiency of the coughs, the closeness of contact, and the adequacy of ventilation in the contact area. Epidemiologic data indicate that large doses or prolonged exposure to smaller infecting doses is usually needed to initiate infection in humans. In some closed environments, such as a submarine or a crowded nursing home, a single open case of pulmonary tuberculosis can infect the majority of nonimmune individuals sharing sleeping accommodations. Infection outdoors is less likely due to ventilation and the susceptibility of MTB to ultraviolet light.
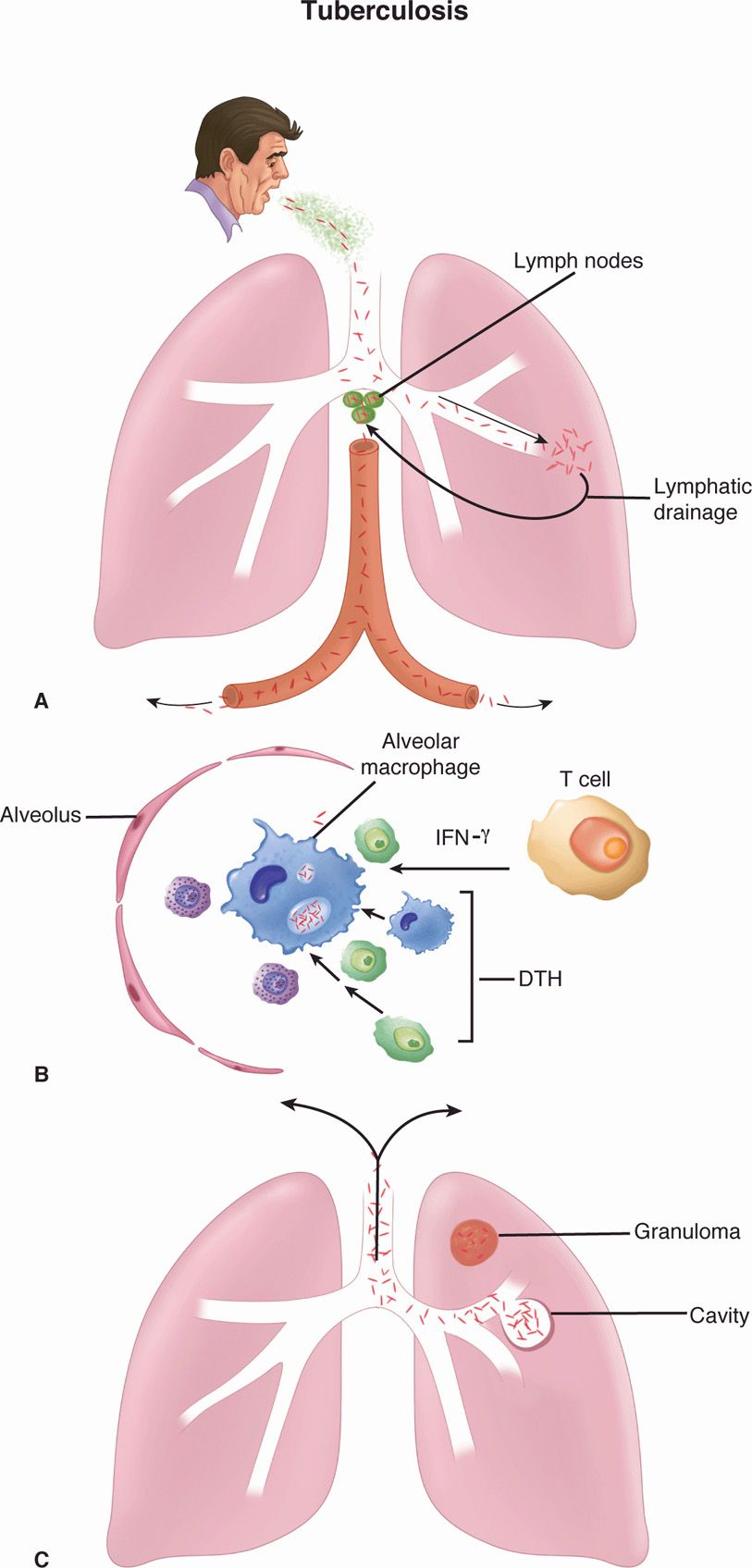
FIGURE 27–4. Tuberculosis. A. Primary tuberculosis. Mycobacterium tuberculosis is inhaled in droplet nuclei from an active case of tuberculosis. Initial multiplication is in the alveoli with spread through lymphatic drainage to the hilar lymph nodes. After further lymphatic drainage to the bloodstream, the organisms are spread throughout the body. B. Alveolar macrophage. The two-front battle being carried out between A and C is shown. Ingested bacteria multiply in the nonactivated macrophage. (1) THI cellular immune responses attempt to activate the macrophage by secreting cytokines (interferon gamma [IFN-γ]). If successful, the disease is arrested. (2) Inflammatory elements of delayed-type hypersensitivity (DTH) are attracted and cause destruction. If activation is not successful, DTH injury and disease continue. C. Reactivation tuberculosis. Reactivation typically starts in the upper lobes of the lung with granuloma formation. DTH-mediated destruction can form a cavity, which allows the organisms to be coughed up to infect another person.
Most infections are by respiratory route
Repeated coughing generates infectious dose into air
Poor ventilation increases risk
The AIDS pandemic and the spread of MTB strains resistant to multiple drugs have added to the tuberculosis burden. Globally, one-third of the world’s population is infected, and 30 million people have active disease. It is estimated that patients with latent tuberculosis increase their risk of reactivation disease by a factor 200 to 300 times with the development of HIV coinfection. HIV-infected persons are also at particularly high risk for primary infection even in the first year when their CD4 T cell counts are still high. With this dark synergy, tuberculosis and AIDS are the leading causes of premature death in the world, and 30% of those deaths are HIV infected.
AIDS and drug resistance enhance spread
PATHOGENESIS
 Primary Tuberculosis
Primary Tuberculosis
Mycobacterium tuberculosis is a facultative intracellular pathogen whose success depends on avoiding the killing mechanisms of professional phagocytes. Primary tuberculosis is the initial infection in which inhaled droplet nuclei containing tubercle bacilli are deposited in the peripheral respiratory alveoli, most frequently those of the well-ventilated middle and lower lobes. At the earliest stages ESAT-6 may facilitate binding to laminin in the basement membrane of alveolar epithelial cells. In the alveoli the bacteria are recognized by alveolar macrophage complement receptors (CR1, CR3, CR4) and phagocytosed. This inaugurates a two-stage battle with the macrophage, which may be resolved in weeks or may last for decades. The first is with the phagosome/lysosome digestive mechanisms of the macrophage. In this process, MTB has the upper hand through its ability to interfere with the acidification of the phagosome, which renders the lysosomal enzymes (which require acidic pH) less effective. This allows the bacteria to multiply freely in the phagosome of the nonactivated macrophage (Figure 27–4). The second stage is the triggering of TH1 immune responses, beginning with digestion and surface presentation of mycobacterial components and ending with cytokine activation of the macrophages. The short- and long-term outcomes of the infection depend on the ability of the macrophage activation process to overcome the intracellular edge that MTB has as a result of its ability to block acidification of the phagosome.
MTB multiplies in alveolar macrophages
Acidification of phagosome blocked
THI responses triggered
In the early stages of infection, MTB-laden macrophages are transported through lymphatic channels to the hilar lymph nodes draining the infected site. From there, a low-level bacteremia disseminates the bacteria to a number of tissues, including the liver, spleen, kidney, bone, brain, meninges, and apices or other parts of the lung. Although the primary site of infection and enlarged hilar lymph nodes can often be detected radiologically, the distant sites usually have no findings. In fact, the primary evidence for their existence is reactivation at nonpulmonary sites later in life. Tuberculous meningitis is the most serious of these.
MTB disseminates to lymph nodes and bloodstream
In the primary lesion as MTB cells multiply, macrophages and dendritic cells release cytokines (tumor necrosis factor, interleukin 12, interferon gamma [IFN-γ]), which attract T cells and other inflammatory cells to the site. The recruited CD4 T cells initiate the TH1-type immune response over the following 3 to 9 weeks in which IFN-γ is the primary activator of macrophages. As the bacteria multiply, they generate mycobacterial proteins which trigger a DTH response with its phagocytes, fluid, and release of digestive enzymes. This adds a destructive component to the process and is the sole known source of injury in tuberculosis. The magnitude of the DTH depends on the size of the MTB population. If the TH1 immune process is effective, the antigenic source of DTH stimulation wanes and the disease resolves. The mycobacterial protein-specific DTH sensitization remains, and its elicitation is the basis of the tuberculin skin test (see Diagnosis).
Cytokines attract T cells and THI response
MTB proteins also trigger DTH and injury
The mixture of the TH1 immune and DTH responses is manifest in a microscopic structure called a granuloma, which is composed of lymphocytes, macrophages, epithelioid cells (activated macrophages), fibroblasts, and multinucleated giant cells all in an organized pattern (Figure 27–5). As the granuloma grows, the destructive nature of the hypersensitivity component leads to necrosis usually in the center of the lesion. This is termed caseous necrosis because of the cheesy, semisolid character of material at the center of large gross lesions, but the term fits the smooth glassy appearance of microscopic granulomas as well.
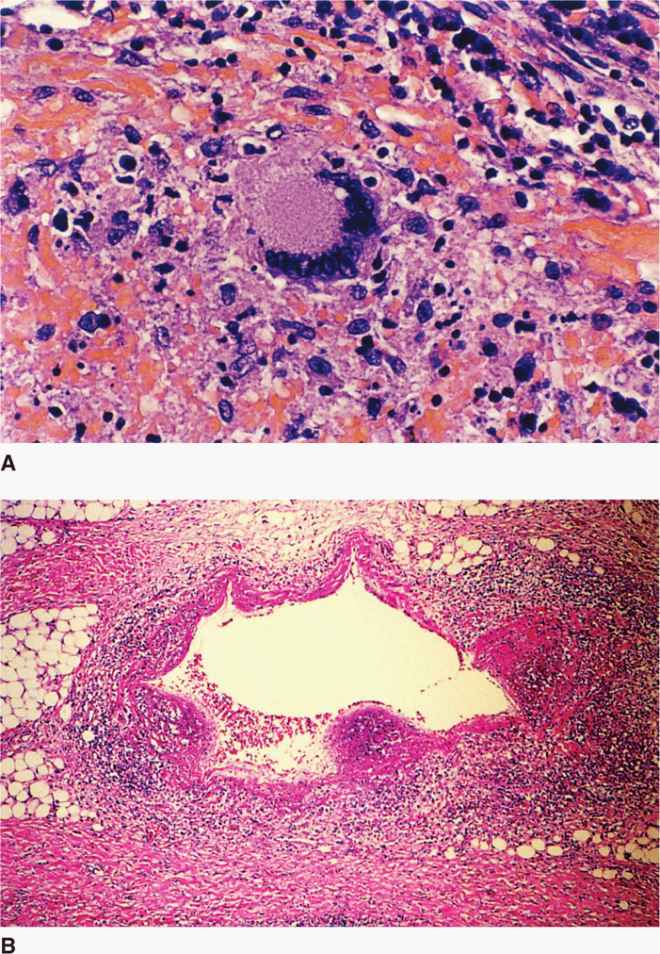
FIGURE 27–5. Tuberculous granulomas. A. Early granuloma with lymphocytes, epithelioid cells, and fibroblasts organizing around a central focus. The multinucleate giant cell in the center is typical of granulomas but not exclusive to Mycobacterium tuberculosis. B. Multiple granulomas surround and invade a vein near the lung hilum. Central degeneration is starting to appear and will eventually become caseous necrosis. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford CT: Appleton & Lange, 1997.)
The granuloma includes macrophages, lymphocytes, fibroblasts
Caseous necrosis is due to DTH
 Latent Tuberculosis
Latent Tuberculosis
Primary infections are handled well once the immune response halts the intracellular growth of MTB. Bacterial multiplication ceases, the lesions heal by fibrosis, and the organisms appear to slowly die. This sequence occurs in infections with multiple other infectious agents for which it is the end of the story. In tuberculosis, some of the organisms, when faced with oxygen and nutrient deprivation, instead of dying enter a prolonged dormant state called latency. Some view the arrival of MTB specific T cells 3 to 4 weeks after infection as the start of containment rather than cure. Specific factors facilitating survival are not known but the waxy nature of the MTB cell wall must be of aid as it is in the environment. It has long been assumed that these latent bacilli are primarily in healed granulomas in the lung, but we now know they are widely distributed with or without evidence of local granulomatous inflammation. These organisms in the lung and elsewhere lie waiting for reactivation months, years, or decades later. For most persons who undergo a primary infection this never happens, either because of the complete killing of the original population or the failure of factors favoring reactivation to materialize.
Primary lesions heal once immunity develops
Some MTB enter dormant state rather than dying
 Reactivation (Adult) Tuberculosis
Reactivation (Adult) Tuberculosis
Although mycobacterial factors have been identified (resuscitation-promoting factor), little is known of the mechanisms of reactivation of these latent foci. It has generally been attributed to some selective waning of immunity. The new foci are usually located in body areas of relatively high oxygen tension that would favor growth of the aerobe MTB. The apex of the lung is the most common, with spreading, coalescing granulomas, and large areas of caseous necrosis. Necrosis often involves the wall of a small bronchus from which the necrotic material is discharged, resulting in a pulmonary cavity and bronchial spread. Frequently, small blood vessels are also eroded. The destructive nature of these lesions cannot be directly attributed to any products or structural components of MTB. The damage is due to the failure of the host to control growth of MTB and thus the rising load of mycobacterial proteins which stimulate the autodestructive DTH response.
Latent MTB reactivates at aerobic sites
Destruction forms pulmonary cavities
Progressive DTH causes injury
IMMUNITY
Humans generally have a rather high innate immunity to the development of disease. This was tragically illustrated in the Lübeck disaster of 1926, in which infants were administered MTB instead of an intended vaccine strain. Despite the large dose, only 76 of 249 died and most of the others developed only minor lesions. Approximately 10% of immunocompe-tent persons infected with MTB develop active disease at any time in their life. There is epidemiologic and historic evidence for differences in the immunity in certain population groups and between identical and nonidentical twins. What is known of the mechanisms of innate immunity is similar to that with other pathogenic bacteria. These include Toll-like receptor responses generated by the recognition of components of the MTB cell wall and phagocytic responses.
Innate immunity is high and genetically variable
Adaptive immunity to tuberculosis is primarily related to the development of reactions mediated through CD4 T lymphocytes via TH1 pathways (see Chapter 2). Intracellular killing of MTB by macrophages activated by INF-γ and other cytokines is the essential step. The specific components of MTB that are important in initiating these reactions are not known. Cytotoxic CD8 T cells are also generated during infection and may play some role. Although antibodies are formed in the course of disease, there is no evidence they play any role in immunity.
THI immunity is most important
Cytotoxic CD8+ lymphocytes may participate
 TUBERCULOSIS: CLINICAL ASPECTS
TUBERCULOSIS: CLINICAL ASPECTS
MANIFESTATIONS
 Primary Tuberculosis
Primary Tuberculosis
Primary tuberculosis is either asymptomatic or manifest only by fever and malaise. Radiographs may show infiltrates in the mid-zones of the lung and enlarged draining lymph nodes in the area around the hilum. When these lymph nodes fibrose and sometimes calcify, they produce a characteristic picture (Ghon complex) on radiograph. In approximately 5% of patients, the primary disease is not controlled and merges into the reactivation type of tuberculosis, or disseminates to many organs. The latter may result from a necrotic tubercle eroding into a small blood vessel.
Mid-lung infiltrates and adenopathy are produced
Primary infection may progress to reactivation or dissemination
 Reactivation Tuberculosis
Reactivation Tuberculosis
Approximately 10% of persons recovering from a primary infection develop clinical disease sometime during their lifetime. In Western countries, reactivation of previous quiescent lesions occurs most often after age 50 and is more common in men. Reactivation is associated with a period of immunosuppression precipitated by malnutrition, alcoholism, diabetes, old age, and a dramatic change in the individual’s life, such as loss of a spouse. In areas in which the disease is more common, reactivation tuberculosis is more frequently seen in young adults experiencing the immunosuppression that accompanies puberty and pregnancy. Recently, reactivation and progressive primary tuberculosis among younger adults have increased as a complication of AIDS.
Reactivation is most common in older men
Predisposing factors include underlying disease and life events
Cough is the universal symptom of tuberculosis. It is initially dry, but as the disease progresses sputum is produced, which even later is mixed with blood (hemoptysis). Fever, malaise, fatigue, sweating, and weight loss all progress with continuing disease. Radiographically, infiltrates appearing in the apices of the lung coalesce to form cavities with progressive destruction of lung tissue. Less commonly, reactivation tuberculosis can also occur in other organs, such as the kidneys, bones, lymph nodes, brain, meninges, bone marrow, and bowel. Disease at these sites ranges from a localized tumor-like granuloma (tuberculoma) to a fatal chronic meningitis. Untreated, the progressive cough, fever, and weight loss of pulmonary tuberculosis creates an internally consuming fire that usually takes 2 to 5 years to cause death. The course in AIDS and other T-cell–compromised patients is more rapid.
Cough is universal
Cavities form in lung apices
Multiple organs are involved
DIAGNOSIS
 Tuberculin Test
Tuberculin Test
The tuberculin skin test (Figure 27–6) measures DTH to an international reference tuber-culoprotein preparation called purified protein derivative (PPD). The test involves an intradermal injection that is read 48 to 72 hours later. An area of induration of 10 mm or more accompanied by erythema constitutes a positive reaction, and no induration indicates a negative reaction. A positive PPD test indicates that the individual has developed DTH through infection at some time with MTB, but carries no implication as to whether the disease is active. Persons who have been infected with another mycobacterial species or immunized with the bacillus Calmette-Guérin (BCG) vaccine may also be reactive, but the induration is usually less than 10 mm. Patients with severe disseminated disease, those on immunosuppressive drugs, or those with immunosuppressive diseases such as AIDS may fail to react due to anergy.
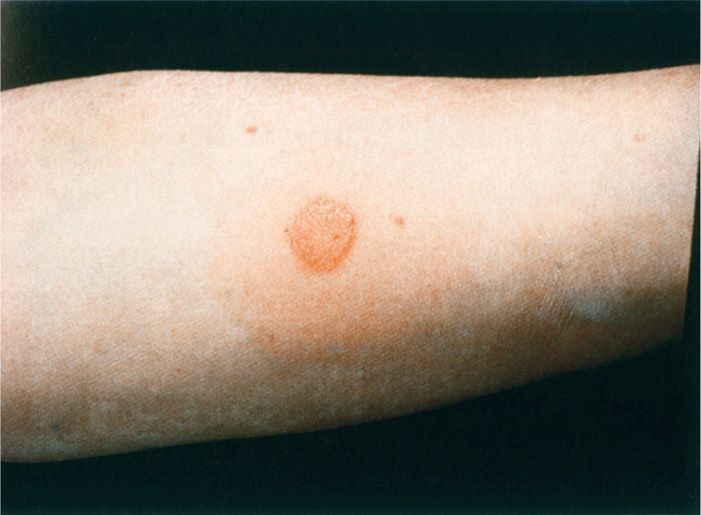
FIGURE 27–6. Tuberculin test. The ppD (purified protein derivative) tuberculoprotein was injected intradermally at this site 48 hours previously. the erythema and induration (>10mm) that are present indicate the development of delayed-type (type IV) hypersensitivity. (reproduced with permission from Nester EW: Microbiology: A Human Perspective, 6th edition. 2009.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


