And he would play his Diphtheria Blues
—Frank Zappa
This chapter includes a variety of highly pathogenic Gram-positive rods that are not currently common causes of human disease. Their medical importance lies in the lessons learned when they were more common, and the continued threat their existence poses. Corynebacterium diphtheriae, the cause of diphtheria, is a prototype for toxigenic disease. Listeria monocytogenes is a sporadic cause of meningitis and other infections in the fetus, newborn, and immunocompromised host. Occurrences in 2001 have served as a painful reminder that Bacillus anthracis, the cause of anthrax, is still the agent with the most potential for use in bioterrorism. The characteristics of these bacilli are presented in Table 26–1.
CORYNEBACTERIA
Corynebacteria (from the Greek koryne, club) are small and pleomorphic. The genus Corynebacterium includes many species of aerobic and facultative Gram-positive rods. The cells tend to have clubbed ends and often remain attached after division, forming “Chinese letter” or palisade arrangements. Spores are not formed. Growth is generally best under aerobic conditions on media enriched with blood or other animal products, but many strains grow anaerobically. Colonies on blood agar are typically small (1-2 mm), and most are nonhemolytic. Catalase is produced, and many strains form acid (usually lactic acid) through carbohydrate fermentation. Surface and cell wall structure is similar to other Gram-positive bacteria.
Pleomorphic club-shaped rods
Corynebacterium diphtheriae
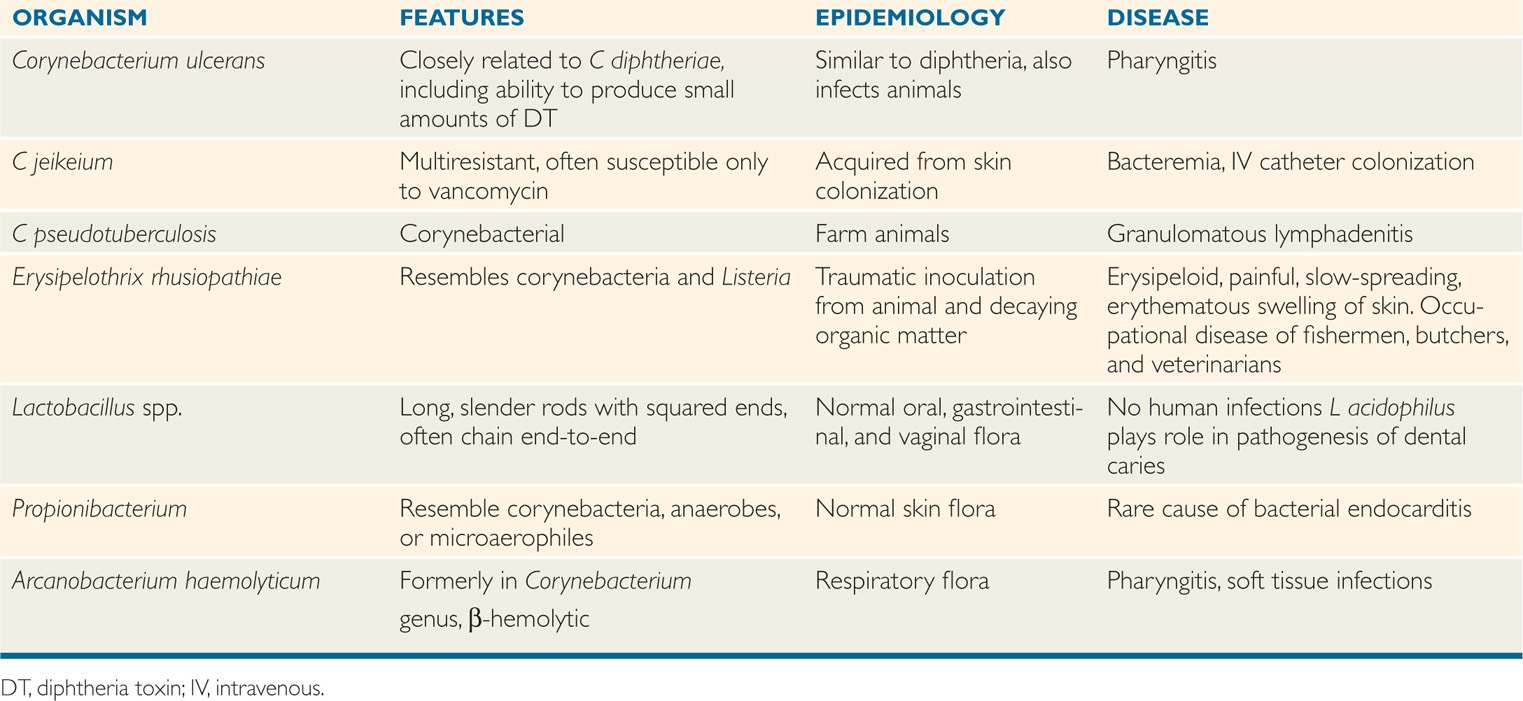
Corynebacterium diphtheriae produces a powerful exotoxin that is responsible for diphtheria. Other corynebacteria are nonpathogenic commensal inhabitants of the pharynx, nasopharynx, distal urethra, and skin; they are collectively referred to as “diphtheroids.” The species that have disease associations are included in Table 26–2.
TABLE 26–2 Other Aerobic and Facultative Gram-Positive Bacilli
C diphtheriae produces exotoxin
Other corynebacteria are called diphtheroids
 BACTERIOLOGY
BACTERIOLOGY
Corynebacterium diphtheriae are differentiated from other corynebacteria by the appearance of colonies on the selective media used for its isolation and a variety of biochemical reactions. Strains of C diphtheriae may or may not produce diphtheria toxin (DT). The gene for DT is contained in the genome of a bacteriophage, which is lysogenic in the C diphtheriae chromosome. For strains with the gene, DT production is controlled by a repressor protein (DtxR), which responds to iron concentrations and also regulates other toxin-related functions.
DT gene contained in a lysogenic phage
DT is an A-B toxin that acts in the cytoplasm to inhibit protein synthesis irreversibly in a wide variety of eukaryotic cells. After binding mediated by the B subunit, both the A and B subunits enter the cell in an endocytotic vacuole. In the low pH of the vacuole, the toxin unfolds, exposing sites that facilitate translocation of the A subunit from the phagosome to the cytosol. The target is elongation factor 2 (EF-2), which transfers polypeptidyl-transfer RNA from acceptor to donor sites on the ribosome of the host cell. The specific action of the A subunit is to inactivate EF-2, by ADP-ribosylation (ADPR), which shuts off protein synthesis. The details of DT action are illustrated in Chapter 1 (Figure 1–7) as a prototype toxin. Corynebacterium diphtheriae itself is unaffected because it uses a protein other than EF-2 for the same steps in protein synthesis.
A subunit enters the cytosol from a vacuole
EF-2 is inactivated by ADP-ribosylation
tRNA blockage on ribosome blocks protein synthesis
EPIDEMIOLOGY
Corynebacterium diphtheriae is transmitted by droplet spread, by direct contact with cutaneous infections, and, to a lesser extent, by fomites (Figure 26–1). Some subjects become convalescent pharyngeal or nasal carriers and continue to harbor the organism for weeks, months, or longer. Diphtheria is rare where immunization is widely practiced. In the United States, for example, fewer than 10 cases are now reported each year. These usually occur as small outbreaks in populations that have not received adequate immunization, such as migrant workers, transients, and those who refuse immunization on religious grounds. It has been more than 25 years since any outbreak exceeded 50 cases.
Transmitted by respiratory droplets
Most cases are in unimmunized transients
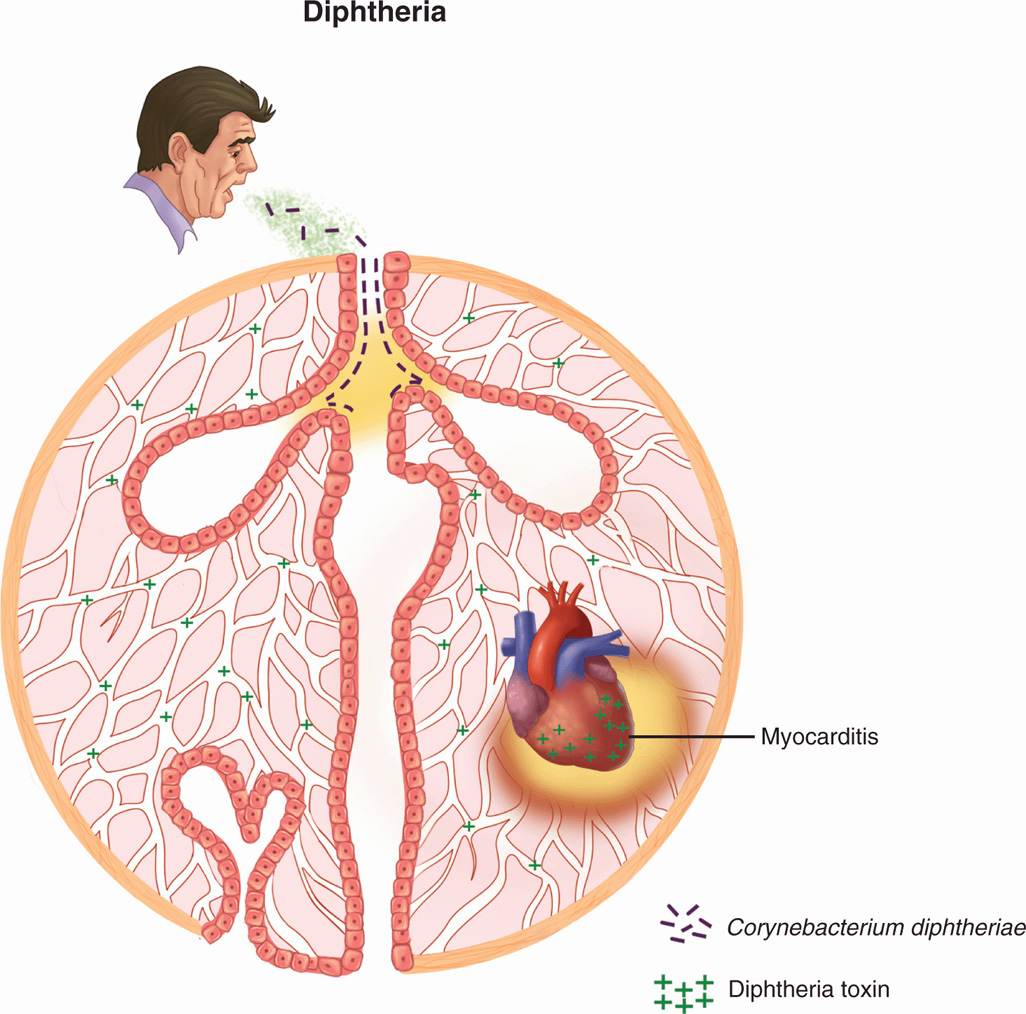
FIGURE 26–1. Diphtheria overview. Infection with Corynebacterium diphtheriae is acquired by respiratory droplet spread. The throat and upper airways are infected, but there is no invasion. Diphtheria toxin (DT) produced at the primary side is absorbed into the bloodstream and affects multiple organs, particularly the heart where acute myocarditis is produced.
Diphtheria still occurs in developing countries and in places where public health infrastructure has been disrupted. For example, in the former Soviet Union, where the annual number of diphtheria cases had been below 200, over 47 000 cases and 1700 deaths occurred between 1990 and 1995. This outbreak followed the reintroduction of C diphtheriae into a population where the public health systems had broken down as a result of the political situation. Reinstitution of effective immunization brought diphtheria rates back to base levels.
Outbreaks occur when immunization rates decrease
PATHOGENESIS
Corynebacterium diphtheriae has little invasive capacity, and diphtheria is due to the local and systemic effects of DT, a protein exotoxin with potent cytotoxic features (Figure 26–2). It inhibits protein synthesis in cell-free extracts of virtually all eukaryotic cells, from protozoa and yeasts to higher plants and humans. Its toxicity for intact cells varies among mammals and organs, primarily due to differences in toxin binding and uptake. In humans, the B subunit binds to one of a common family of eukaryotic receptors that regulate cell growth and differentiation, thus exploiting a normal cell function.
A subunit inhibits protein synthesis
B subunit binding determines cell susceptibility
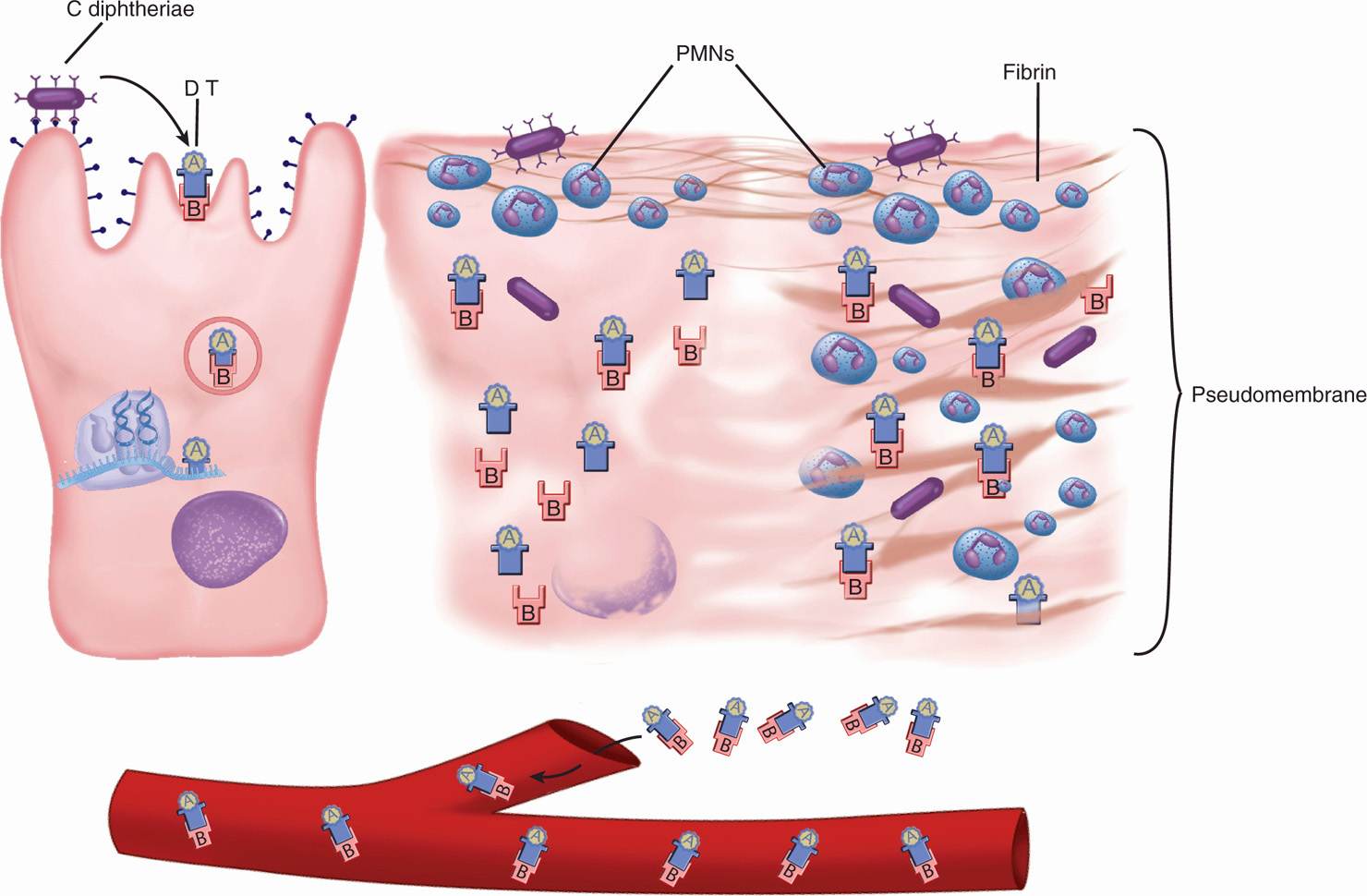
FIGURE 26–2. Diphtheria cellular view. (Left) Corynebacterium diphtheriae binds to epithelial cells and secretes diphtheria toxin (DT). The A-B toxin enters the cell, and the A subunit exits the endocytotic vacuole. In the cytoplasm, the A subunit catalyzes the ADP-ribosylation of EF-2, which inhibits protein synthesis at the ribosome (see Figure 1–7). (Middle) The cell is dying and superficial inflammation brings polymorphonuclear neutrophils (PMNs) and fibrin. (Right) The cell is destroyed and the inflammatory components have coalesced into a pseudomembrane. The bacteria do not invade, but DT enters the bloodstream.
The production of DT has both local and systemic effects. Locally, its action on epithelial cells leads to necrosis and inflammation, forming a pseudomembrane composed of a coagulum of fibrin, leukocytes, and cellular debris. The extent of the pseudomembrane varies from a local plaque to an extensive covering of much of the tracheobronchial tree. Absorption and circulation of DT allow binding throughout the body. Myocardial cells are most affected; eventually, acute myocarditis develops.
Local effects produce pseudomembrane
Absorption of DT leads to myocarditis
IMMUNITY
Diphtheria toxin is antigenic, stimulating the production of protective antitoxin antibodies during natural infection. Formalin treatment of toxin produces toxoid, which retains the antigenicity but not the toxicity of native toxin and is used in immunization against the disease. It is clear that this process functionally inactivates fragment B. Whether it also inactivates fragment A or prevents its ability to dissociate from fragment B is not known. Molecular studies of the A subunit structure and action suggest that another approach to immunization may be by genetic engineering of the A subunit so that it fails to bind EF-2 but retains its antigenicity.
Antibodies neutralize toxin
Toxoid is formalin inactivated DT
 DIPHTHERIA: CLINICAL ASPECTS
DIPHTHERIA: CLINICAL ASPECTS
MANIFESTATIONS
After an incubation period of 2 to 4 days, diphtheria usually manifests as pharyngitis or tonsillitis. Typically, malaise, sore throat, and fever occur, and a patch of exudate or membrane develops on the tonsils, uvula, soft palate, or pharyngeal wall. The gray-white pseudomembrane (Figure 26–3) adheres to the mucous membrane and may extend from the oropharyngeal area down to the larynx and into the trachea. Associated cervical adenitis is common, and in severe cases cervical adenitis and edema produce a “bull neck” appearance. In uncomplicated cases, the infection gradually resolves, and the membrane is coughed up after 5 to 10 days.
Severe pharyngitis may have exudate or membrane
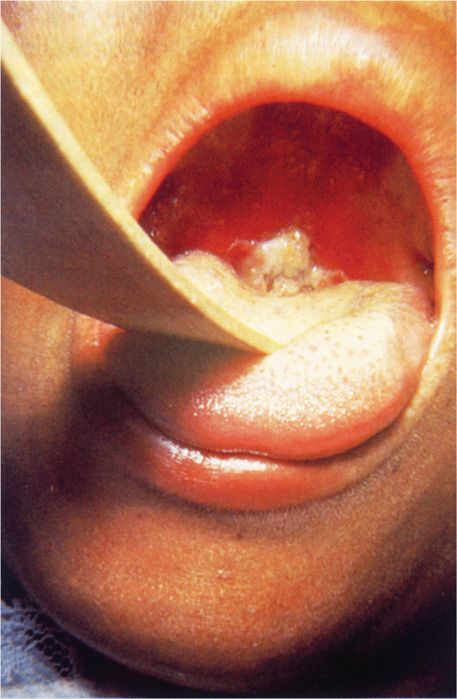
FIGURE 26–3. Diphtheria. Typical appearance of a diphtheritic pseudomembrane adherent to the oropharynx of this child. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford CT: A ppleton & Lange, 1997.)
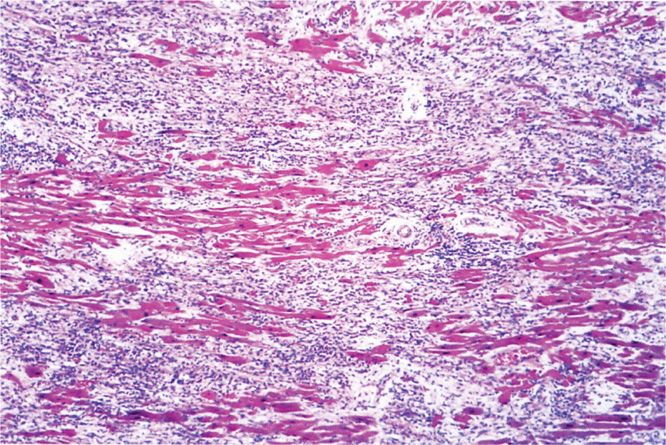
The complications and lethal effects of diphtheria are caused by respiratory obstruction or by the systemic effect of DT absorbed at the site of infection. Mechanical obstruction of the airway produced by the pseudomembrane, edema, and hemorrhage can be sudden and complete and can lead to suffocation, particularly if large sections of the membrane separate from the tracheal or laryngeal epithelial surface. The DT absorbed into the circulation causes injury to various organs, most seriously the heart. Diphtheritic myocarditis (Figure 26–4) can be detected by electrocardiography in two-thirds of patients and is serious enough to cause cardiac malfunction in up to 25%. It appears during the second or third week and is manifested by cardiac enlargement, arrhythmia, and congestive heart failure with dyspnea. Nervous system involvement appears later in the course of disease, most often involving paralysis of the soft palate, oculomotor (eye) muscles, or select muscle groups. The paralysis is reversible and is generally not serious unless the diaphragm is involved. The disease resolves with the formation of antitoxin antibody.
FIGURE 26–4. Diphtheritic myocarditis. Necrosis and inflammation are present in this section of myocardium from a fatal case of diphtheria. (Reproduced with permission from Connor DH, Chandler FW, Schwartz DQ, et al: Pathology of Infectious Diseases. Stamford CT: Appleton & Lange, 1997.)
Pseudomembrane can block the airway
DT myocarditis may lead to congestive heart failure
Corynebacterium diphtheriae may produce nonrespiratory infections, particularly of the skin. The characteristic lesion, ranges from a simple pustule to a chronic nonhealing ulcer, and is most common in tropical and hot, arid regions. Cardiac and neurologic complications from these infections are infrequent, suggesting that the efficiency of toxin production or absorption is low compared with that in respiratory infections.
Cutaneous diphtheria produces ulcerative lesion
DIAGNOSIS
The initial diagnosis of diphtheria is entirely clinical. There are presently no rapid laboratory tests of sufficient value to influence the decision regarding antitoxin administration. Direct smears of infected areas of the throat are not reliable diagnostic tools. Definitive diagnosis is accomplished by isolating and identifying C diphtheriae from the infected site and demonstrating its toxigenicity. Isolation is usually achieved with a selective medium containing potassium tellurite (eg, Tinsdale medium).
Primary diagnosis is clinical
Culture requires special medium
It should be recognized that although the diagnosis of diphtheria could once be made and confirmed with great confidence, it is now more difficult because experience with the disease is rare. Most physicians have never seen a case of diphtheria, and most laboratories have never isolated the organism and do not even stock the required medium. Because routine throat culture procedures do not detect C diphtheriae, the physician must advise the laboratory of the suspicion of diphtheria in advance. Generally, 2 days are required to exclude C diphtheriae (ie, no colonies isolated on Tinsdale agar); however, more time is needed to complete identification and toxigenicity testing of a positive culture.
Laboratory must be notified of suspicion in advance
TREATMENT
Treatment of diphtheria is directed at neutralization of the toxin with concurrent elimination of the organism. The former is most critical and is accomplished by promptly administering a diphtheria antitoxin, an antiserum produced in horses. It must be administered early because it only neutralizes circulating toxin and has no effect on toxin already fixed to or within cells. Corynebacterium diphtheriae is susceptible to a variety of antimicrobials, including penicillins, cephalosporins, erythromycin, and tetracycline. Of these, erythromycin has been the most effective. The complications of diphtheria are managed primarily by supportive measures.
Antitoxin therapy aimed at neutralizing free toxin
Erythromycin is the most effective antimicrobial therapy
PREVENTION
The mainstay of diphtheria prevention is immunization. The vaccine is highly effective. Three to four doses of diphtheria toxoid produce immunity by stimulating antitoxin production. The initial series is begun in the first year of life. Booster immunizations at 10-year intervals maintain immunity. Fully immunized individuals may become infected with C diphtheriae because the antibodies are directed only against the toxin, but the disease is mild. Serious infection and death occur only in unimmunized or incompletely immunized individuals. Immunization with DT toxoid prevents serious toxin-medicated disease.
DT toxoid with 10-year boosters
LISTERIA MONOCYTOGENES
 BACTERIOLOGY
BACTERIOLOGY
Listeria monocytogenes is a Gram-positive rod with some bacteriologic features that resemble those of both corynebacteria and streptococci. In stained smears of clinical and laboratory material, the organisms resemble diphtheroids. Listeria are not difficult to grow in culture, producing small, β-hemolytic colonies on blood agar. An unusual feature for human pathogens is the ability of L monocytogenes to grow slowly in the cold, even at temperatures below 0°C. This is due to the action of enzymes (RNA helicases) induced at low temperatures. Growth at refrigerator temperatures turns out to be important in the foodborne transmission of L monocytogenes (see epidemiology below). Listeria species are catalase-positive, which distinguishes them from streptococci, and they produce a characteristic tumbling motility in fluid media at temperatures below 30°C, which distinguishes them from corynebacteria.
Rods resemble corynebacteria
Colonies are β-hemolytic
Induced enzymes allow growth in cold
Listeria monocytogenes is the only one of six Listeria species pathogenic for humans. There are 13 serotypes based on flagellar and surface antigens, but most human cases are limited to only three (1/2a, 1/2b, 4b). The major virulence factors are a group of invasion-associated surface proteins called internalins and a pore-forming cytotoxin, listeriolysin O (LLO).
Internalin and LLO enhance virulence
![]() LISTERIOSIS
LISTERIOSIS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree