FIGURE 24–1. Staphylococcus aureus. Gram stain showing the Gram-positive cocci in clusters resembling bunches of grapes (arrows) and neutrophils (arrowhead). (Image contributed by Professor Shirley Lowe, University of California, San Francisco School of Medicine, with permission.)
STAPHYLOCOCCI: GROUP CHARACTERISTICS
Although staphylococci have a marked tendency to form clusters (from the Greek staphyle, bunch of grapes), some single cells, pairs, and short chains are also seen. Staphylococci have a typical Gram-positive cell wall structure. Like all medically important cocci, they are nonflagellate, nonmotile, and non–spore-forming. Staphylococci grow best aerobically but are facultatively anaerobic. In contrast to streptococci, staphylococci produce catalase. More than one dozen species of staphylococci colonize humans; of these, S aureus is by far the most virulent. The ability of S aureus to form coagulase separates it from other, less virulent species (Table 24–1). It is common to lump the other species together as coagulase-negative staphylococci (CoNS).
TABLE 24–1 Features of Human Staphylococci
Staphylococci form clusters and are catalase-positive
Coagulase distinguishes S aureus from other species
Staphylococcus aureus
 BACTERIOLOGY
BACTERIOLOGY
STRUCTURE
In growing cultures, the cells of S aureus are uniformly Gram-positive and regular in size, fitting together in clusters with the precision of pool balls. In older cultures, in resolving lesions, and in the presence of some antibiotics, the cells often become more variable in size, and many lose their Gram positivity.
The cell wall of S aureus consists of a typical Gram-positive peptidoglycan interspersed with considerable amounts of teichoic acid. The peptidoglycan of the cell wall is commonly overlaid with polysaccharide and surface proteins. Although thin polysaccharide capsules are frequently present, their significance in human infections is unknown, and they will not be discussed further. Surface proteins such as clumping factor (Clf), which binds to fibrinogen, and fibronectin-binding proteins (FnBPs) likely play a role in the early stages of infection. Another protein, protein A, is unique in that it binds the Fc portion of IgG molecules, leaving the antigen-reacting Fab portion directed externally (turned around). It is present in most clinical isolates of S aureus. Protein A is also able to stimulate cytokines (TNF-α), platelets, and B cells.
Surface proteins bind fibrinogen and fibronectin
Protein A binds IgG and stimulates cytokines and B cells
 Metabolism
Metabolism
After overnight incubation on blood agar, S aureus produces white colonies that tend to turn a buff-golden color with time, which is the basis of the species epithet aureus (golden). Most, but not all, strains show a rim of clear β-hemolysis surrounding the colony. The most important test used to distinguish S aureus from other staphylococci is the production of coagulase, an enzyme which binds prothrombin in a manner that provides for the cleavage of fibrinogen to fibrin. It is demonstrated by incubating staphylococci in plasma; this produces a fibrin clot in a few hours.
Colonies are white or golden and hemolytic
Coagulase produces a fibrin clot
TOXINS AND BIOLOGICALLY ACTIVE EXTRACELLULAR ENZYMES
 Toxins
Toxins
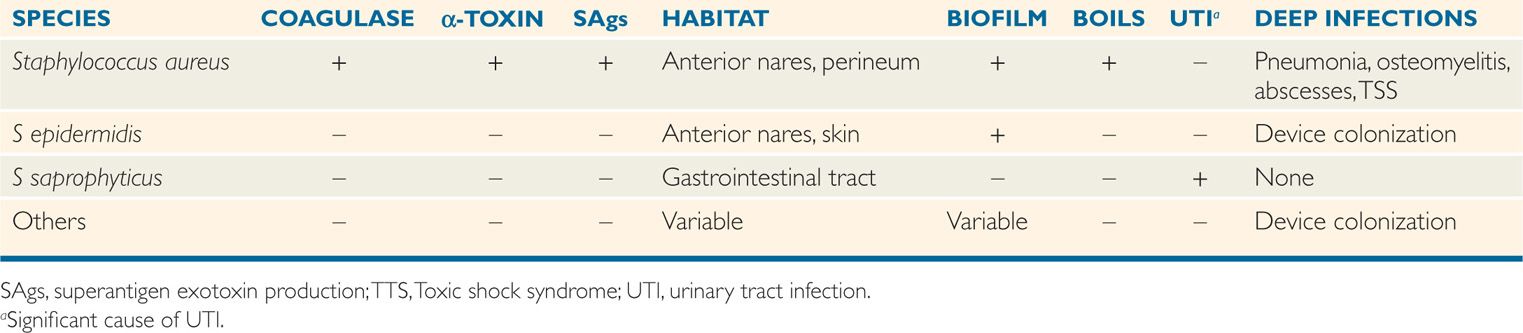
Staphylococcus aureus produces a number of named cytolytic toxins (α, β, ô, γ), of which α-toxin is the most important. α-Toxin, sometimes called α-hemolysin, is a protein secreted by almost all strains of S aureus, but not by coagulase-negative staphylococci. It is a pore-forming cytotoxin that lyses the cytoplasmic membranes by direct insertion into the lipid bilayer to form transmembrane pores (Figure 24–2). The resultant egress of vital molecules leads to cell death. This action is similar to other biologically active cytolysins such as streptolysin O, complement, and the effector proteins of cytotoxic T lymphocytes. α-Toxin is not active against neutrophils but does lyse a wide variety of other cells including keratinocytes. Another pore-forming toxin is active against neutrophils and known as a leukocidin (Panton-Valentine leukocidin or PVL), causes tissue necrosis but is found in only a small portion of clinical isolates (<10%).
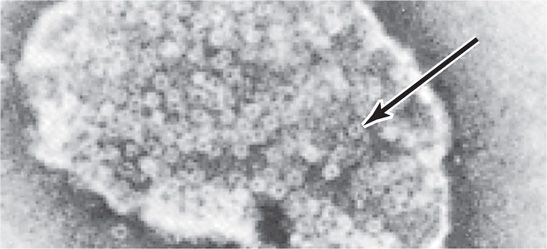
FIGURE 24–2. Staphylococcus aureus α-toxin. A fragment of a rabbit erythrocyte lysed with α-toxin is shown. Note the ring-shaped pores in the membrane created by insertion of the toxin. (Bhakdi S, Tranum-Jensen J: Mechanism of complement cytolysis and the concept of channel-forming proteins, Philos Trans R Soc Lond B Biol Sci 1984 Sep 6;306(1129):311-324.)
α-Toxin inserts in lipid bilayer to form transmembrane pores
PV leukocidin attacks neutrophils
 Exfoliatin
Exfoliatin
Exfoliatin is produced by a small proportion of S aureus strains. It binds to a specific cell membrane ganglioside found only in the stratum granulosum of the keratinized epidermis of the skin. There it causes intercellular splitting of the epidermis between the stratum spinosum and stratum granulosum, presumably by disruption of intercellular junctions. The toxin itself is a protease which acts on desmosomes important to interkeratinocyte adhesion. Two variants of exfoliatin are antigenic in humans, and the circulating antibody confers immunity to their effects.
Exfoliatin splits intraepidermal junctions
 Staphylococcal Superantigen Toxins
Staphylococcal Superantigen Toxins
The superantigens (SAgs) are a family of secreted proteins that are able to stimulate systemic effects as a result of absorption from the gastrointestinal tract after ingestion or at a site where they are produced in vivo by multiplying bacteria. There are now more than 15 described staphylococcal superantigen toxins (StaphSAgs), the most important of which in human disease are antigenic variants of the long-known staphylococcal enterotoxins (SEA, SEB, etc) and the more recently discovered toxic shock syndrome toxin (TSST-1). An individual strain may produce one or more toxins, but less than 20% of S aureus strains produce any StaphSAg. As superantigens they are strongly mitogenic for T cells and do not require proteolytic processing before binding with class II major histocompatibility complex (MHC) molecules on antigen-presenting cells. This process not only bypasses the specificity of antigen processing but results in massive cytokine release. The StaphSAg toxins share physiochemical and biologic activity similarities with each other and StrepSAgs produced by group A streptococci.
StaphSAgs bind MHC II without processing
Superantigens cause massive cytokine release
Staphylococcal Enterotoxins
The ability of S aureus enterotoxins to stimulate gastrointestinal symptoms (primarily vomiting) in humans and animals has long been known. Once formed, these toxins are quite stable, retaining activity even after boiling or exposure to gastric and jejunal enzymes. In addition to their superantigen actions, they appear to act by stimulating reflexes in the abdominal viscera, which are transmitted to medullary emetic centers in the brain stem via the vagus nerve.
Once formed, enterotoxins are stable to boiling and digestive enzymes
Vomiting is stimulated by brain stem mechanism
![]() STAPHYLOCOCCAL DISEASE
STAPHYLOCOCCAL DISEASE
In many ways, S aureus is the “all-time champion” of microbial pathogens. Although tuberculosis and malaria have greater global prevalence and the spread of AIDS is more ominous, the ferocity of staphylococcal infections has remained constant for as long as we can tell. In Shakespeare’s Lear (1606) quoted above, the king is not himself infected. He has just chosen two prototype staphylococcal lesions (boil, carbuncle) as the vilest of symbols to characterize his ungrateful daughters and his treatment at their hands. Today, in any hospital in the world S aureus still heads the list of pathogens isolated from the bloodstream of seriously ill patients.
EPIDEMIOLOGY
The basic human habitat of S aureus is the anterior nares. Ten to thirty percent of the population carry the organism at this site at any given time, and rates among hospital personnel and patients may be much higher. From the nasal site, the bacteria are shed to the exposed skin and clothing of the carrier and others with whom they are in direct contact. Spread is augmented by touching the face and, of course, nose picking. It is blocked by handwashing. Once present on the skin, even transiently, S aureus can gain deeper access either through skin appendages or trauma (Figure 24–3). Although outbreak investigations show that some strains have enhanced virulence, still no laboratory tests can be used to separate them from the large pool of colonized individuals.
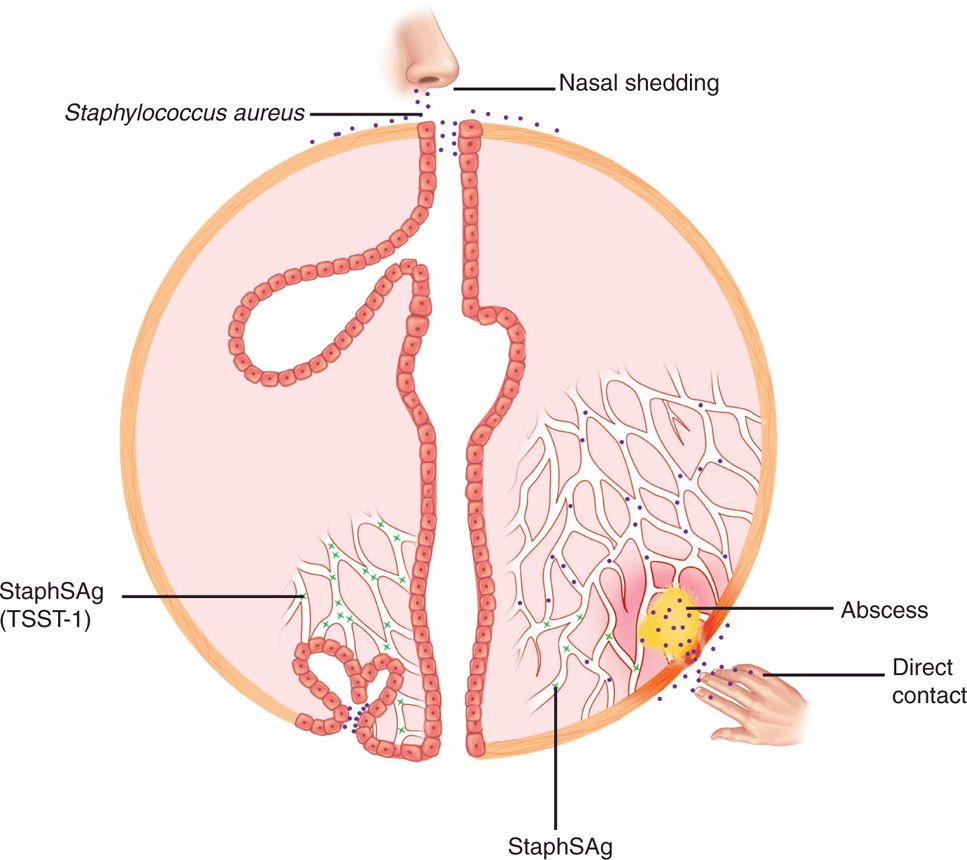
FIGURE 24–3. Staphylococcal disease. The source of infection is most commonly endogenous from colonized anterior nares or by direct contact with someone carrying S aureus. An abscess (boil) is the typical lesion. In a small proportion of cases, the strain may produce a circulating exotoxin similar to the staphylococcal superantigens (StaphSAgs), which can produce toxic shock syndrome in association with a local infection (lower right) or with menses (lower left). For details of menstrual-associated toxic shock syndrome, see Figure 24–8.
Anterior nares colonization is common
Strains with increased virulence cannot be distinguished
Most S aureus infections acquired in the community are autoinfections with strains that the subject has been carrying in the anterior nares, on the skin, or both. Community outbreaks are usually associated with poor hygiene and fomite transmission from individual to individual. Unlike many pathogenic bacteria, S aureus can survive periods of drying; for example, recurrent skin infections can result from use of clothing contaminated with pus from a previous infection.
Community infections are endogenous
S aureus survives drying
Hospital outbreaks caused by a single strain of S aureus most commonly involve patients who have undergone surgical or other invasive procedures. The source of the outbreak may be a patient with an overt or unapparent staphylococcal infection (eg, decubitus ulcer), which is then spread directly to other patients on the hands of hospital personnel. A nasal or perineal carrier among medical, nursing, or other hospital personnel may also be the source of an outbreak, especially when carriage is heavy and numerous organisms are disseminated. The most hazardous source is a medical attendant who works despite having a staphylococcal lesion such as a boil. Hospital outbreaks of S aureus infection can be self-perpetuating: infected patients and those who attend them frequently become carriers, and the total environmental load of the causative staphylococcus is increased.
Hospital spread is on the hands of medical personnel
Outbreaks involve nasal carrier or worker with lesion
Staphylococcal food poisoning is one of the most common foodborne illnesses in the world. It has been an unhappy and embarrassing sequel to innumerable group picnics and wedding receptions in which gastronomic delicacies have been exposed to temperatures that allow bacterial multiplication. Characteristically, the food is moist and rich (eg, red meat, poultry, creamy dishes). The food becomes contaminated by a preparer who is a nasal carrier or has a staphylococcal lesion. If the food is left unrefrigerated for hours between preparation and serving, the staphylococci are able to multiply and produce enterotoxin in the food. Because of the heat resistance of the toxin, toxicity persists even if the food is subsequently cooked before eating.
Enterotoxin is produced in rich foods before they are ingested
PATHOGENESIS
 Primary Infection
Primary Infection
A boil (or furuncle) is an abscess and a prototype for the purulent lesions produced by many other bacteria. The initial stages of attachment by S aureus are mediated by a number of surface proteins, which bind to host cells or elements on their surface. Proteins that bind to the glycoprotein fibronectin that is ubiquitous on mucosal surfaces are of particular importance in the early stages of infection. These FnBPs mediate adhesion to and perhaps invasion of mammalian cells. This allows S aureus to persist and to produce α-toxin and other cytolysins, which injure the cell (Figure 24–4). As the lesions become destructive and spread below the surface, other proteins that bind to collagen and other elements of the extracellular matrix may play a role. At this stage, actions of coagulase and Clf on fibrinogen-binding, and the antiphagocytic effect of protein A binding to IgG, all combine to limit the effectiveness of host phagocytes. If the strain produces the PV leukocidin the compromise of innate defenses would be enhanced. The continued production of α-toxin destroys keratinocytes and other cells allowing the lesion to expand. The inflammatory cells, fibrin, and other tissue components form a wall, which becomes the painfully familiar boil (Figure 24–5). A carbuncle (Figure 24–6) is an extension of this process in which, rather than discharging at the surface, the process forms multiple compartments. There is evidence that S aureus can regulate this multifactored process deploying adhesions and extracellular products at the stages they are needed.
FIGURE 24–4. Staphylococcal disease cellular view. Initial attachment to fibronectin is mediated by fibronectin-binding proteins (FnBP), and the major injury is caused by the pore-forming α-toxin. Cells are destroyed by leaking their cytosol. The α-toxin also inserts in the polymorphonuclear neutrophils. resistance to phagocytosis and the formation of a wall are aided by fibrinogen-binding Clf.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


