FIGURE 2–1. Human blood cells. Stem cells in the bone marrow divide to form two blood cell lineages: (1) the lymphoid stem cell gives rise to B cells that become antibody-secreting plasma cells, T cells that become activated T cells, and natural killer cells. (2) The common myeloid progenitor cell gives rise to granulocytes and monocytes that give rise to macrophages and dendritic cells. (Reproduced with permission from Willey JM: Prescott, Harley, & Klein’s Microbiology, 7th edition. McGraw-Hill, 2008.)
INNATE (NONSPECIFIC) IMMUNITY
Innate immunity acts through a series of specific and nonspecific mechanisms, all working to create a series of hurdles for the pathogen to navigate (Table 2–1). The first are mechanical barriers such as the tough multilayered skin or the softer but fused mucosal layers of internal surfaces. As discussed in Chapter 1, microbial flora on these surfaces present formidable competitors for space and nutrients. Turbulent movement of the mucosal surfaces and enzymes or acid secreted on their surface make it difficult for an organism to persist. Organisms that are able to pass the mucosa encounter a population of cells with the ability to engulf and destroy them. In addition, body fluids contain chemical agents such as complement, which can directly injure the microbe. The entire process has cross-links to the adaptive immune system. The endpoint of phagocytosis and digestion in a macrophage is the presentation of the antigen on its surface; the first step in specific immune recognition.
TABLE 2–1 Features of Innate Immunity in Infection
Skin, mucosa are barriers
Cells engulf, digest, and presen antigens from microbes
PHYSICAL BARRIERS
The thick layers of the skin containing insoluble keratins present the most formidable barrier to infection. The mucosal membranes of the alimentary and urogenital tract are not as tough but, often, are bathed in secretions inhospitable to invaders. Lysozyme is an enzyme that digests peptidoglycan—a unique structural component of the bacterial cell wall. Lysozyme is secreted onto many surfaces and is particularly concentrated in conjunctival tears. The acid pH of the vagina and particularly the stomach makes colonization difficult for most organisms. Only small particles (5-10 μm) can be inhaled deep into the lung alveoli because the lining of the respiratory includes cilia that trap and move them toward the pharynx.
Lysozyme digests bacterial walls
Cilia move particles away from the alveoli
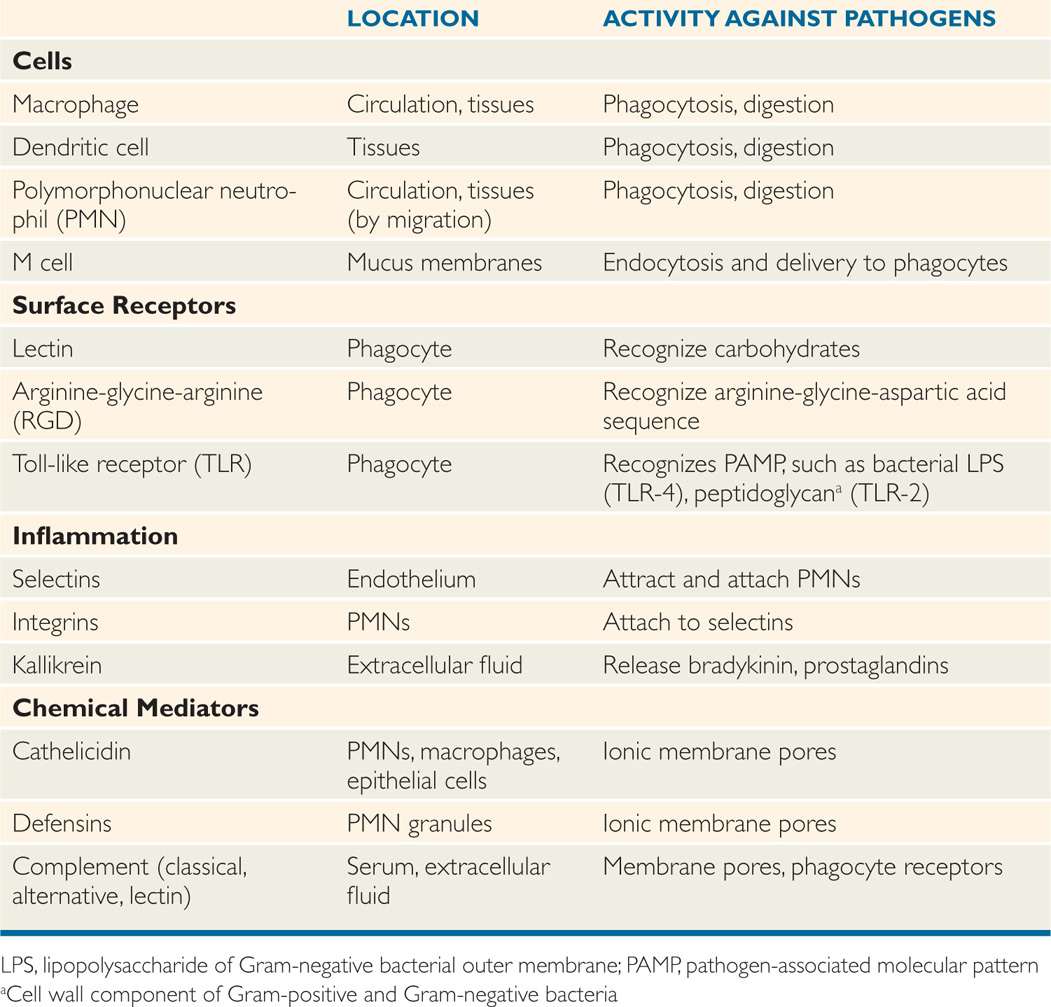
The skin and mucosal surfaces of the intestinal and respiratory tract also contain concentrations of lymphoid tissue within or just below their surfaces, which provide a next-level defense for invaders surviving the above-described defenses. These lymphoid collections are designed to entrap and deliver invaders to some of the phagocytes described in the following text. For example, in the intestine, M cells (Figure 2–2) that lack the villous brush border of their neighbors endocytose bacteria and then release them into a pocket containing macrophages and lymphocytic components (T and B cells) of the adaptive immune system. The enteric pathogen Shigella exploits this receptiveness of the M cell to attack the adjacent enterocytes from the side.
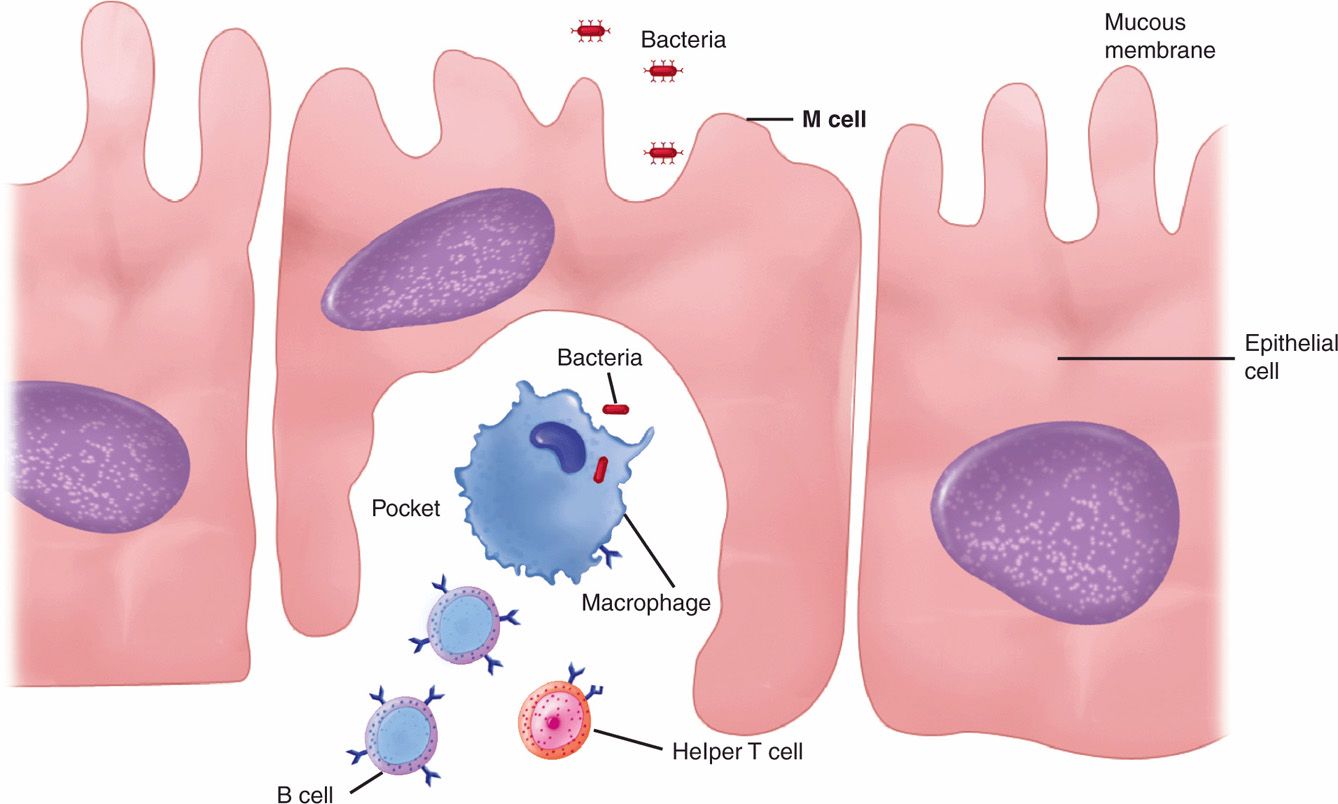
FIGURE 2–2. M cell. An M cell is shown between two epithelial cells in a mucous membrane. It has endocytosed a pathogen and released it into a pocket containing macrophages and other immune cells.
M cells deliver to macrophages and lymphocytes
IMMUNORESPONSIVE CELLS AND ORGANS
Not all the cells shown in Figure 2–1 are involved in the immune system; of those that are, not all respond to infection. What the immunoresponsive cells have in common is derivation from hematopoietic stem cells in the bone marrow, which create the myeloid and lymphoid series followed by further differentiation into their mature cell types. Of the types shown, the erythroblast and megakaryocte do not participate in immune reactions. In the myeloid series, basophils and mast cells are primarily involved in allergic reactions rather than infection. The immunoresponsive cells are found throughout the body in the circulation or at fixed locations in tissues. They are concentrated in the lymph nodes and spleen, and form a unified filtration network designed as a sentinel warning system. In the lymphoid series, cells destined to become T cells mature in the thymus (the source of their name). Thus, the thymus, spleen, and lymph nodes might be thought of as the organs of the immune system. These are collectively referred to as the lymphoid tissues.
Stem cells differentiate to myeloid and lymphoid series
Thymus, spleen, and lymph nodes are immune organs
 Cells Responding to Infection
Cells Responding to Infection
Monocytes
Monocyte is a general morphologic term for cells that include or quickly (hours) differentiate into macrophages or dendritic cells. These are the cells of the immune system that both phagocytose invaders and process them for presentation to the adaptive immune system. Macrophages are found in the circulation and tissues, where they are sometimes given regional names such as alveolar macrophage. They possess surface receptors such as mannose and fructose, which nonspecifically recognize components commonly found on pathogens and more specialized receptors able to recognize unique components of microbes such as the lipopolysaccharide (LPS) of Gram-negative bacteria. They also have receptors that recognize antibody and complement.
Macrophages in circulation or tissues
Surface receptors recognize pathogens
Dendritic cells have a distinctive star-like morphology, and are present in the skin and in the mucous membranes of the respiratory and intestinal tracts. Similar to macrophages, they phagocytose and present foreign antigens. Surface recognition includes a process called pathogen-associated molecular patterns (PAMPs), in which selective molecular patterns unique to pathogens are recognized and bound. After binding and phagocytosis, dendritic cells migrate to lymphoid tissues where specific immune responses are triggered.
Star-like tissue phagocytes
Migrate to lymphoid tissues
Granulocytes
Of the cells in the granulocyte series, the most active is the polymorphonuclear neutro-phil or PMN. These cells have a distinctive multilobed nucleus and cytoplasmic granules that contain lytic enzymes and antimicrobial substances including peroxidase, lysozyme, defensins, collagenase, and cathelicidins. PMNs have surface receptors for antibody and complement and are active phagocytes. In addition to the digestive enzymes, PMNs have other oxygen-dependent and oxygen-independent pathways for killing microorganisms. Unlike macrophages, they only circulate and are not present in tissues except by migration as part of an acute inflammatory response.
PMNs have digestive and killing pathways
In circulation unless they migrate in inflammation
Eosinophils are nonphagocytic cells that participate in allergic reactions along with basophils and mast cells. Eosinophils are also involved in the defense against infectious parasites by releasing peptides and oxygen intermediates into the extracellular fluid. It is felt that these products damage membranes of the parasite.
Eosinophils damage parasites
Lymphocytes
Lymphocytes are the primary effector cells of the adaptive immune system. They are produced from a lymphocyte stem cell in the bone marrow and leave in a static state marked to become T, B, or null cells after further differentiation (Figure 2–3). This requires activation mediated by surface binding, which then stimulates further replication and differentiation.
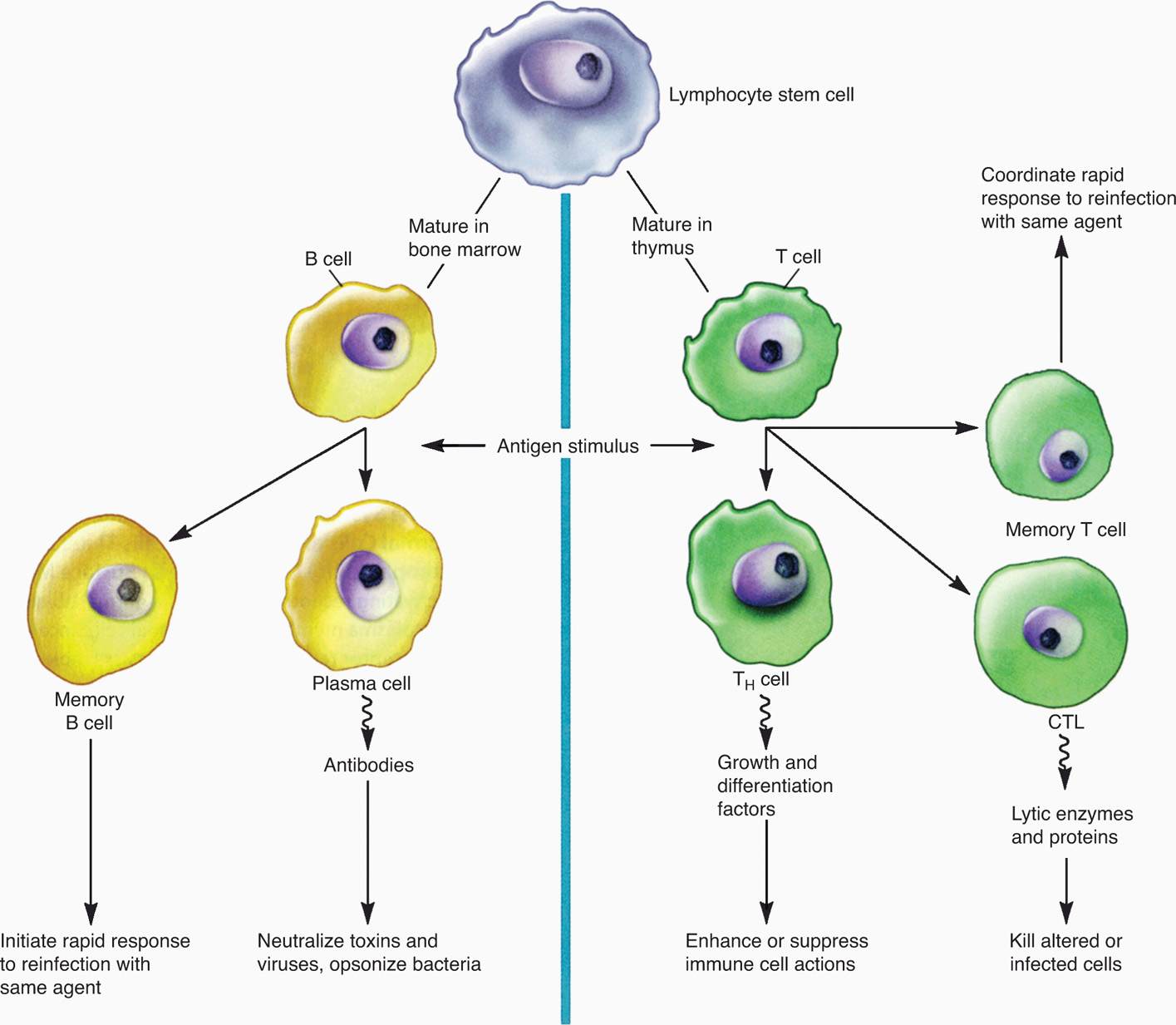
FIGURE 2–3. B and T lymphocytes. B cells and Τ cells arise from the same cell lineage but diverge into two functional types. Immature B cells and Τ cells are indistinguishable by morphology (Reproduced with permission from Willey JM: Prescott, Harley, & Klein’s Microbiology, 7th edition. McGraw-Hill, 2008.)
T, B, and null cells initially static
B cells mature in the bone marrow and then circulate in the blood to lymphoid organs. At these sites, they may become activated to a form called a plasma cell, which produces antibodies. T cells mature in the thymus and then circulate awaiting activation. Their activation results in production of cytokines, which are effector molecules for multiple immunocytes and somatic cells. Some of the uncommitted null cells become natural killer (NK) cells, which have the capacity to directly kill cells infected with viruses.
B cells make antibody
T cells secrete cytokines
Phagocytosis
Phagocytosis is one of the most important defenses against microbial invaders (Figure 2–4). The major cells involved are PMNs, macrophages, and dendritic cells. For all, the process begins with surface–pathogen recognition mechanisms, which may be either dependent on opsonization of the organism with complement or antibody or independent of opsonization. At this point, only the opsonin-independent mechanisms are considered. These use the nonspecific mechanisms already described and hydrophobic interactions between bacteria and the phagocyte surface. More powerful mechanisms include lectins, which bind carbohydrate moieties and protein–protein interactions based on a specific peptide sequence (arginineglycine-aspartic-acid or RGD). These RGD receptors are present on virtually all phagocytes.
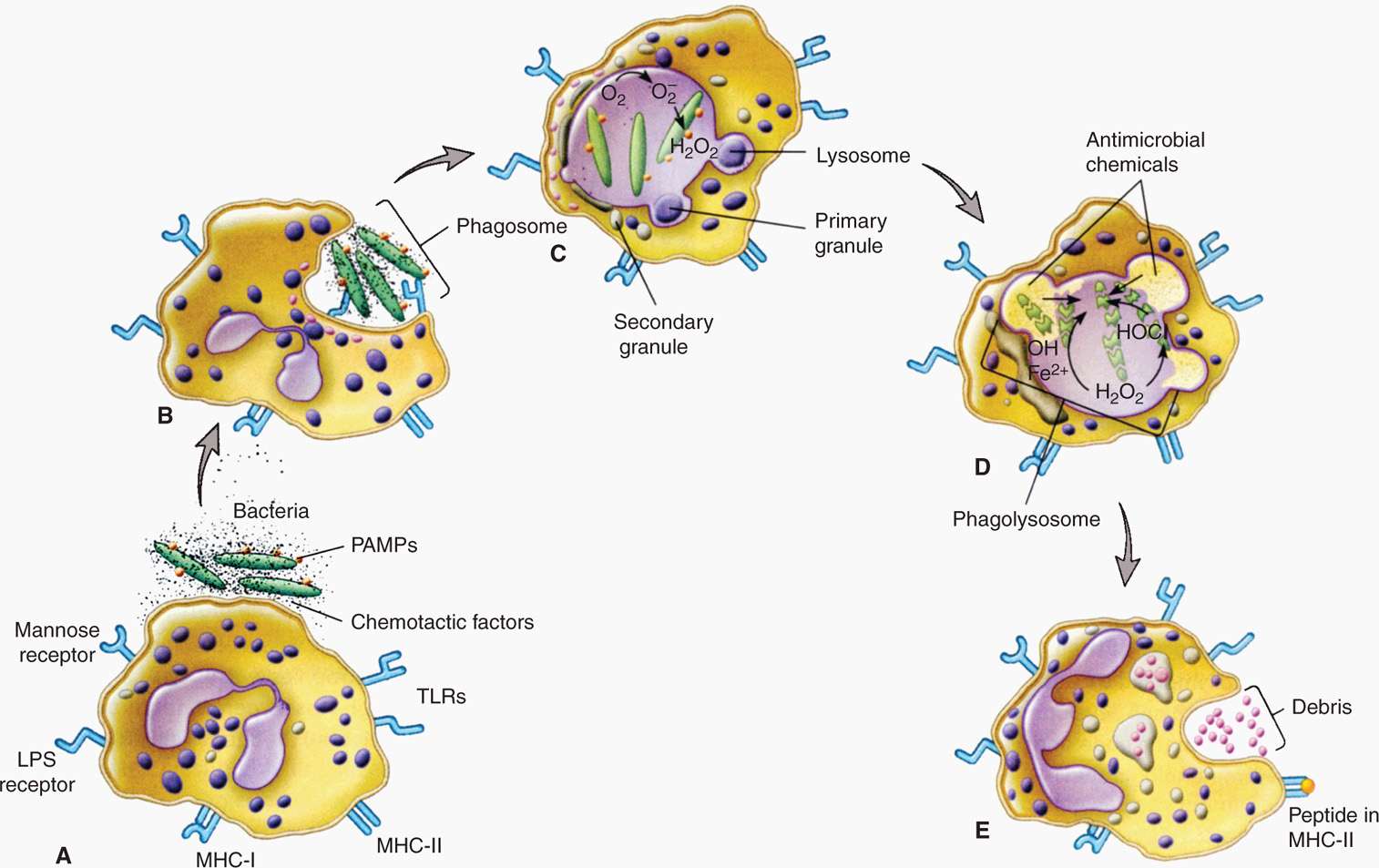
FIGURE 2–4. Phagocytosis. A. Drawing shows receptors on a phagocytic cell, such as a macrophage, and the corresponding PAMPs participating in phagocytosis. The schematic depicts the process of phagocytosis showing ingestion. B. Participation of primary and secondary granules and, C., O2-dependent killing events. D. Intracellular digestion. E. Endocytosis LPS receptor, lipopolysaccharide receptor; TLRs, toll-like receptors; MHCI, class I major histocompatibility protein; MHCII, class II major histocompatibility protein; PAMPs, pathogen-associated molecular patterns. (Reproduced with permission from Willey JM: Prescott, Harley, & Klein’s Microbiology, 7th edition. McGraw-Hill, 2008.)
Opsonization not required
Carbohydrate and peptide sequence recognized
Another mechanism is use of the PAMPs already mentioned. Phagocytes have evolved a distinct class called Toll-like receptors (TLRs), of which at least 10 sets are known. These include sets that recognize a molecular pattern in bacterial peptidoglycan (TLR-2) and LPS (TLR-4). TLRs not only bind, but also trigger signaling pathways leading to induction of cytokines and other directors of the specific immune response.
TLRs bind LPS, peptidoglycan, and induce cytokines
Bound organisms are taken inside the phagocyte in a membrane-bound phagosome destined to fuse with lysosomes inside to form a phagolysosome. This is the main killing ground of the phagocyte. The lysosomal enzymes include hydrolases and proteases that have maximum activity at the acidic pH inside the phagolysosome. In addition, inside the phagocyte are oxidative killing mechanisms created by enzymes that produce reactive oxygen intermediates (superoxide, hydrogen peroxide, singlet oxygen) driven by a metabolic respiratory burst in the cell cytoplasm. These mechanisms are particularly used for killing bacteria. Bacterial pathogens whose pathogenesis involves multiplication rather than destruction inside the phagocyte have mechanisms to block one or more of the preceding steps. For example, some pathogens are able to block fusion of the phagosome with the lysosome; others interfere with the acidification of the phagolysosome.
Enzymes digest in acidic phagolysosome
Reactive oxygen driven by respiratory burst
Another mechanism effective with some viruses, fungi, and parasites is the formation of reactive nitrogen intermediates (nitric oxide, nitrate, and nitrite) delivered into a vacuole or in the cytoplasm. PMN granules contain a variety of other antimicrobial substances, including peptides called defensins. Defensins act by permeabilizing membranes and, in addition to bacteria, are active against enveloped viruses.
Reactive nitrogen affects viruses
INFLAMMATION
Inflammation encompasses a series of events in which the above mentioned cells are deployed in response to an injury—such as a new microbial invader. At the first insult, chemical signals mobilize cells, fluids, and other mediators to the site to contain, combat, and heal. In acute inflammation, the first events may be noticed in minutes, and the entire process resolved over a matter of days to a couple of weeks. Chronic inflammation may follow the incomplete resolution of an acute process or arise as a slow insidious process of its own. The natural history of infections such as tuberculosis, which follow this pattern, run for months, years, even decades.
Acute = hours to days
Chronic = weeks to months
The first event in acute inflammation is the release of chemical signals (chemokines) that act on adhesion molecules (selectins) in local capillaries. This slows the movement of passing PMNs and activates adhesive integrins on their surface. This leads to tight adhesion to the endothelium followed by squeezing past the endothelial wall to the tissues below. There, chemotactic factors released by the bacteria lead them to the primary site. Increasing acidity of local fluids releases enzymes (kallikrein, bradykinin) that open junctions in capillary walls and allow increased flow of fluids and more leukocytes. Histamine (from mast cells), arachidonic acid, and prostaglandin release complete the picture of swelling and pain.
PMNs migrate from capillaries
Enzymes and chemical mediators facilitate swelling
Chronic inflammation bridges the innate and adaptive immune responses. An acute phase, if present, is usually not noticed, and the cellular infiltrate is composed of lymphocytes and macrophages with relatively few PMNs. It is generally associated with slower-growing pathogens such as mycobacteria, fungi, and parasites in which cell-mediated immunity (TH1) is the primary adaptive defense. Many of these pathogens have mechanisms that allow them to multiply in nonactivated macrophages. If the macrophages are effectively activated by T cells, the multiplication ceases and the inflammation and injury are minimal. If not, multiplication and chronic inflammation continue sometimes in the form of a granuloma, which is an indication of a destructive hypersensitivity component to the inflammation.
Lymphocytes and macrophages predominate
Granulomas indicate failure to resolve by adaptive cellular mechanisms
CHEMICAL MEDIATORS
Chemical mediators of innate immunity that have direct antimicrobial activity include cationic proteins and complement. The cationic proteins (cathelicidin, defensins) act on bacterial plasma membranes by the formation of ionic pores, which alter membrane permeability. The complement system is a series of glycoproteins, which can directly insert in bacterial membranes or act as receptors for antibody. Cytokines are proteins or glycoproteins released by one cell population that act as signaling molecules for another. They are generally thought of in the context of the adaptive immune system, but they can be stimulated directly by microorganisms.
Peptides alter membrane permeability
 The Complement System
The Complement System
The complement system consists of more than 30 distinct components and several other precursors. All are in the plasma of healthy individuals in inactive forms that must be enzymatically cleaved to become active. When this happens, a cascade of reactions is triggered, which activates the various components in a fixed sequence (Figure 2–5). The difference between the pathways is in the mechanisms for their initiation. Once started, any pathway can produce the same effects on pathogens, which include enhancing phagocytosis, activation of leukocytes, and lysis of bacterial cell walls. An important step in the process is coating of the organism with serum components, a process called opsonization. The coatings may be mannose-binding proteins, complement components, or antibody. There is no immunologic specificity in complement activation or in its effects.
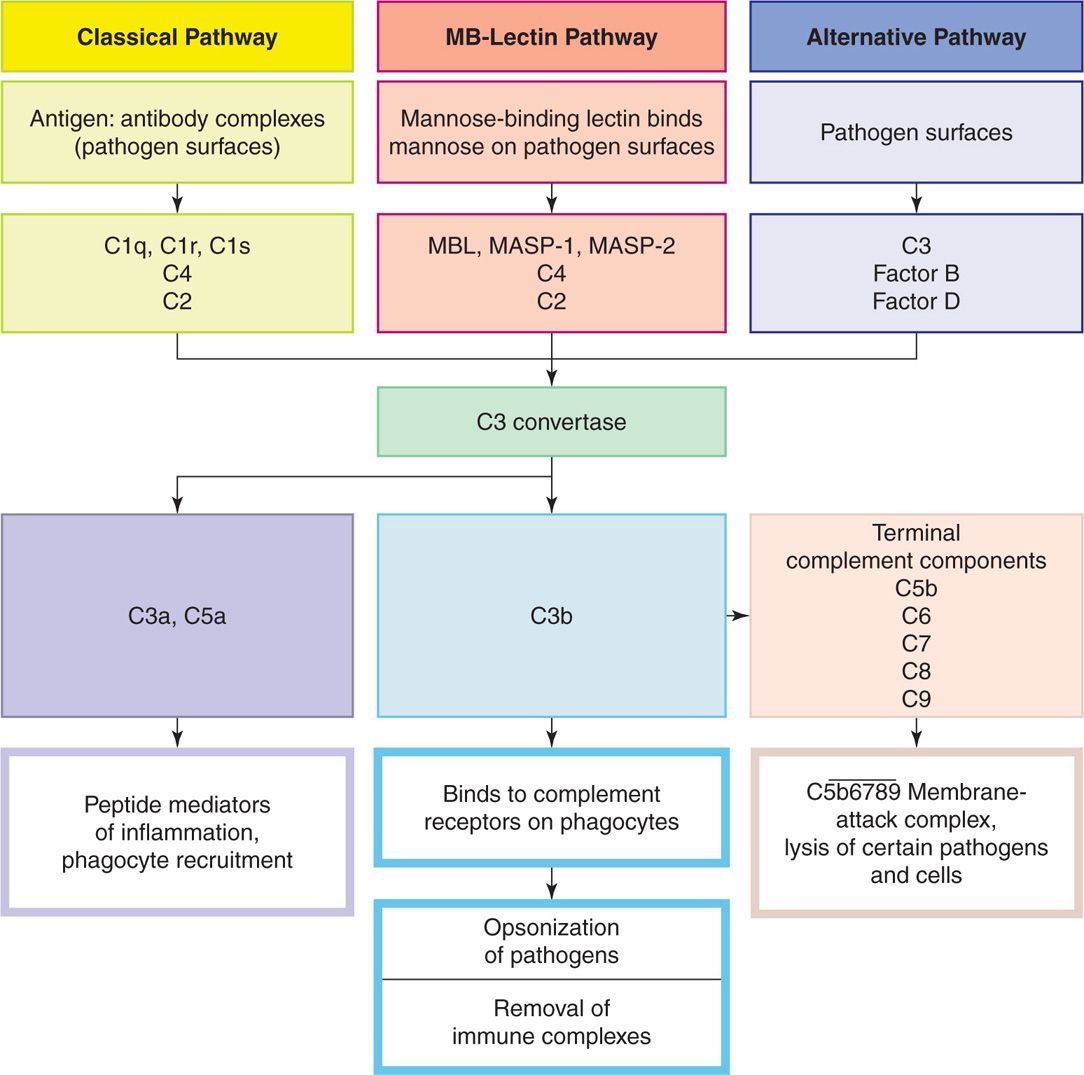
FIGURE 2–5. Components and action of complement. Complement activation involves a series of enzymatic reactions that culminate in the formation of C3 convertase, which cleaves complement component C3 into C3b and C3a. The production of the C3 convertase is where the three pathways converge. C3a is a peptide mediator of local inflammation. C3b binds covalently to the bacterial cell membrane and opsonizes the bacteria, enabling phagocytes to internalize them. C5a and C5b are generated by the cleavage of C5 by a C5 convertase. In addition, C5a is a powerful peptide mediator of inflammation. C5b promotes the terminal components complement to assemble into a membrane-attack complex. (Reproduced with permission from Willey JM: Prescott, Harley, & Klein’s Microbiology, 7th edition. McGraw-Hill, 2008.)
Multiple components activated in cascade fashion when triggered
Pathways differ in initiation mechanism
Opsonization is serum coating of pathogens
Alternative Pathway
The alternative pathway is activated by bacterial cell wall components with repetitive surface structures such as LPS. The multiple components come together in the formation of the membrane-attack complex, which inserts directly into bacterial membranes (Figure 2–6), particularly the outer membrane of Gram-negative bacteria. This not only injures the organism, but also enhances phagocytosis because the other end of the molecule has receptors for phagocytes. Gram-positive bacteria are less affected because they have no exposed membrane (see Chapter 21). These actions are particularly important for the effectiveness of innate immunity in the early stages of acute infections before the adaptive immune system has time to act. The key complement component for alternate pathway activity is C3b. C3b activation and degradation are regulated by a number of serum factors (factors B, D, and H) that can modulate its activity. A major mechanism for pathogens to block alternate pathway attack is by binding factor H to their surface. This is accomplished by bacterial capsules and surface proteins. This concentration of factor H causes local degradation of C3b (see Chapter 22, Figure 22–4).
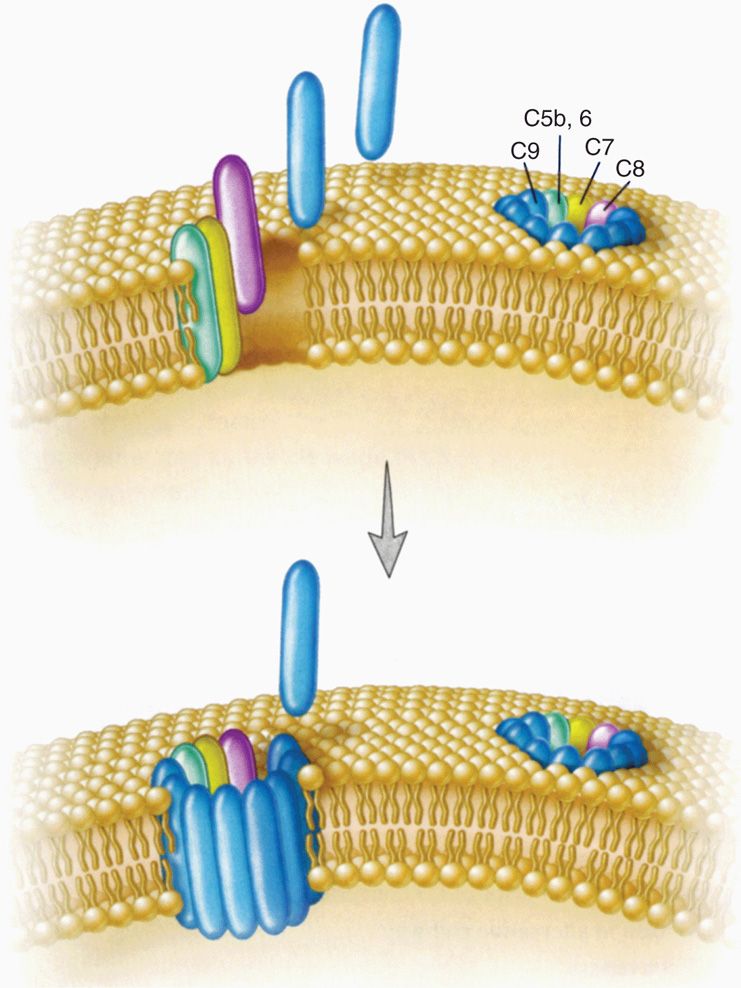
FIGURE 2–6. Complement membrane-attack complex. The membrane-attack complex (MAC) is a tubular structure that forms a transmembrane pore in the target cell’s plasma membrane. The subunit architecture of the MAC shows that the transmembrane channel is formed by multiple polymerized molecules. (Reproduced with permission from Willey JM: Prescott, Harley, & Klein’s Microbiology, 7th edition. McGraw-Hill, 2008.)
Activation is by pathogen surfaces
Membrane-attack complex inserts and provides phagocyte receptors
Factor H binding accelerates C3b degradation on capsules
Lectin Pathway
Another means of activating the complement system is based on the carbohydrate building of lectins. In this case, the lectins bind to mannose—a common surface component of bacteria, fungi, and some virus envelopes. This binding opsonizes the pathogen and enhances phagocytosis. Thus, as in the alternative pathway, the activation comes from pathogen surfaces and proceeds through the same C3 convertase (Figure 2–5).
Lectins bind mannose on pathogens
Classic Pathway
The classic complement pathway is initiated by the binding of antibodies formed during the adaptive immune response (as described further) with their specific antigens on the surface of a pathogen. This binding is highly specific but amounts to another case of opsonization activating the complement cascade. In this case, specific sites on the Fc portion of immunoglobulin molecules bind and activate the C1 component of complement to start the process. The pathway and sequence of individual complements are characteristic of the classic pathway, but it still reaches C3b, the common point for microbial directed action. As with the alternative pathway, this creates the membrane-attack complex, the mediators of inflammation, and receptors for phagocytes on C3b.
Antigen–antibody reaction exposes complement binding sites
C3b has receptors for phagocytes
 Cytokines
Cytokines
Cytokine is a broad term referring to molecules released from one cell population destined to have an effect on another cell population (Table 2–2). As these proteins and glycoproteins have been discovered, they have been named and classified in relation to biologic effects observed initially only to discover that they have multiple other actions. For infectious diseases, the operative subcategories are chemokines, which are cytokines chemotactic for inflammatory cell migration, and interleukins (IL-1, -2, -3, etc), which regulate growth and differentiation between monocytes and lymphocytes. Tumor necrosis factor (TNF), so named for its cytotoxic effect on tumor cells, can also induce apoptosis (programmed cell death) in phagocytes—a useful feature for pathogens they have taken in. Interferons (INF-α, -β, and -γ) were originally named for their interference with viral replication (Figure 2–7), but are now known to be central to activation of T cells and macrophages. Unless commanded to understand specific situations, cytokine is used to represent all these mediators in these pages.
ILs, IFNs, TNF, chemokines are all cytokines
TABLE 2–2 Some Cytokines Acting in Infection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


