VIROLOGY
STRUCTURE
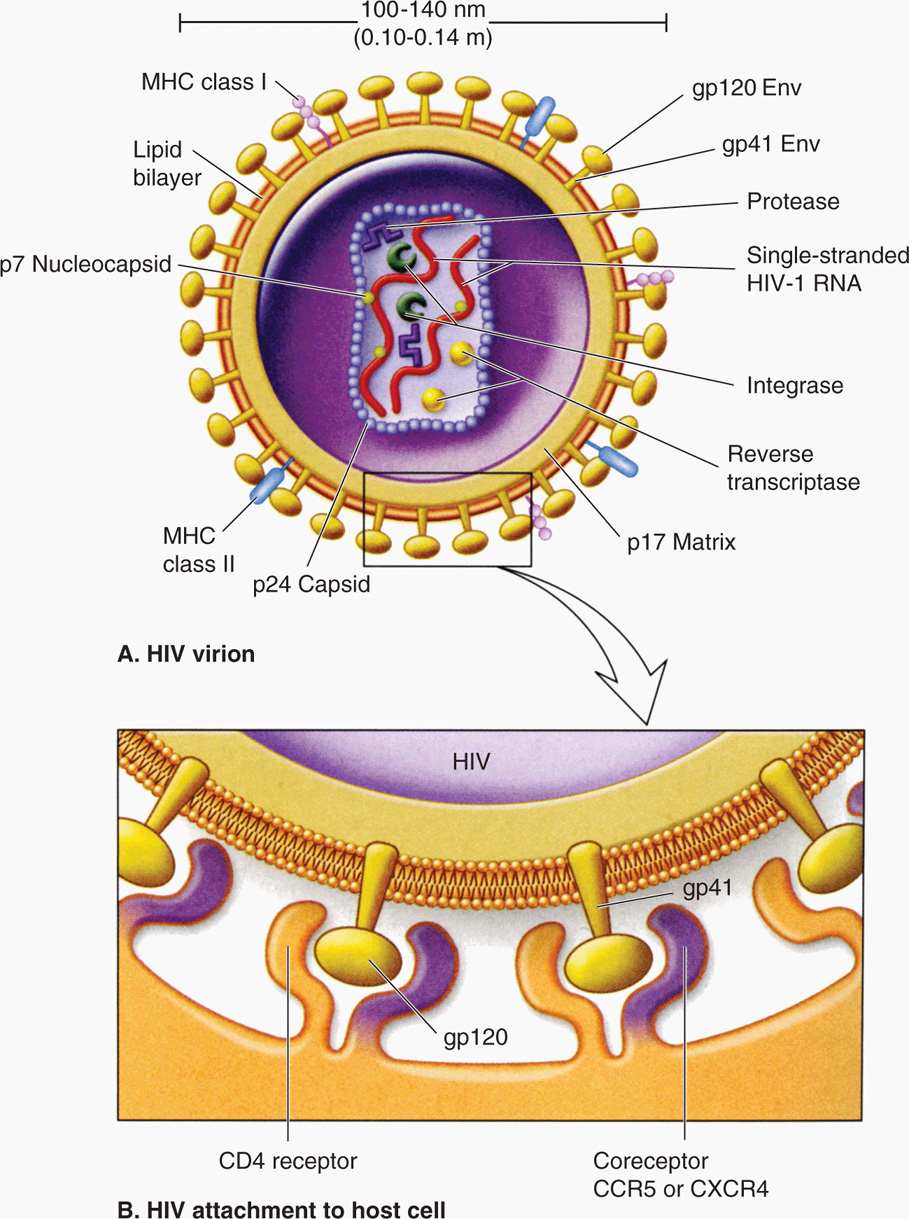
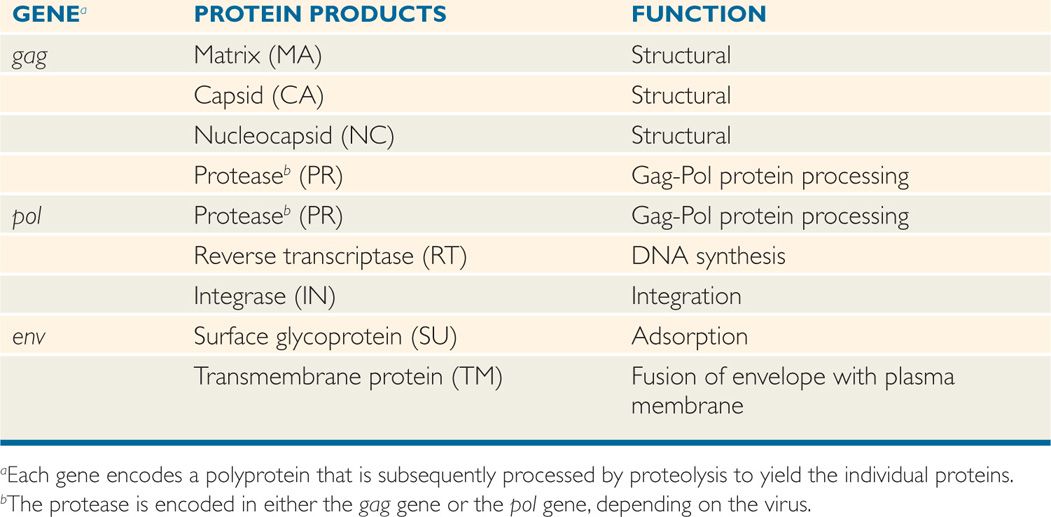
All retroviruses are remarkably similar in their basic composition and structure. The structure of HIV-1 is depicted in Figure 18–1. The virion size is about 100 nm in diameter, and because it contains two copies of the RNA genome, it is diploid. The RNA genome is coated with the nucleocapsid protein (NC), and the RNA-protein complexes are enclosed in a capsid (CA, also called p24) composed of multiple subunits in an icosahedral symmetry, which is covered by a membrane-associated matrix (MA, also called p17) protein. Like all enveloped viruses, the membrane is acquired during budding from the host cell plasma membrane, but the surface (SU, also called gp120) and transmembrane (TM, also called gp41) glycoproteins found in the envelope are virally encoded. In addition to the structural proteins shown in Figure 18–1, the virion core contains three virus-specific proteins (enzymes) that are essential for viral replication: Reverse transcriptase (RT), protease (PR), and integrase (IN). The relation between the viral genes found in all retroviruses (gag, pol, and env) and the proteins they encode are presented in Table 18–1. Some retroviruses, including HTLV and HIV, encode additional regulatory and accessory proteins. Based on SU gp120 sequence, HIV-1 can be T-lymphotropic (X4), macrophage tropic (R5), or both X4/R5 (dual tropic).
FIGURE 18–1. Structure of HIV particle. The two RNA molecules enclosed within the capsid are coated with the nucleocapsid protein. The matrix protein lies just inside the membrane envelope. B. The envelope contains two membrane glycoproteins, gp41 and gp120, also called transmembrane protein and surface protein, respectively. CCR5; CXCR4, chemokine receptors, acting as coreceptors.
TABLE 18–1. Major Retroviral Genes and Proteins
Virion contains two single-stranded, positive-sense RNA molecules (diploid genome)
Three critical enzymes, reverse transcriptase, protease, and integrase, are virus-encoded
Envelope acquired during budding contains two viral glycoproteins, gp120 and gp 41
RETROVIRAL REPLICATION CYCLE
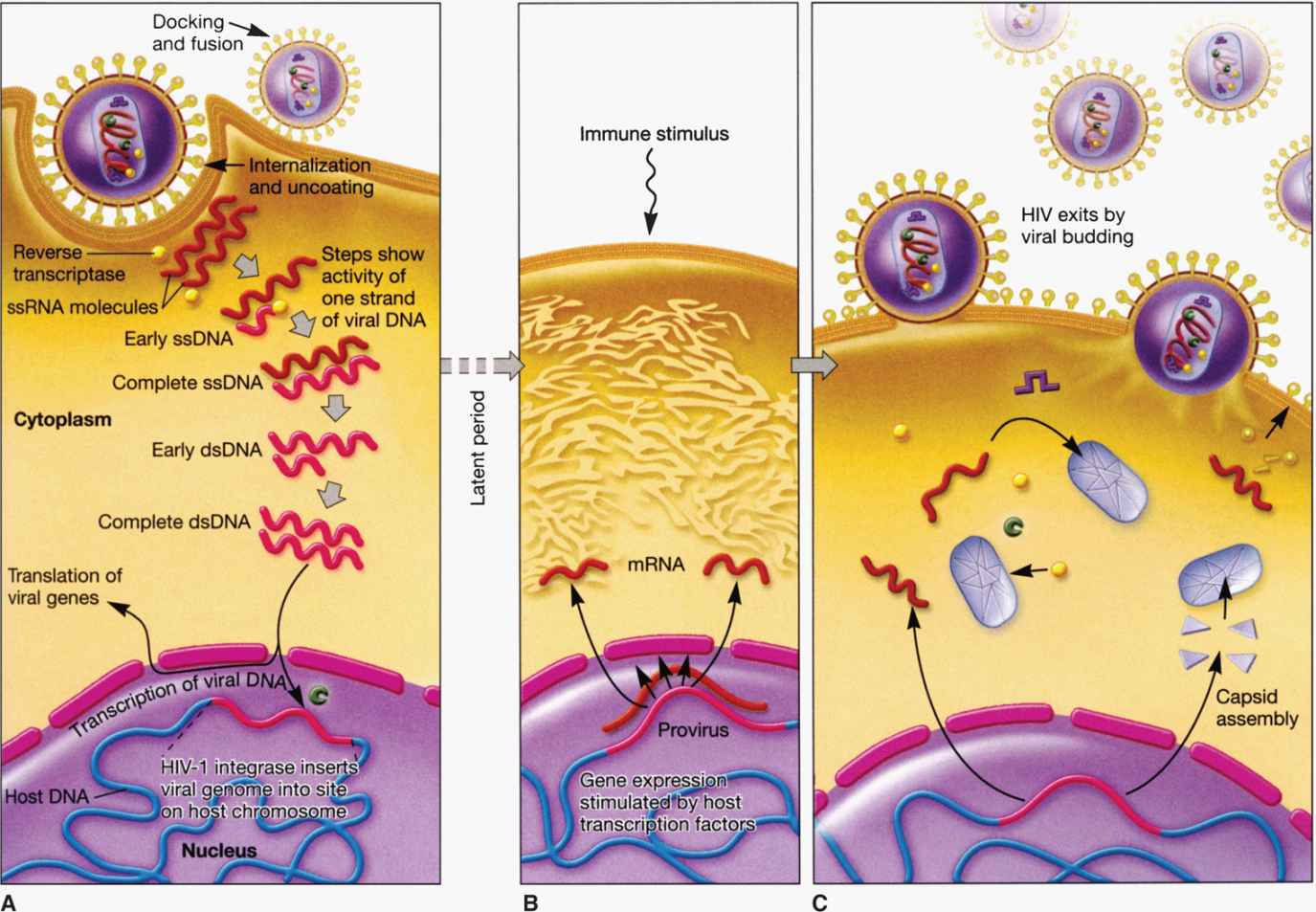
Figure 18–2 depicts the life cycle of a typical retrovirus (eg, HIV-1) and serves to illustrate the many unique aspects of retroviral replication that are targets for current antiviral agents and could be potential targets of new and effective therapeutic intervention.
FIGURE 18–2. Retroviral (HIV-1) life cycle. A. Viral entry and post entry (reverse transcription, DNA synthesis, and integration) events; B. Viral gene expression (transcription and protein synthesis); C. Virus assembly and release.
 Viral Entry
Viral Entry
Retroviral virions are adsorbed to cellular membrane receptors through an interaction of viral surface protein and cellular receptors and enter the cell by direct fusion of the viral envelope with the plasma membrane of the host cell. For HIV-1, the virion attachment protein is the SU glycoprotein gp120, and the cellular receptor is the CD4 molecule with one of the chemokine receptors, CXCR4 or CCR5, acting as coreceptors. These receptors and coreceptors occur primarily in the plasma membrane of CD4+ T lymphocytes, but also on cells of the monocyte-macrophage lineage, and some other target cells such as Langerhans cells, dendritic cells, and certain brain cells. The CD4+ T-lymphocytes express higher levels of CD4 and CXCR4 and somewhat lower levels of CCR5. However, the monocytes/macrophages express lower levels of CD4 and CXCR4 but higher levels of CCR5. Inhibitors of CCR5 coreceptor are available to be used in combination therapy. Early in infection, the viruses are often macrophage tropic (R5 viruses) because R5 viruses that use CCR5 coreceptor are predominantly transmitted to recipients. The emergence of syncytia-forming variants that use the CXCR4 coreceptor and are T-lymphotropic (X4 viruses) appears to correlate with rapid advancement to AIDS. The HIV-1 transmembrane TM protein gp41 is responsible for fusion of the viral and cell membranes, leading to entry of the virion core complex into the cytoplasm of the cell. Fusion inhibitor to gp41 function is a peptide-based antiviral agent approved as a part of combination therapy when other first-line drugs have failed.
HIV-1 surface glycoprotein gp120 attaches to CD4 cell and chemokine coreceptors, CCR5 or CXCR4
Whereas R5 HIV-1 binds to CD4 and CCR5, X4 HIV-1 interacts with CD4 and CXCR4
Transmembrane gp41 protein mediates fusion of viral and cellular membranes
Inhibitors to CCR5 and gp41 are approved and available for HIV therapy
HIV-1 can also infect cells that lack the CD4 surface molecule such as certain brain cells and other cells types with a low efficiency, apparently because the chemokine receptors in combination with the fusion-inducing activity of the TM protein is sufficient in these cases to promote entry. Fusion activity may also play an important role in amplification of the effects of the virus infection, particularly during the later stages of the infection, because infected cells expressing viral glycoproteins in their membranes readily fuse with uninfected CD4+ T lymphocytes to form large syncytia. This process appears to provide a means for cell-to-cell transmission of the virus that bypasses the usual extracellular phase and may contribute to the overall depletion of CD4+ T lymphocytes in an infected person.
HIV-1 can infect cells expressing chemokine receptors without the CD4 molecule but with a very low efficiency
Fusion provides direct cell-to-cell transmission
 Viral Postentry Events
Viral Postentry Events
Among the RNA viruses, retroviral replication is unique because it involves reverse transcription. Soon after the entry of the viral core into the cytoplasm of the infected cell, there is partial uncoating and the viral RNA is reverse transcribed (converted) into a complementary DNA (cDNA) by the action of reverse transcriptase enzyme, the virion-associated RNA-dependent DNA polymerase. The cDNA is converted into double-stranded DNA by the action of the DNA-dependent DNA polymerase activity of the same reverse transcriptase enzyme. The viral RNA template is removed from the RNA-DNA hybrid by RNAase H activity of the same reverse transcriptase enzyme. The overall process is referred to as reverse transcription. Currently, there are several antiviral agents that are inhibitors of reverse transcriptase enzyme used in combination therapy (as the first line of drugs) to treat HIV infection. Following reverse transcription, the resultant linear DNA molecule circularizes and makes a preintegration complex with the help of viral and host factors. The preintegration complex enters the nucleus and integrates more or less at random sites into the host cell chromosome catalyzed by viral integrase. Once the viral genetic information has been converted to DNA and integrated, it essentially becomes part of the cellular genome, and the cell is permanently infected. The viral genome, called the provirus, is therefore replicated and faithfully inherited as long as the infected cell continues to divide. Integrase inhibitors have been developed and approved as a part of combination HIV therapy in those patients who have developed resistance to first-line drugs.
Reverse transcriptase enzyme copies RNA to double-stranded DNA
Reverse transcriptase inhibitors are available as part of combination antiretroviral therapy (ART)
DNA integrates into the host chromosome and replicates with the cell as a provirus
Integrase inhibitors have been approved for HIV therapy
Special sequences contained within the RNA are duplicated during the reverse transcription process so that the integrated provirus contains identical long terminal repeats (LTRs) at its ends (Figure 18–3). The LTR sequences contain the appropriate promoter, enhancer, and other signals required for transcription of the viral genes by the host RNA polymerase II. Transcription produces a full-length RNA genome and one or more spliced mRNAs. For the oncoviruses, the predominant spliced mRNA is translated to produce the envelope glycoproteins, but in HIV-1, a series of spliced mRNAs are produced that encode, in addition to the envelope proteins, a series of viral regulatory and accessory proteins. Unlike most retroviruses, HIV-1 and the other lentiviruses apparently exert considerable control over whether the primary transcripts are allocated to full-length RNA or are spliced to produce mRNAs (see text that follows). With the exception of these regulatory and accessory proteins, all retroviral proteins are initially translated as polyproteins that are subsequently processed by proteolysis into the individual protein molecules. Although the HIV-1 envelope precursor proteins (gp160) are cleaved by host cell protease, the enzyme responsible for cleavages of Gag and Gag-Pol precursors is the virus-specific protease (PR) that is encoded by the pol gene of HIV-1.
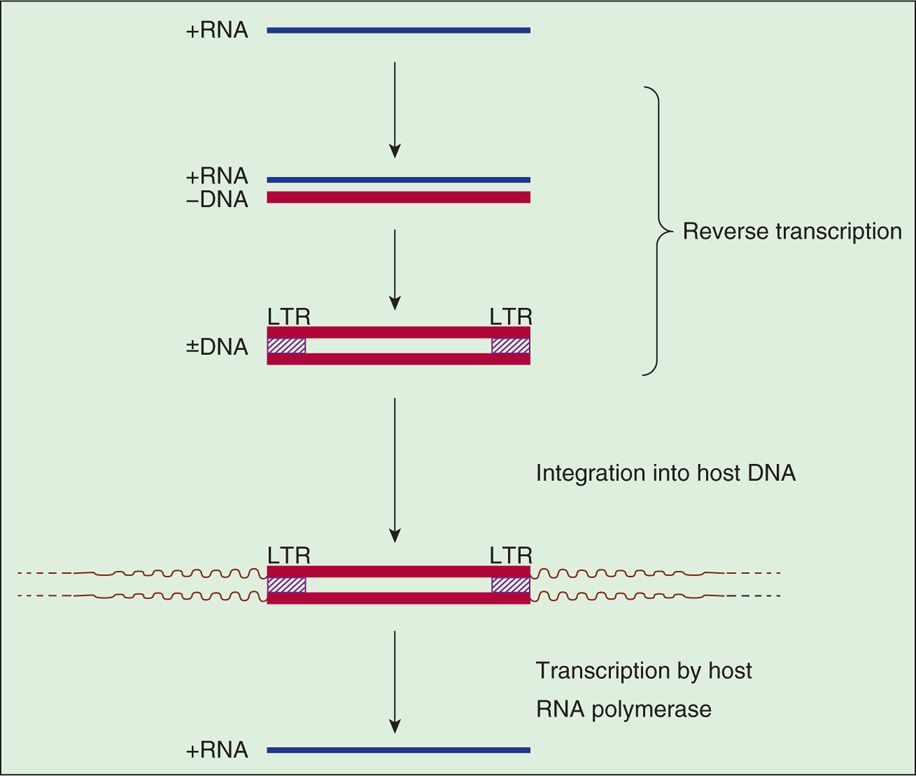
FIGURE 18–3. Retroviral RNA replication. LTR, long terminal repeat.
Provirus includes its own promoter and signals that control transcription by host RNA polymerase
LTR contains promoter and enhancer signals required for transcription and regulation of gene expression
Genomic RNA and spliced mRNAs are both produced: The latter encode envelope glycoproteins and regulatory proteins
HIV-1 can control extent of genomic or spliced mRNA production
A simplified view of retroviral RNA replication is presented in Figure 18–3. In addition to DNA polymerase activity, the reverse transcriptase possesses an RNase H activity that is responsible for degrading the RNA portion of the DNA-RNA hybrid (+RNA/-DNA) produced in the first phase of reverse transcription. The immediate product of reverse transcription is a linear, double-stranded DNA molecule that is flanked by the LTR sequences. The viral integrase (IN) catalyzes the integration of the linear DNA into host DNA. The integration process is highly specific with respect to the viral DNA, and two base pairs are generally lost from each end of the DNA. The choice of a target site for integration into the cellular DNA appears, however, to be nearly random but preferably in actively transcribed genes. A short sequence of base pairs in the target DNA (four to six, depending on the virus) is duplicated during the integration process, and these repeat sequences immediately flank the integrated provirus. The replication process is completed by transcription of the proviral DNA by the host RNA polymerase II.
RNase H activity degrades original RNA genome
Integrase-catalyzed integration is random in host DNA
Integrated HIV DNA is transcribed by host RNA polymerase
Of all the known retroviruses, HIV-1 possesses the most error-prone reverse transcriptase. The consequence of this high error rate is that each time the viral RNA is reverse transcribed, three to four new mutations are introduced into the resulting DNA. Because the process of transcription of the integrated proviral DNA to produce new viral genomes is also error-prone, mutant genomes accumulate rapidly over the course of an infection. The end result is a quasispecies that accounts for the many nucleotide differences observed between different isolates (even from the same infected individual) and for the variability of the SU envelope protein gp120. It may explain, in part, the failure of the immune system to control the infection, the increases in viral virulence that appear to occur during the course of the infection, and the difficulty of developing an effective vaccine.
HIV reverse transcriptase is error-prone which generates viral quasispecies
Isolates from the same patient can differ in multiple genotypic and phenotypic properties
RETROVIRAL GENES
The genome organization of different types of retroviruses is shown in Figure 18–4 (see also Table 18–1). All retroviruses contain the same structural genes in the order of gag-pol-env genes. The gag (group-specific antigen) gene encodes the structural proteins (capsid, nucleocapsid, matrix) of the virus and, in some animal retroviruses, the protease. The pol (polymerase) gene in human retroviruses and HIV encodes the reverse transcriptase, the integrase, and the protease. The env (envelope) gene encodes the two membrane glycoproteins found in the viral envelope, SU gp120 and TM gp41. HIV-1 gp120 has five variable regions and several constant regions. The CD4-binding domains on gp120 are localized in the constant regions, whereas the coreceptor (CXCR4/CCR5) binding regions on gp120 are confined in the variable region 3 (V3 loop). The V3 region is also the principal neutralizing domain of the virus, and therefore contributes to antigenic variation and varying degrees of neutralization. However, gp41 is embedded in the envelope and mediates fusion of the viral envelope with the plasma membrane at the time of viral infection and less variable than gp120. The fusion of gp41 with the plasma membrane can be blocked by gp41 inhibitor.
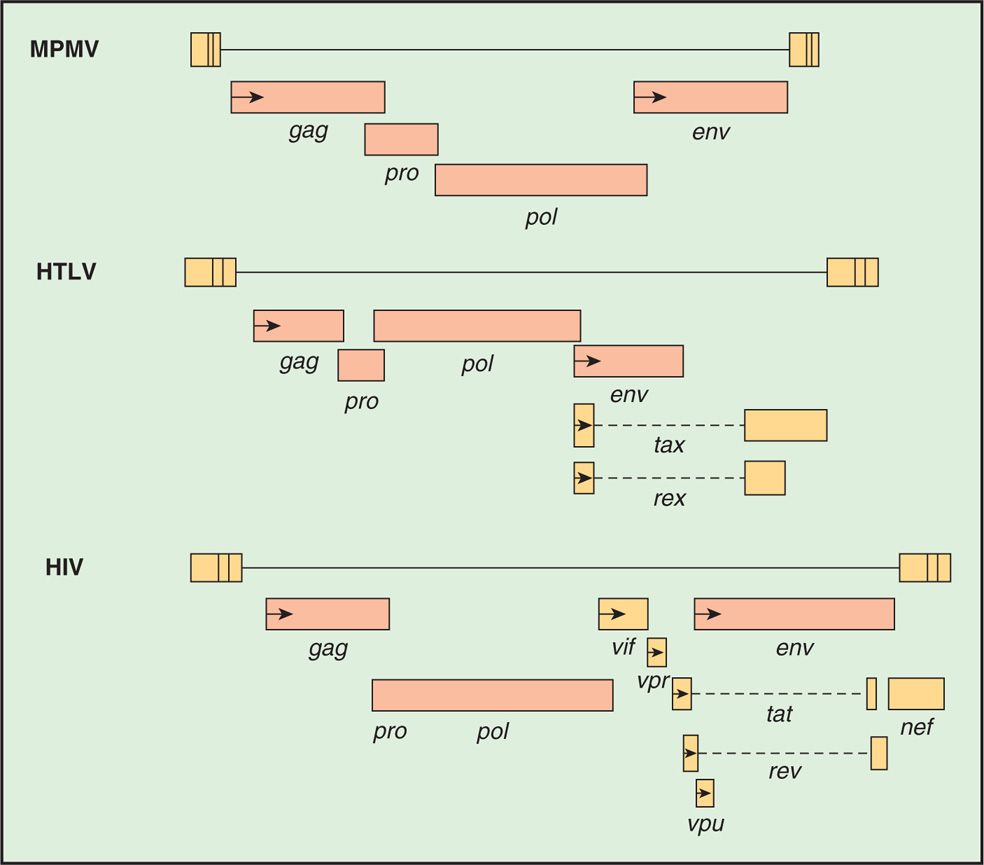
FIGURE 18–4. Structure of retroviral genes of a mouse retrovirus (MPMV), HTLV. and HIV.
Genome is organized into gag, pol, and env genes
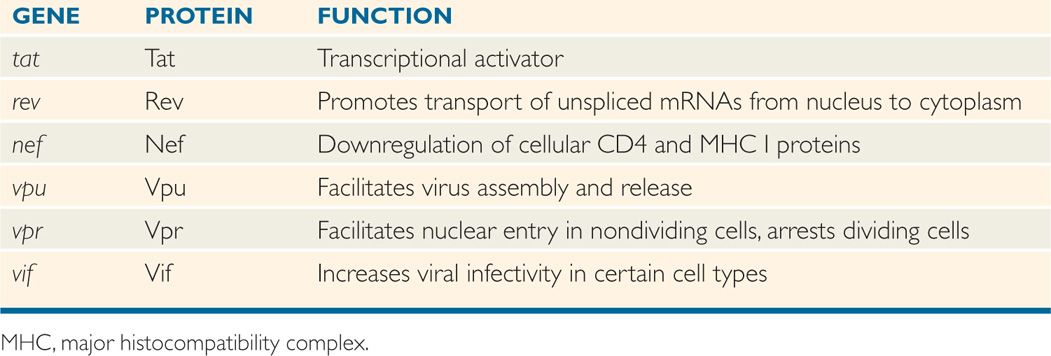
A comparison of the genetic makeup of HIV-1 with that of a typical retrovirus (Figure 18–4) reveals a larger number of genes and a much more complex organization. HIV-1 contains, in addition to the gag, pol, and env genes, an array of other genes (tat, rev, nef, vif, vpr, and vpu). Expression of these genes requires mRNA splicing, and all apparently encode proteins that serve regulatory or accessory roles during the infection (see text that follows). HTLV-I encodes the regulatory proteins, Tax and Rex, which are analogous to the HIV-1 Tat and Rev proteins. The names of the genes that have been best characterized and the proteins and functions they determine are listed in Table 18–2.
TABLE 18–2 Roles of HIV-1 Regulatory and Accessory Proteins
HIV-1 has multiple regulatory and accessory genes; tat, rev, nef, vif, vpu, and vpr
ROLES OF HIV-1 REGULATORY AND ACCESSORY PROTEINS
HIV-1 has the ability to produce a complex array of regulatory and accessory proteins that appear to be involved in viral replication, pathogenesis, and disease progression. These proteins also appear to interact with cellular factors to modulate the infection differently in different host cells. The roles of the two HIV-1 regulatory genes, tat and rev, and the four accessory proteins, nef, vpu, vpr, and vif, are discussed below and summarized in Table 18–2. Although the four accessory proteins are dispensable in many cell line culture systems, they appear to be important for the maximum pathogenic potential of the virus in infected individuals.
The products of the tat and rev regulatory genes are the Tat and Rev proteins, respectively. Both of these proteins are essential for viral replication. When the infected T lymphocyte is stimulated, for example, by antigen presentation, Tat and Rev play a positive role in promoting viral gene expression. In the absence of high levels of Tat, the host RNA polymerase initiates properly at the LTR promoter, but transcription is usually prematurely terminated leading to the production of short, dead-end transcripts. Tat is a transcriptional activator that acts at a sequence near the beginning of the viral mRNA, called Tat-acting responsive (TAR) element, to recruit cellular proteins to the transcribing RNA polymerase, resulting in a modification to the polymerase that prevents premature termination and allows complete transcription of the proviral genome.
Tat is a transcriptional activator that promotes synthesis of full-length and subgenomic viral transcripts
The Rev protein acts at the level of mRNA splicing and transport. Normally, unspliced cellular transcripts are retained in the nucleus, and only fully spliced mRNAs are transported to the cytoplasm for translation. The only viral proteins that are made from fully spliced mRNAs are Tat, Rev, and Nef, and consequently only these proteins are found early after infection, when there is no mechanism to prevent complete splicing of pre-mRNAs. To express the Vif, Vpr, and Vpu proteins, and the Env polyprotein, all of which are made from singly spliced transcripts, as well as the Gag and Pol polyproteins, which are translated from the unspliced genomic RNA, it is necessary to transport incompletely spliced RNAs to the cytoplasm. Transport of partially spliced transcripts is accomplished by Rev binding to a site on the viral RNA within the env gene called the Rev-responsive element (RRE). The RNA-bound Rev then interacts with normal cellular machinery responsible for protein export from the nucleus to mediate the movement of the RNA through the nuclear pore. By promoting translation of the virion structural proteins and some of the accessory proteins, Rev turns up late gene expression that leads directly to a high rate of virus production.
Rev promotes export of unspliced and partially spliced transcripts to cytoplasm
The Nef accessory protein appears to interfere with immune recognition of infected cells. Nef causes the internalization and degradation of the CD4 protein, which likely prevents superinfection and contributes to virus release by preventing the formation of complexes between the cellular receptor and newly synthesized virions. Nef also causes the down-regulation of cell surface major histocompatibility complex (MHC) I molecules, which may prevent recognition of infected cells by cytotoxic T lymphocytes (CTLs). In addition, virions produced in the absence of the Nef protein are at least partially blocked at some step before integration. The combination of these and perhaps other effects allows the Nef protein to play an essential pathogenic role in an infected individual.
Nef downregulates CD4 to avoid superinfection and also downregulates MHC I to interfere with immune recognition
The Vpu protein of HIV-1 appears to play two separate roles during the late stages of infection. In the absence of Vpu, the Env protein forms complexes with CD4 in the endoplasmic reticulum and fails to reach the plasma membrane of the cell. One of the roles of Vpu is to target the destruction of CD4 in the endoplasmic reticulum to allow for incorporation of Env into newly synthesized virions. The second role of Vpu is to promote the release of virions from the infected cell. The most likely mechanism is that Vpu counteracts the function of a host factor, BST-2 (bone marrow stromal antigen 2, CD137 or tetherin). BST-2 tethers HIV to the cell and prevents virus release, and thus has antiviral activity.
Vpu targets CD4 destruction and virion release
BST-2 has antiviral activity, because it prevents virus release from infected cells
Vpu neutralizes the function of the host factor (BST-2) to facilitate virus release
The Vpr protein is dispensable for HIV-1 replication in T-cell lines, but required for efficient viral replication in monocytes/macrophages. Several possible roles for Vpr in HIV-1 replication have been suggested, including modest transactivation of HIV-1 LTR, enhancement of the nuclear migration of the preintegration complex in the newly infected nondividing cells, inhibition of establishment of chronic infection, arrest of cells in the G2/M phase of the cell cycle, and inducing latent cells into a high level of virus production. Furthermore, successful infection of nondividing cells such as macrophages and resting T-lymphocytes requires Vpr to allow the newly synthesized viral DNA to reach the nucleus and be integrated into the cellular DNA.
Vpr promotes transport of the preintegration complex into the nucleus of nondividing cells
Vpr arrests cells in G2/M phase of the cell cycle
HIV-2 encodes Vpx and not Vpu. Vpx has homology to Vpr and shares the functions of Vpr. The functions of HIV-2 Vpr and Vpx have been segregated, including HIV-2 Vpr maintaining the ability to induce G2 arrest, whereas Vpx retains the ability to enhance infection of nondividing cells such as macrophages.
Vif (virion infectivity factor) increases the infectivity of HIV-1 in primary T cells and certain “nonpermissive” cells (macrophages) in culture. In the absence of Vif, the virus fails to complete reverse transcription in these cell types. “Permissive” cell lines infected by mutants defective in the vif gene produce normal yields of infectious virus. One possible explanation for this observation is that “permissive” cells contain a factor that can substitute for the missing Vif protein. Thus, one role of Vif may be to extend the host range of HIV-1 to cell types that would otherwise not be infected. Vif inhibits an RNA editing enzyme, APOBEC3G (apolipoprotein B, a member of innate immune system), which causes hyper-mutation in HIV DNA after reverse transcription and inhibiting viral replication.
Vif increases efficiency of infection and yield of virus
APOBEC3G has antiviral activity which is disrupted by Vif
Superimposed on this complex regulatory network is the fact that the viral promoter contains elements that are sensitive to specific cellular transcription factors. This observation may help explain why virus production in CD4+ T lymphocytes is greatly increased when the cells are activated. Clearly, the outcome of an HIV-1 infection is determined by a complex interplay among very large number of different factors.
Activation of CD4+ T lymphocytes increases virus production
![]() ACQUIRED IMMUNODEFICIENCY SYNDROME EPIDEMIOLOGY
ACQUIRED IMMUNODEFICIENCY SYNDROME EPIDEMIOLOGY
AIDS was first recognized in the United States in 1981, when it became apparent that an unusual number of rare skin cancers (Kaposi sarcoma) and opportunistic infections were occurring among male homosexuals. These patients were found to have a marked reduction in CD4+ T lymphocytes and were subject to a wide range of opportunistic infections normally controlled by an intact immune system. The disease was found to progress relentlessly to a fatal outcome and was first identified in male homosexuals, hemophiliacs, who were receiving blood-derived coagulation factors, and injection drug users.
First recognized in male homosexuals, hemophiliacs, and drug abusers
Retrospective serologic studies with material saved from patients in various studies indicate that HIV-1 infection was already occurring in Africa in the 1950s and in the United States in the 1970s. In 1985, HIV-2 was found to be endemic in parts of West Africa and to cause a milder immunodeficiency at a slower pace. To date, this virus has been relatively restricted geographically, although HIV-2 infections have occurred in the Western Hemisphere.
HIV-2 is endemic in West Africa
 Transmission
Transmission
HIV is transmitted between humans in three ways: Sexually, perinatally or vertically, and by exposure to contaminated blood or blood-derived products. The virus has been demonstrated in particularly high titers in semen and cervical secretions, and the majority of cases result from sexual contact—both homosexual and heterosexual. Heterosexual contact is the major route of transmission worldwide. Infection is facilitated by breaks in epithelial surfaces, which provide direct access to the underlying tissues or bloodstream. The relative fragility of the rectal mucosa and the large numbers of sexual contacts are probable contributing factors to the predominance of the disease among promiscuous male homosexuals. HIV-1 is transmitted heterosexually to females by vaginal or cervical routes, despite natural barriers, such as multicellular layers of squamous epithelial cells of vaginal mucosa and antimicrobial activity of cervicovaginal secretions.
The risk of transmission further increases with the disruption of integrity of the vaginal mucosa because of dry or traumatic sex and other infectious and inflammatory diseases. Once the virus is deposited in the vaginal mucosa, the virus can also traverse the vaginal mucous layer and probably reach the dendritic projections of Langerhans cells followed by infection of submucosal cells such as macrophages, T lymphocytes, and dendritic cells. Transmission appears to be more efficient from men to women, but the reverse is clearly documented. The probability of HIV transmission per unprotected sexual act is estimated at 0.0003 to 0.0015. The risk of perinatal transmission from an infected mother to her child has been estimated to range from 15% to 40% (average around 30%) without any ART. Mother-to-child transmission can occur prepartum (via transplacental route), intrapartum (through birth canal), and postpartum (through breast milk). It is important to note that ART during pregnancy can significantly reduce the risk of mother-to-child transmission of HIV-1.
Transmission is sexual and by exposure to infective fluids
Perinatal or vertical transmission can readily occur
Use of ART during pregnancy significantly reduces the risk of HIV-1 vertical transmission
Propagation of HIV-1 in cell culture and characterization of viral antigens allowed development of effective test procedures for detecting HIV infection. These almost eliminated the risk of transmission by blood transfusion; testing of donors and the use of recombinant or specially treated coagulation factors have now virtually eliminated these sources of infection. Until serologic tests for the infection became available in 1985, more than 10 000 cases of AIDS were probably acquired in the United States through blood transfusion, and about 80% of hemophiliacs treated with coagulation factors derived from pooled blood sources became infected. Transmission of infection by blood is now largely associated with sharing of needles and syringes by injecting drug users, and this has been an increasing source of the disease. In some areas of the world, the seroprevalence of HIV positivity among injecting drug users has been as high as 70%.
Testing of blood supply reduced risk
Intravenous drug abusers are at extremely high risk
Transmission of infection to healthcare workers through accidental needlesticks that are potentially contaminated is very rare (considerably <1%), presumably because the amount of infectious virus in the blood of infected person is small and because larger volumes or repeated exposures are needed for a significant chance of infection. Nevertheless, transmission has occurred from both clinical and laboratory exposure, and extreme care in handling needles, sharps, and so on, is necessary. Transmission does not occur through day-to-day nonsexual contact with infected individuals or through insect vectors, because of the fragility of the virus and the need for direct mucosal or blood contact. It is of interest that the virus has been detected in saliva, tears, urine, and breast milk. With the possible exception of breast milk, these sources have not been shown to be infectious. Breast milk is considered the major route of HIV-1 vertical transmission in developing countries.
Accidental needlesticks among healthcare workers mandate extreme care in prevention
Shed in breast milk, where it may infect breastfeeding infants
 Occurrence
Occurrence
By the end of 2012, 35.3 million (32.2-38.8 million) people were living with HIV globally, 2.3 million people (260 000 children) were newly infected with HIV (33% lower than 2001), and 1.6 million people died of AIDS (30% decline from 2005) worldwide. Of 35.3 million people globally living with HIV/AIDS at the end of 2012, 32.1 million were adults (17.3 million women) and 3.3 million were children. Although sub-Saharan Africa has 70% of all HIV-1-infected people in the world, about 5 million people are living with HIV in South, Southeast and East Asia. After sub-Saharan Africa, the most heavily affected area regions where 1% of the people are living with HIV in 2012 are the Caribbean, Eastern Europe, and Central Asia. Since 2001, the number of newly infected people in the Middle East and North Africa has increased by 35%. One of the striking trends of the HIV epidemic is that 45% of infected people are between the ages of 15 and 24 years.
Thirty-five million people living with HIV/AIDS worldwide
New infection declined by 33% in 2012 than 2001 worldwide
AIDS-related deaths decreased by 30% from 2005 to 2012 worldwide
At the end of 2010, approximately 1.1 million (1,144,500) people have been living with HIV/AIDS in the United States, including 44% blacks/African American, 33% whites, 19% Hispanics, and about 1.3% Asians/Pacific Islanders, and American Indians. Males accounted for 75.7% of the HIV-infected population, and more than one-half million people have died with HIV/AIDS. From 2006 to 2009, the annual rate of HIV transmission has declined by 9% (4.48 in 2006 and 4.19 in 2009) in the United States. The highest prevalence rates (51.5%) have been in men who have sex with men (MSM) followed by high-risk heterosexual contact (26.7%), intravenous drug users (15.9%), and those infected with both male-to-male and injection drug use (5.2%). In 2010, 47,500 new cases of HIV-1 infection were reported in the United States. The overall rate of HIV perinatal (mother-to-child) transmission in the United States decreased from 3.4 per 100 000 live births in 2007 to 2.1 in 2009.
United States has 1.1 million people living with HIV/AIDS
Black/African American represents 44% of all HIV infection
Males account for 75% of all HIV-infected population
Highest prevalence rates of 51.5% in MSM
Significant drop in mother-to-child transmission rates
The epidemiology of HIV infection is changing in the United States as the pandemic evolves and as the modes of transmission become more generally understood. The numbers and proportions of heterosexually transmitted, and/or drug abuse-related, cases are increasing, particularly among the poor and disadvantaged racial minorities. Antibody rates in prostitutes may be as high as 40%, depending partly on the degree of associated intravenous drug abuse. Prevalence rates in the heterosexual population, in general, are currently less than 1% but have been increasing. In 1985, in the United States, only 7% of the AIDS cases were in women; by 2010, the percentage had risen to 25%. Approximately 2000 newborns per year used to be infected by HIV perinatally, but this number has significantly decreased because more pregnant women receive ART during pregnancy. Black/African American patients now account for 44% of the cases, exceeding the percentages in non-Hispanic white men.
Prevalence rates have shifted over time, with increasing cases among women and economically disadvantaged minority groups
In contrast to the situation in the United States and Western Europe, heterosexual transmission is the primary route of transmission in Africa and Asia, where there is an approximately equal distribution of infection and disease between the sexes. This may be due to a high incidence in these areas of ulcerative genital lesions caused by other sexually transmitted diseases. These lesions facilitate passage of virus into the tissues of others during intercourse. In central and eastern Europe, where there is an emerging epidemic, the most common risk factor is intravenous drug use.
Men and women nearly equally infected in Africa and Asia
AIDS has been reported in more than 186 countries. The rate of new infection has dropped by 33% in 2012 compared with 2001. The sharpest declines since 2001 have occurred in the Caribbean (42% decline) and sub-Saharan Africa (25% decline). However, the epidemics in Latin America, Eastern Europe, and Central America have remained unchanged. In the Middle East and North Africa, the new infection has increased by 35% in 2012 from 2001, which is a reason of concern. Until recently, the Far East had few cases, but now there is epidemic spread and more than 5 million people are living with HIV/AIDS in 2012, especially in South and Southeast Asia (India, South China, Burma, Thailand, Cambodia, Vietnam, and Malaysia). HIV-2 infection is found primarily in West Africa and is spread by heterosexual transmission. Infection by this virus has, however, been reported in Europe in homosexual men, injection drug users, transfusion recipients, and hemophiliac men. For example, in Russia, there were between 730 000 and 1.3 million AIDS cases at the end of 2012 and a prevalence rate of HIV among adults of 1.4%, higher than before.
Increasingly widespread in Africa, South America, parts of Asia, and Russia
New infection declined in sub-Saharan Africa and the Caribbean
 HIV Clades and Geographic Distribution
HIV Clades and Geographic Distribution
Based on genetic variation, three classes of HIV-1 have developed worldwide, including M (major), O (outlying), and N (new). However, class M accounts for more than 90% of all HIV-1 cases globally and is further classified into several subtypes or clades, including A to H and recombinants. In addition, the demographic distribution of individuals infected with particular clades is becoming heterogeneous with the progressing pandemic. However, several clades predominate in a given region of the world, including clade B (Americas, Europe, and Australia), clade C (India and South Africa), clade E (Southeast Asia), and most major clades and recombinants (Africa). Among all clades circulating worldwide, clade C is found in more than 50% of HIV-1-infected people. The interclade variation in the envelope gene is in the range of 20% to 30%, whereas intraclade variation is 10% to 15%. There is also some argument that certain clades may have increased risk of transmission and progress to AIDS more rapidly than others. Understanding the immunopathogenesis of the emerging HIV-1 clades is key to vaccine development.
Class M most common
Clade or subtype B found in the United States
All clades and their recombinants found in Africa
Clade C in more than 50% of the infected population
PATHOGENESIS
HIV infection is typically characterized by: (1) an inefficient transmission of HIV (common route: sexual transmission); (2) an acute phase of intense viral replication and dissemination to lymphoid tissues (antiretroviral syndrome; flu- or mononucleosis-like illness in infected individuals); (3) activation of innate and adaptive immune response but unable to contain the highly replicating and mutating virus; (4) a chronic (persistent) asymptomatic phase (clinical latency) of continued viral replication and immune activation; and (5) an advanced phase of marked depletion of CD4 T lymphocytes (immune deficiency) leading to development of AIDS (opportunistic infections). Figure 18–5 summarizes the immunopathogenic events of HIV infection. Although the pathogenesis of HIV-1 infection is very complex, the following factors are likely to be important in the disease-causing process.
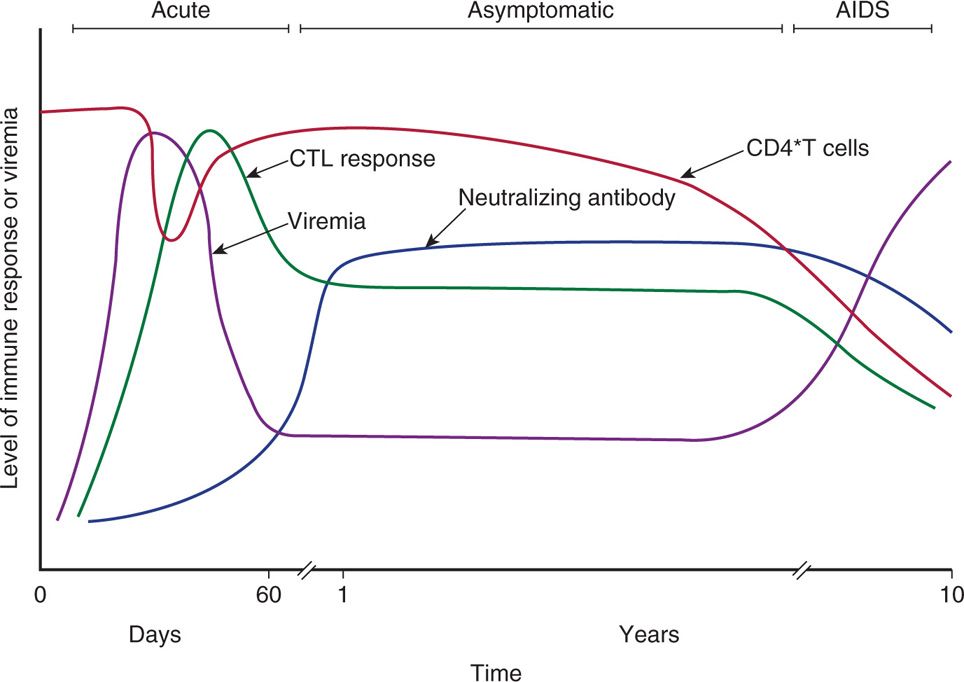
FIGURE 18–5. Temporal changes in viral load, anti-HIV immune responses, and total CD4 T-cell counts during various stages of HIV infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree