Large, enveloped, double-stranded DNA viruses
Eight HHVs cause a range of diseases
HERPESVIRUSES: GROUP CHARACTERISTICS
 VIROLOGY
VIROLOGY
All herpesviruses are morphologically similar, with an overall size of 180 to 200 nm. An example of a HSV virion is shown in Figure 14–1 as a representative virion structure for herpesviruses. The linear, double-stranded DNA genome and core proteins are encapsidated by an icosahedral capsid. The capsid is surrounded by the tegument, a relatively amorphous protein-filled region unique to herpesviruses. The tegument contains viral proteins and enzymes that play a structural role and are required immediately for viral replication upon initial infection. Virions have also been shown to contain both host and viral mRNAs that can be translated upon entry, but their role for infection is unknown. Surrounding the tegument is a lipoprotein envelope originally derived from the nuclear membrane of the infected host cell. The envelope contains multiple viral glycoproteins that act as viral binding, fusion, and entry proteins.
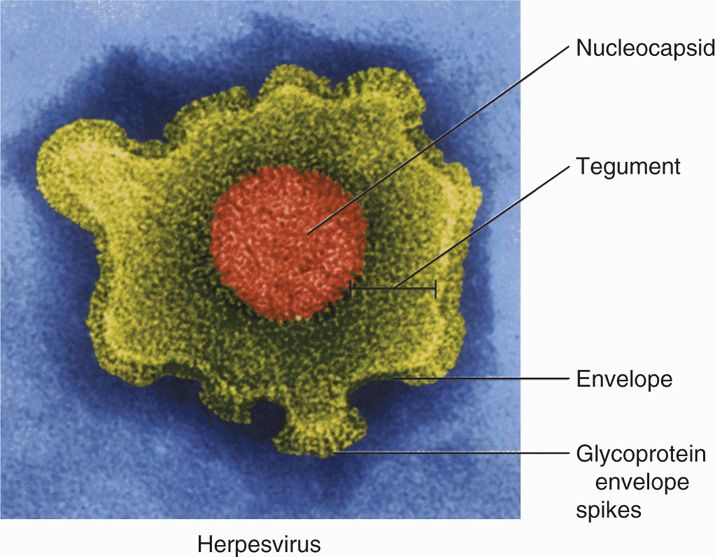
FIGURE 14–1. Virion structure of herpes simplex virus. (Reproduced with permission from Willey JM: Prescott, Harley, & Klein’s Microbiology, 7th edition. McGraw-Hill, 2008.)
Herpesviruses have an icosahedral capsid surrounded by a tegument and a lipid envelope
Herpesvirus genomes range from 125 kbp (VZV) to 240 kbp (CMV) of DNA, and code from around 75 viral proteins to over 200. However, it is now clear from next-generation RNA sequencing and proteomics that the coding capacity is much more complex than originally thought and many more genes may be expressed in the infected cell. Herpesviruses express the enzymes necessary for viral DNA synthesis allowing herpesviruses to infect both dividing and quiescent cells. The HHVs have six blocks of orthologous genes with interspersed species-specific viral genes. There are substantial differences in their genomic sequences particularly in the unique coding regions of each herpesvirus. Antigenic analysis of both conserved and nonconserved genes is an important means for differentiation among herpesviruses despite some cross-reactions (eg, between HSV-1 and HSV-2).
Herpesviruses encode a large number of proteins
Based on certain virologic similarities, the herpesviruses may be divided into three subfamilies α, β, and γ herpesviruses. HSV-1 and HSV-2, as well as VZV, are in the α subfamily, characterized by relatively rapid replication time and neuronal latency; CMV, HHV-6, and HHV-7 are in the β subfamily, characterized by slow replication rates and extremely limited host range; EBV and KSHV (HHV-8) are in the γ subfamily characterized by relatively rapid replication, replication in lymphocytes, and restricted host range. These characterizations are now made on the basis of genomic sequences but the original classifications have held up in the genomic era.
There are three genus of herpesvirus, α, β, and δ
Cell tropism for the individual viruses varies significantly. HSV has the widest range; it can infect many different animal hosts and replicates in numerous animal and human host cells, although in nature it is only found in humans. VZV infects only humans and is best grown in cells of human origin, although some laboratory-adapted strains can grow in primate cell lines. Human CMV replicates well only in limited human cell lines including human foreskin fibroblasts. HHV-6 and HHV-7 preferentially grow in T-lymphocyte cell cultures. EBV does not replicate in most commonly used cell culture systems, but can be grown in continuous human or primate lymphoblastoid cell cultures where it is present in the latent state. KSHV infects many cell types but generally establishes latency in cultured cells, where only a low percentage of the cells support active replication.
Herpes simplex has widest range of cell tropism
Human γ-herpesviruses generally establish latency in cultured cells
 Replication
Replication
The replication of HSV has been comprehensively studied and is representative of all herpesviruses, as shown in Figure 14–2. HSV generally causes lytic infection in epithelial cells and subsequently establishes latency in neuronal cells. The glycoproteins in the HSV envelope interact with cellular receptors, including initial binding to heparan sulfate and subsequent interaction with higher affinity receptors, leading to fusion with the cell membrane. For most herpesviruses fusion occurs at the cytoplasmic membrane but for some viruses or in specific cell types, the virus is first endocytosed and fusion occurs in the endosome. Fusion delivers tegument proteins into the cytoplasm as well as the capsid containing viral DNA. The capsid migrates to the nucleus where the genome is then extruded into the nucleus. In the nucleus, the viral genome circularizes and viral gene expression can be initiated. Transcription of the large, complex genome is sequentially regulated in three distinct classes of mRNAs: (1) immediate early (IE) mRNAs, also known as α genes, are synthesized 2 to 4 hours after infection. IE genes do not require de novo viral protein synthesis prior to expression and generally encode for proteins involved in regulation of viral gene expression and host defense; (2) early (E) mRNAs, or β genes, require prior protein synthesis of IE genes and generally encode proteins involved in viral replication (DNA binding proteins, DNA polymerase, thymidine kinase, etc), and (3) late (L) mRNAs, or γ genes, require viral genome replication for full expression and encode major structural proteins: capsid subunits, tegument proteins, and envelope glycoproteins. The early (E) proteins thymidine kinase and DNA polymerase are distinct from host cell enzymes and are, therefore, important targets of antiviral chemotherapy as discussed later. Synthesis of IE genes is required for E genes and E genes shut off the IE genes. The E genes are required for viral genomic replication, which in turn is required for optimal synthesis of most L genes. However, some of the late structural proteins are produced to lower levels independently of genome replication. Viral DNA replication occurs in a rolling circle fashion producing high–molecular-weight DNA concatemers. Genomic concatemers are cleaved and packaged into preassembled capsids in the nucleus.
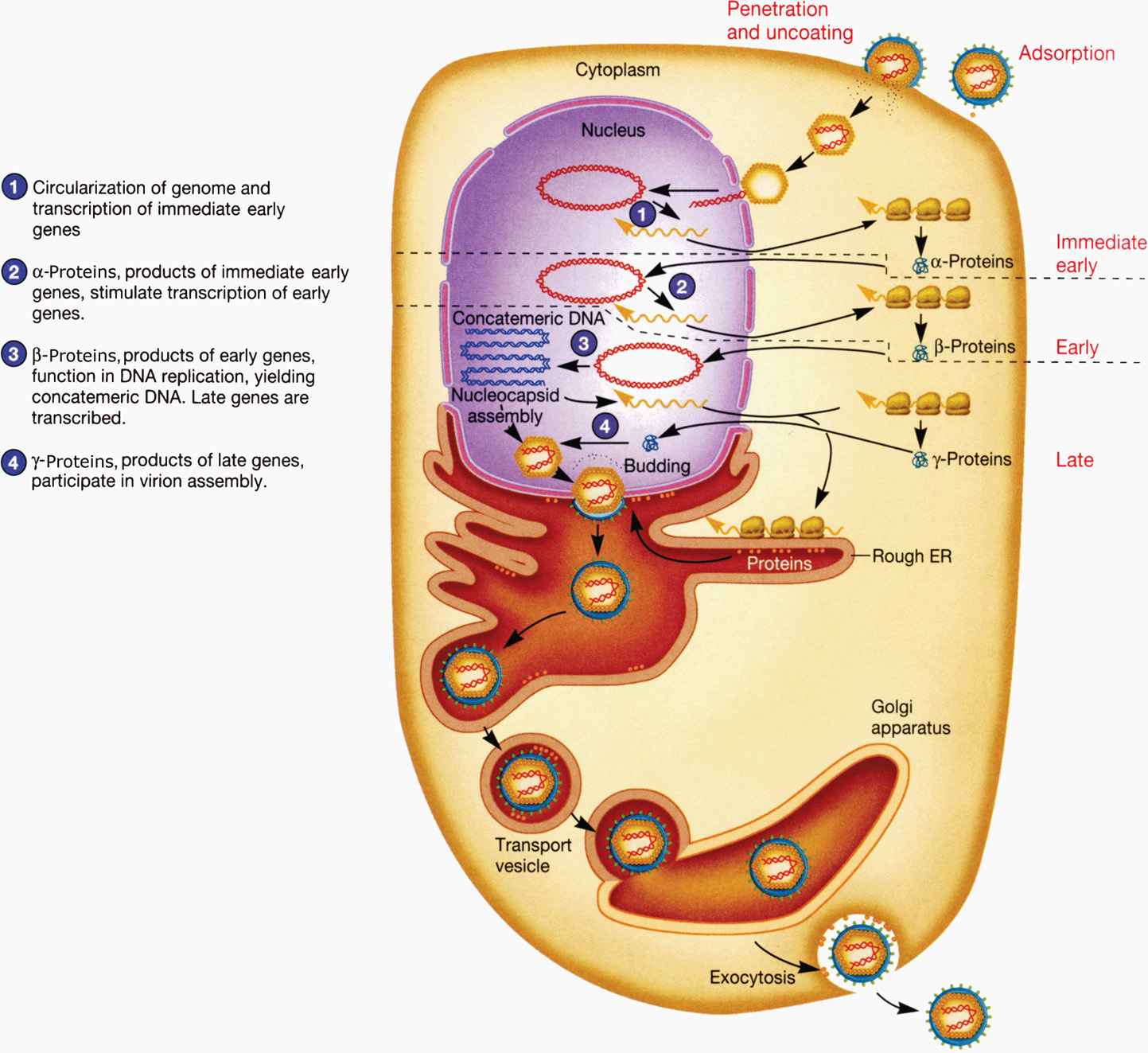
FIGURE 14–2. Replication cycle of herpes simplex virus 1. (Reproduced with permission from Willey JM: Prescott, Harley, & Klein’s Microbiology, 7th edition. McGraw-Hill, 2008.)
Three classes of mRNAs produced
Coordinated, sequential gene expression of the three classes occurs
Herpesvirsues assemble in the nuclei and a proteolytic cleavage event is necessary for the maturation of the capsid. A viral protease is responsible for the maturation. The envelope is acquired from the inner lamella of the nuclear membrane. Budding occurs at the nuclear membranes, and virions are then transported through the ER and Golgi. Re-envelopment and de-envelopment through the ER and Golgi and ultimately the cytoplasmic membrane is thought to occur. Host cell protein synthesis shut-off occurs for both α- and γ-herpesviruses and is thought to occur by cleavage of mRNAs by viral protein complexes. Ultimately, viral replication and host cell shut-off leads to death of the infected cell. Due to their long replication cycle, β-herpesviruses do not exhibit host cell shut-off.
Herpesvirus capsids assemble in the nucleus
α- and γ-Herpesviruses shut-off host cell protein synthesis
 Latency
Latency
In vivo, herpesviruses generally produce an initial lytic infection which is eventually controlled by the host immune system. However, during the initial infection, latent infection is also established. Latent infection allows all herpesvirus infection to be maintained for the life of the host. During latency, the genome of the virus is present in cells, but infectious virus is not recovered. The viral DNA is maintained as an episome in the nucleus. Integration is extremely rare. Latent infection is different from chronic infection in that the viral genome is not replicated and virions are not produced. During latency there is minimal viral gene expression with only 1 to 10 latent genes being regularly expressed, depending on the virus. Latent genes encode functions for maintenance of the viral episome, preventing host cell death and inhibiting the host immune response. Many herpesviruses also express microRNAs during latency. MicroRNAs are small regulatory RNAs that control gene expression without producing a peptide product. This allows the virus to alter host and viral gene expression without producing antigens that could be recognized by the host immune system. HSV-1 expresses only miRNAs during latent infection and no proteins, minimizing the ability of the immune system to recognize the latently infected cells. Periodic reactivation provides a constant source of new infections in the population. There is a range of reactivation rates depending on the virus and the host. In immunosuppressed patients, reactivation is more common and severe, indicating that the immune system must play a role in the suppression of reactivation.
All HHVs exhibit latent infection for the life of the host
Periodic reactivation provides a source of viral spread
HERPES SIMPLEX VIRUS
 VIROLOGY
VIROLOGY
The genomes of herpes simplex 1 and 2 (HSV-1 and -2, respectively) are both approximately 150 kbp of DNA. Although they are distinct epidemiologic and antigenic viruses, their genomes contain approximately 50% homology, making them the most closely related HHVs. Nearly all of the genes of HSV-1 have co-linear homologs in HSV-2. HSV-1 and HSV-2 share many glycoprotein and structural antigens, but differences in glycoprotein B, among other glycoproteins, enable them to be distinguished antigenically. The viruses can also be distinguished by PCR assays as well.
HSV-1 and HSV-2 are closely related
HSV-1 and HSV-2 can be distinguished epidemiologically, antigenically, and by DNA homology
![]() HERPES SIMPLEX DISEASE
HERPES SIMPLEX DISEASE
EPIDEMIOLOGY
HSV-1 is more often associated with disease “above the waist” or facial herpes, whereas HSV-2 is most often associated with genital infections or “below the waist” infections. However, an increasing number of genital infections are caused by HSV-1. HSV-1 is most often spread by direct contact of mucosal tissue, especially the lip area. Both HSV-1 and HSV-2 are prevalent worldwide. There are no known animal vectors for HSV-1 or HSV-2. Seroepidemiologic studies indicate that the prevalence of HSV antibody varies by age and socioeconomic status of the population studied. In most developing countries, up to 90% of the population has HSV-1 antibody by the age of 30 years. In the United States, HSV-1 antibody is found in 18% to 35% of children by the age of 5 years with the percentages varying according to the population studied. In the United States, the seroprevalence rises to approximately 60% to 70% by the age of 30 years for middle-class populations; among lower socioeconomic groups, however, the percentage is higher. Detection of HSV-2 antibody before puberty is less common. Direct sexual transmission is the major mode of spread. Approximately 15% to 30% of sexually active adults in Western industrialized countries have HSV-2 antibody and seropositive rates are positively correlated with the number of sexual partners. The virus can be isolated from the cervix and urethra of approximately 5% to 12% of adults attending sexually transmitted disease clinics; many of these patients are asymptomatic or have small, unnoticed lesions on penile or vulvar skin. Asymptomatic shedding accounts for transmission from a partner who has no active genital lesions and often no history of genital herpes. Genital herpes is not a reportable disease in the United States, but it is estimated that more than 1 million new cases occur per year.
HSV-1 is highly prevalent in the population
HSV-2 is associated with sexual activity
There are no known animal vectors for HSV-1 or HSV-2
PATHOGENESIS
 Acute Infections
Acute Infections
Both HSV-1 and HSV-2 initially infect and replicate in the mucoepithelial cells and initiate lytic or productive infection at the site of contact. Pathologic changes during acute infections consist of development of multinucleated giant cells (Figure 14–3), ballooning degeneration of epithelial cells, focal necrosis, eosinophilic intranuclear inclusion bodies, and an inflammatory response characterized by an initial polymorphonuclear neutrophil (PMN) infiltrate and a subsequent mononuclear cell infiltrate. Subsequently, the virus spreads to local sensory neurons and travels in retrograde fashion to the sensory ganglia that innervate the site of infection. In the case of facial herpes, the virus infects neurons in the trigeminal ganglia and in the case of genital herpes, the dorsal root or sacral ganglia. Latency is established in the ganglionic neurons. A round of replication may occur in the ganglia, but is not necessary for the establishment of latency.
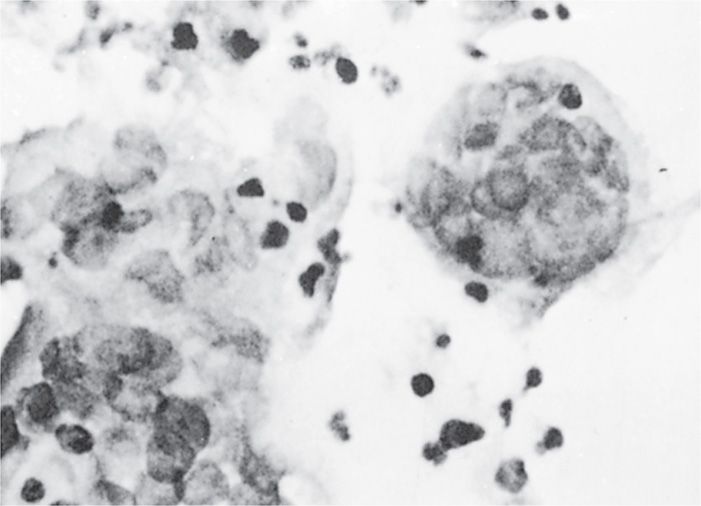
FIGURE 14–3. Multinucleated giant cells from herpes simplex virus lesion.
Lytic replication at the site of infection produces inflammation and giant cells
Virus can infect and spread to neurons and establishes latency in sensory ganglia
 Latent Infection
Latent Infection
In humans, latent infection by HSV-1 has been demonstrated in trigeminal, superior cervical, and vagal nerve ganglia, and occasionally in the S2-S3 dorsal sensory nerve root ganglia. Latent HSV-2 infection has been demonstrated in the sacral (S2-S3) region. Latent infection of neurons by HSV does not result in the death of the cell. Multiple viral genomes exist in a circular extrachromosomal form in the nucleus, and transcription of only a small portion of the viral genome occurs, limited to a single viral transcript, the latency-associated transcript (LAT). The LAT encodes a number of miRNAs that serve as regulatory RNAs that can alter host cell gene expression without expressing foreign proteins. Because latency is established in nondividing neurons, HSV does not encode direct functions to maintain the viral episome. Latent infection does not require synthesis of early or late viral polypeptides and, therefore, antiviral drugs directed at the thymidine kinase enzymes or viral DNA polymerase do not eradicate the virus in its latent state.
HSV genomes exist as episomes during latency
There is no synthesis of early or late viral polypeptides during latent infection
A subset of patients exhibit overt clinical disease from reactivation of the virus. This can occur over the entire life of the host. However, people without clinical disease will also reactivate and spread the virus through subclinical shedding. The mechanisms by which latent infection is reactivated are unknown. Precipitating factors that are known to initiate reactivation of HSV and subsequent clinical disease include exposure to ultraviolet light, sunlight, fever, excitement, emotional stress, and trauma (eg, oral intubation). However, it is clear that reactivation and viral shedding between overt disease episodes is common, and may account for much of the spread of the virus. Upon reactivation, the virus initiates some form of lytic replication and virus travels down the neuronal axons, most often to a site near the site of initial infection. The epithelium is subsequently infected and leads to localized spread and ulceration in a subset of reactivations.
Reactivation can be induced by sun exposure, fever, trauma, or stress
Only a subset of infected patients exhibit overt clinical disease
IMMUNITY
Host factors have a major effect on clinical manifestations of HSV infection. Many episodes of HSV infection are either asymptomatic or mildly symptomatic. Initial symptomatic clinical episodes of the disease are often more severe than recurrent episodes, likely due to the presence of anti-HSV antibodies and immune lymphocytes in persons with recurrent infections. Prior infection with HSV-1 may provide some protection against or shorten the duration of symptoms and lesions from subsequent infection with HSV-2 as a result of some degree of cross-protection, though dual infections certainly occur.
Both cellular and humoral immune responses are important in immunity to HSV. Neutralizing antibodies directed against HSV envelope glycoproteins appear to be important in preventing exogenous reinfection. Antibody-dependent cellular cytotoxicity (ADCC) may be important in limiting early spread of HSV. By the second week after infection, cytotoxic T lymphocytes can be detected, which have the ability to destroy HSV-infected cells before completion of the replication cycle. Conversely, in immunosuppressed patients, especially those with depressed cell-mediated immunity, reactivation of HSV may be associated with prolonged viral excretion and persistence of lesions. During latency, the HSV-1 and HSV-2 do not express viral proteins and are thus effectively hidden from the immune system. However, the immune system plays a role in keeping latency in check as immunosuppression leads to more common reactivation. It is possible that the virus may initiate reactivation more often than previously thought and that the adaptive immune system shuts down those cells once they reactivate.
ADCC may limit early spread of HSV; cytotoxic T lymphocytes destroy HSV-infected cells
Reactivation is controlled by the adaptive immune system
HSV express a number of genes that have evolved to inhibit innate and adaptive immunity. There are a number of genes capable of inhibiting interferon pathways at different stages. HSV-1 also encodes an IE protein that blocks peptide loading onto MHC-I and prevents the complex from reaching the cell surface. Additionally, HSV inhibits apoptosis during both latent and lytic phases.
HSV encodes inhibitors of innate and adaptive immunity
 CLINICAL ASPECTS
CLINICAL ASPECTS
MANIFESTATIONS
 Herpes Simplex Type 1
Herpes Simplex Type 1
Infection with HSV-1 is more often, associated with facial disease though it causes an increasing number of genital infections. It consists characteristically of grouped or single vesicular lesions that become pustular and coalesce to form single or multiple ulcers. On dry surfaces, these ulcers scab before healing; on mucosal surfaces, they reepithelialize directly. HSV can be isolated from almost all ulcerative lesions, but the titer of virus decreases as the lesions evolve. Infections generally involve ectoderm (skin, mouth, conjunctiva, and the nervous system).
Vesicular lesions become pustular and then ulcerate
Primary infection with HSV-1 is most often asymptomatic. When symptomatic, typically in children, it appears most frequently as gingivostomatitis, with fever and ulcerative lesions involving the buccal mucosa, tongue, gums, and pharynx. The lesions are painful, and the acute illness usually lasts 5 to 12 days. During this initial infection, HSV spreads to the sensory neurons and becomes latent within neurons of the trigeminal ganglia, the ganglia that innervated the oral and nasal area.
Primary infections are often asymptomatic
Lesions usually recur on a specific area of the lip and the immediate adjacent skin; these lesions are referred to as mucocutaneous and are commonly called “cold sores” or “fever blisters” (Figure 14–4). Lesions are typically unilateral. Their recurrence may be signaled by premonitory tingling or burning in the area. Systemic complaints are unusual, and the episode generally lasts approximately 7 days. It should be noted that HSV may be reactivated and excreted into the saliva with no apparent mucosal lesions present. HSV has been isolated from saliva in 5% to 8% of children and 1% to 2% of adults who were asymptomatic at the time.
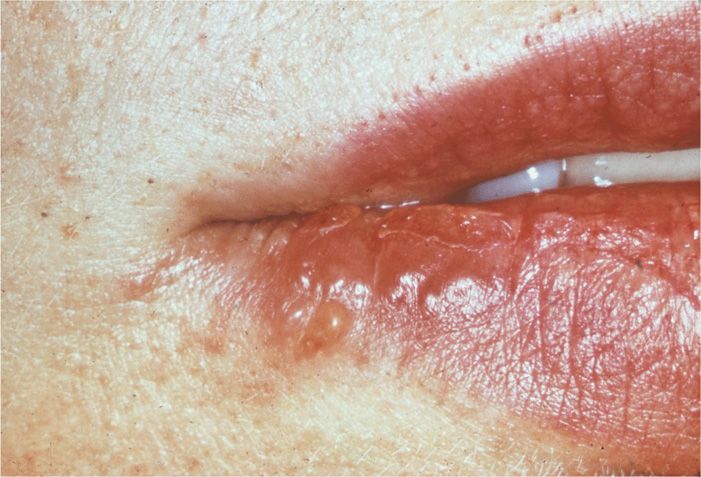
FIGURE 14–4. Coalesced, localized lesions characteristic of reactivated herpes simplex virus type 1 (HSV-1) infection.
Recurrent cold sores are usually unilateral
Virus in saliva with asymptomatic reactivation
In rare instances, HSV infects the finger or nail area. This infection, termed herpetic whitlow, usually results from the inoculation of infected secretions through a small cut in the skin or from needle sticks. Painful vesicular lesions of the finger develop and pustulate; they are often mistaken for bacterial infection and mistreated accordingly.
Herpetic whitlow mimics bacterial paronychia
HSV infection of the eye is one of the most common causes of corneal damage and blindness in the developed world. Infections usually involve the conjunctiva and cornea, and characteristic dendritic ulcerations are produced. With recurrence of disease, there may be deeper involvement with corneal scarring. Occasionally, there may be extension into deeper structures of the eye, especially when topical steroids are used.
Herpetic corneal and conjunctival infection can cause blindness
In rare cases, encephalitis may result from HSV-1 infection. Most cases occur in adults with high levels of anti-HSV-1 antibody, suggesting reactivation of latent virus in the trigeminal nerve root ganglion and extension of productive (lytic) infection into the temporoparietal area of the brain. Primary HSV infection with neurotropic spread of the virus from peripheral sites up the olfactory bulb into the brain may also result in parenchymal brain infection. Classically, HSV encephalitis affects one temporal lobe, leading to focal neurologic signs and cerebral edema. If untreated, mortality rate is approximately 70%. Clinically, the disease can resemble brain abscess, tumor, or intracerebral hemorrhage. Rapid diagnosis by polymerase chain reaction (PCR) of cerebrospinal fluid (CSF) has replaced brain biopsy as the diagnostic test. Intravenous acyclovir reduces the morbidity and mortality of the disease, especially if treatment is initiated early. There are a small number of familiar genetic mutations leading to increased herpes encephalitis. These mutations appear to be in genes involved in specific innate immune responses.
HSV encephalitis typically localized to temporal lobe and has high mortality without treatment
Rapid PCR diagnosis of CSF allows antiviral therapy
 Herpes Simplex Type 2
Herpes Simplex Type 2
Genital herpes is a significant sexually transmitted disease. Both HSV-1 and HSV-2 can cause genital disease, and the symptoms and signs of acute infection are similar for both viruses. Seventy percent of the first episodes of genital HSV infection in the United States are caused by HSV-2, and genital HSV-2 disease is also more likely to recur than genital HSV-1 infection. Ninety percent of the HSV-2 antibody-positive patients have never had a clinically evident genital HSV episode. In many instances, the first clinical episode is years after primary infection.
HSV-2 associated with genital infections
HSV-2 infection patients often do not exhibit overt disease
Primary Genital Herpes Infection
For individuals who develop clinically evident primary genital HSV disease, the mean incubation period from sexual contact to onset of lesions is 5 days. Lesions begin as small erythematous papules, which soon form vesicles and then pustules (Figure 14–5). Within 3 to 5 days, the vesiculopustular lesions break to form painful coalesced ulcers that subsequently dry; some form crusts and heal without scarring. With primary disease, the genital lesions are usually multiple (mean number 20), bilateral, and extensive. The urethra and cervix are also infected frequently, with discrete or coalesced ulcers on the exocervix. Bilateral enlarged tender inguinal lymph nodes are usually present and may persist for weeks to months. About one-third of patients show systemic symptoms such as fever, malaise, and myalgia, and approximately 1% develop aseptic meningitis with neck rigidity and severe headache. First episodes of disease last an average of 12 days.
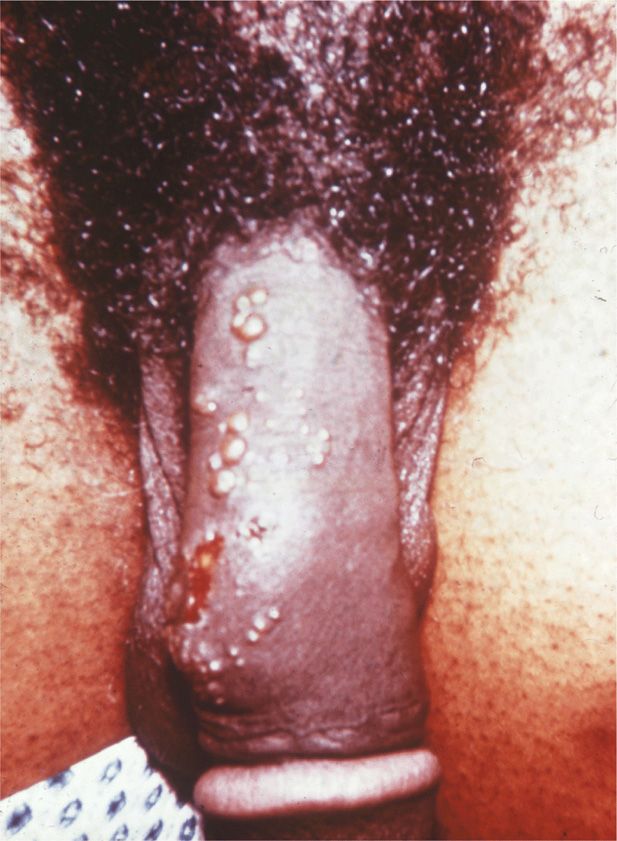
FIGURE 14–5. Multiple grouped vesicles of primary genital herpes.
Multiple painful vesiculopustular lesions
Systemic symptoms and adenopathy can occur
Recurrent Genital Herpes Infection
In contrast to primary infection, recurrent genital herpes is a disease of shorter duration, usually localized in the genital region and without systemic symptoms. A common symptom is prodromal paresthesias in the perineum, genitalia, or buttocks that occur 12 to 24 hours before the appearance of lesions. Recurrent genital herpes usually presents with grouped vesicular lesions in the external genital region. Local symptoms such as pain and itching are mild, lasting 4 to 5 days, and lesions usually last 2 to 5 days.
Prodromal paresthesias and shorter duration
At least 80% of patients with primary, symptomatic, genital HSV-2 infection develop recurrent episodes of genital herpes within 12 months. In patients whose lesions recur, the median number of recurrences is four or five per year. They are not evenly spaced, and some patients experience a succession of monthly attacks followed by a period of quiescence. Over time, the number of recurrences decreases by a median of one-half to one recurrence per year. Recurrences result from reactivation of virus from dorsal root ganglia. Recurrent infections due to reinfection with a different strain of HSV-2 are extremely rare. Recurrent viral shedding from the genital tract often occurs without clinically evident disease.
Recurrent episodes common; may involve shedding without lesions
 Neonatal Herpes
Neonatal Herpes
Neonatal herpes usually results from transmission of virus during delivery through infected genital secretions from the mother. In utero infection, though possible, is uncommon. In most cases, severe neonatal herpes is associated with primary infection of a seronegative woman at or near the time of delivery. This results in an intense viral exposure of a seronegative infant as it passes through the birth canal. The incidence of symptomatic neonatal herpes simplex infection varies greatly among populations, but it is estimated at between 1 per 6000 and 1 per 20 000 live births in the United States. Because a normal immune response is absent in the neonate born to a mother with recent primary infection, neonatal HSV infection is an extremely severe disease with an overall mortality rate of approximately 60%, and neurologic sequelae are high in those who survive. Manifestations vary. Some infants show disseminated vesicular lesions with widespread internal organ involvement and necrosis of the liver and adrenal glands; others have involvement of the central nervous system only, with listlessness and seizures.
Primary infection of mother late during pregnancy is the most common cause
Usually transmitted during birth and leads to high mortality if disseminated
DIAGNOSIS
Herpes simplex viruses (HSVs) can be cultured in cell lines inoculated with infected secretions or lesions. The cytopathic effects of HSV can usually be demonstrated 24 to 48 hours after inoculation of the culture. Isolates of HSV-1 and HSV-2 can be differentiated by staining virus-infected cells with type-specific monoclonal antibodies. A direct smear prepared from the base of a suspected lesion and stained by either Giemsa or Papanicolaou method may show intranuclear inclusions or multinucleated giant cells typical of herpes (Tzanck test), but this is less sensitive than viral culture and not specific. Similar changes can be seen in cells infected with VZV. Enzyme immunoassays and immunofluorescence are rapid and relatively sensitive assays for direct detection of herpes antigen in lesions. Although early versions of these noncultural tests lacked sensitivity, more recent procedures have correlations with culture that approach 90%. Serology should not be used to diagnose active HSV infections, such as those affecting the genital or central nervous systems; frequently there is no change in antibody titer when reactivation occurs. Serology can be useful in detecting those with asymptomatic HSV-2 infection. PCR of CSF and blood is the best test to diagnose HSV encephalitis.
Virus can be isolated from lesions and grown in cell culture
HSV-1 and HSV-2 distinguished by type-specific monoclonal antibodies
PCR of CSF used for diagnosis of herpes encephalitis
TREATMENT
Several antiviral drugs that inhibit HSV have been developed. The most commonly used is the nucleoside analog acyclovir, which is converted by a viral enzyme (thymidine kinase) to a monophosphate form and then by cellular enzymes to the triphosphate form, which is a potent inhibitor of the viral DNA polymerase through chain termination. Acyclovir significantly decreases the duration of primary infection and has a lesser but definite effect on recurrent mucocutaneous HSV infections. If taken daily, it can also suppress recurrences of genital and oral-labial HSV. In its intravenous form, it is effective in reducing mortality of HSV encephalitis and neonatal herpes. Acyclovir-resistant HSV has been recovered from immunocompromised patients with persistent lesions, especially those with acquired immunodeficiency syndrome (AIDS). Foscarnet is active against acyclovir-resistant HSV.
Intravenous acyclovir effective in HSV encephalitis and neonatal disease
The US Food and Drug Administration has approved both valacyclovir and famciclovir for the treatment of recurrent genital HSV. Valacyclovir is an oral prodrug of acyclovir with better bioavailability than acyclovir (54% compared with 15-20%). It is rapidly converted to acyclovir and, in every characteristic except absorption, it is identical with the parent compound. Valacyclovir is not more effective than acyclovir, but can be given in lower doses and less frequently (500 mg twice daily). Famciclovir is the prodrug of another guanosine nucleoside analog, penciclovir. The bioavailability of famciclovir is also high (77%). After conversion, penciclovir must be phosphorylated, similarly to acyclovir. Penciclovir has a longer tissue half-life than acyclovir and can be given as 125 mg twice daily for treatment of recurrent genital HSV. Valacyclovir and famciclovir are now also approved for chronic suppression of recurrent genital HSV. Valacyclovir taken daily was shown to decrease spread between discordant partners in a long-term study.
Acyclovir or prodrugs can decrease duration of acute and recurrent disease
PREVENTION
Avoiding contact with individuals with lesions reduces the risk of spread; however, virus may be shed asymptomatically and transmitted from the saliva, urethra, and cervix by individuals with no evident lesions. Safe sexual practices should reduce transmission. Acyclovir has been shown to reduce asymptomatic shedding and transmission of genital herpes, especially from males to females. Because of the high morbidity and mortality rates of neonatal infection, special attention must be paid to preventing transmission during delivery. Where active HSV lesions are present on maternal tissues, Cesarean section delivery may be used to minimize contact of the infant with infected maternal genital secretions, but Cesarean delivery may not be effective if rupture of the membranes precedes delivery by more than several hours. Avoiding the birth canal is particularly important if the mother has a primary HSV infection late during pregnancy. There is no current HSV vaccine available though a number have been under study for years.
Cesarean section may be performed to avoid neonatal infection
VARICELLA-ZOSTER VIRUS
 VIROLOGY
VIROLOGY
Varicella-zoster virus (VZV) has the same general structural and morphologic features of herpes simplex and other HHVs, but it contains distinct glycoproteins and is antigenically different. The genome of VZV is approximately 125 kbp, which is the smallest genome of the HHVs. Similar to HSV, VZV encodes a thymidine kinase and is responsive to acyclovir. Cellular features of infected cells such as multinucleated giant cells and intranuclear eosinophilic inclusion bodies are similar to those of HSV. VZV is more difficult to isolate in cell culture than HSV. The virus often remains attached to the membrane of the host cell with less release of virions into fluids and thus does not spread well in culture. However, this is not the case in vivo, where it is the most infectious human herpes virus.
![]() VARICELLA-ZOSTER DISEASE
VARICELLA-ZOSTER DISEASE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


