MUMPS
 VIROLOGY
VIROLOGY
Mumps virus is a paramyxovirus, and only one major antigenic type is known. Like fellow members of its genus, it contains a single-stranded, negative-sense RNA genome, and a nucleocapsid that is surrounded by a matrix protein followed by a lipid bilayer envelope (see Figure 9–4). Two glycoproteins are on the surface of the envelope; one mediates hemagglutination and neuraminidase (HN) activity, and the other is responsible for viral lipid membrane fusion (F) to the host cell. Similar to other paramyxoviruses, mumps virus initiates infection by attachment of the HN spike to sialic acid on the cell surface, and F protein promotes fusion with the plasma membrane. It replicates in the cytoplasm by using its own RNA-dependent RNA polymerase, and the progeny viruses are released by budding from the cell membranes. Details about the structure of the virus are described in Chapter 9 and replication of negative sense RNA viruses (paramyxoviruses) are in Chapter 6.
Enveloped, negative sense single-stranded RNA virus with hemagglutinating and neuraminidase activity (HN) and fusion protein F
![]() MUMPS INFECTION
MUMPS INFECTION
EPIDEMOLOGY
Mumps infection is observed to occur most frequently in the 5- to 15-year age group. Infection is rarely seen in the first year of life. Mumps is transmitted from person-to-person through the respiratory route (aerosol), such as in respiratory viruses. Although approximately 85% of susceptible household contacts acquire infection, approximately 30% to 40% of these contacts do not develop clinical disease. The disease is communicable from approximately 7 days before until 9 days after onset of illness; however, virus has been recovered in urine for up to 14 days after onset. The highest incidence of infection is usually during the late winter and spring months, but it can occur during any season.
High frequency in 5-15 years age group
Person-to-person transmission via respiratory route
High infectivity is present before and after onset of illness
PATHOGENESIS
After initial entry into the respiratory tract, the virus replicates locally in the respiratory tract epithelium and local lymph nodes. Replication is followed by viremic dissemination to target tissues such as the salivary glands (parotid glands) and central nervous system (CNS). It is also possible that, before development of immune responses, a secondary phase of viremia may result from virus replication in target tissues (eg, initial parotid glands involvement with later spread to other organs). There is painful swelling of one or both parotid glands. Viruria is common, probably as a result of direct spread from the blood into the urine, in addition to active viral replication in the kidney. The tissue response is that of cell necrosis and inflammation, with predominantly mononuclear cell infiltration. In the salivary glands, swelling and desquamation of necrotic epithelial lining cells, accompanied by interstitial inflammation and edema, may be seen within dilated ducts. The pathogenesis, clinical disease, and immune response are summarized in Figure 10–1.
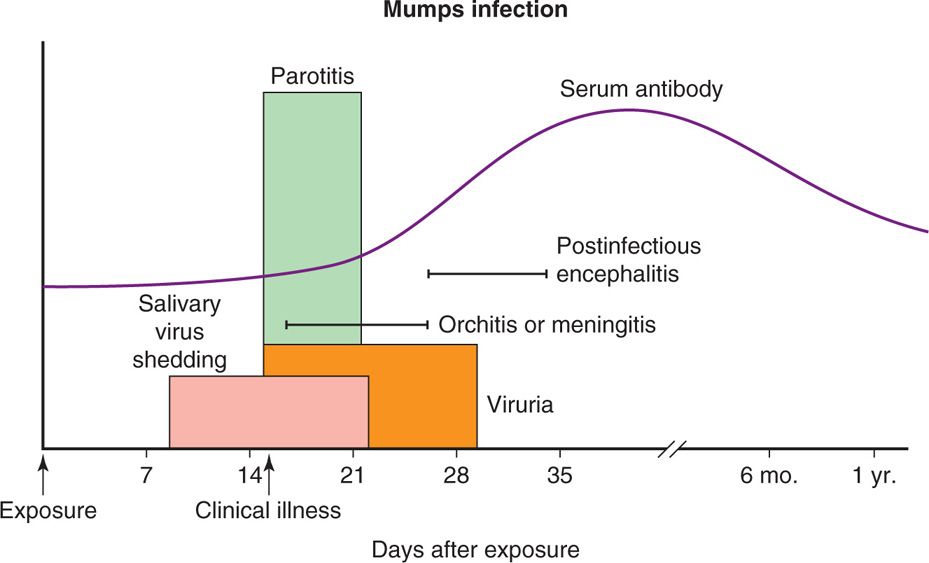
FIGURE 10–1. Pathogenesis of mumps virus infection. After exposure, the virus multiplies in the respiratory tract epithelium (incubation period of 16-18 days on average) and spreads to local lymph nodes followed by viremia, which spreads throughout the body. The virus is also shed in salivary glands and urine. Fever follows painful swelling of one or both parotid glands (parotitis). The symptoms lasts 7 to 10 days. Humoral and cellular immune responses eliminate the virus from the infected hosts. IgM appears early in infection followed by IgG that persists for life. Some of the common complications of the mumps include meningitis, encephalitis, orchitis, oophoritis, pancreatitis, and myocarditis.
Viremic phase follows local replication
Virus replication in salivary glands with painful swelling of parotid glands
Viruria is common
Virus dissemination to almost all body organs
IMMUNITY
As in most viral infections, the early antibody response in mumps is predominantly with immunoglobulin M (IgM), which is replaced gradually over several weeks by a specific IgG antibody. The latter persists for a lifetime, but can often be detected only by specific neutralization assays. Immunity is associated with the presence of neutralizing antibody. The role of cellular immune responses has also been investigated and found to contribute both to the pathogenesis of the acute disease and to recovery from infection. After primary infection, immunity to reinfection is, virtually, always permanent.
Neutralizing antibody is protective
 CLINICAL ASPECTS
CLINICAL ASPECTS
MANIFESTATIONS
After an incubation period of 12 to 29 days (average, 16-18 days), the typical case of mumps is characterized by fever and swelling with tenderness of the salivary glands, especially the parotid glands (Figure 10–2). Swelling may be unilateral or bilateral and persists for 7 to 10 days. Several complications can occur, usually within 1 to 3 weeks of onset of illness. All appear to be a direct result of virus spread to other sites and illustrate the extensive tissue tropism of mumps.
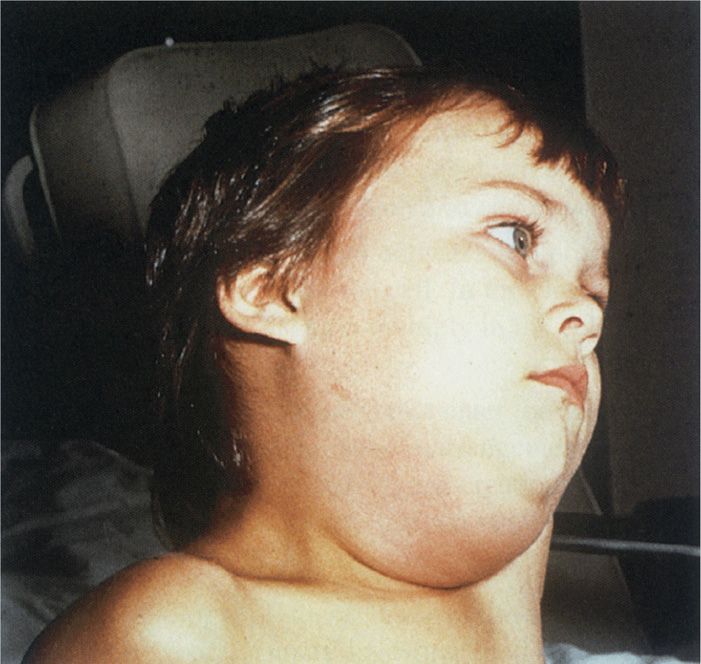
FIGURE 10–2. Mumps parotitis. The swelling just below the earlobe is due to enlargement of the parotid gland. (Reproduced with permission from Nester EW: Microbiology: A Human Perspective, 6th edition. 2009.)
Incubation period is 12 to 29 days
Parotitis is unilateral or bilateral
The common complications of mumps infection, which can occur without parotitis, include the following:
Meningitis: Approximately 10% of all infected patients develop meningitis. It is usually mild, but can be confused with bacterial meningitis. In approximately one third of these cases, associated or preceding evidence of parotitis is absent.
Encephalitis: Encephalitis is occasionally severe.
Spinal cord and peripheral nerves are involved, causing transverse myelitis and polyneuritis in rare cases.
Pancreatitis: Pancreatitis is suggested by upper abdominal pain, nausea, and vomiting.
Orchitis: Orchitis (inflammation of the testes) is estimated to occur in 10% to 20% of infected men, which could be unilateral or bilateral in postpubertal men. Although subsequent sterility is a concern, it appears that this outcome is rare.
Oophoritis: Oophoritis (inflammation of ovaries) is an unusual, usually benign, inflammation of the ovarian glands.
Other rare and transient complications include myocarditis, nephritis, arthritis, thyroiditis, thrombocytopenic purpura, mastitis, and pneumonia. Most complications resolve without sequelae within 2 to 3 weeks. However, occasional permanent effects have been noted, particularly in severe CNS infection, in which sensorineural hearing loss and other impairment can occur.
DIAGNOSIS
Mumps virus can be readily isolated early in the illness from the saliva, pharynx, and other affected sites, such as the cerebrospinal fluid (CSF). In addition, the urine is an excellent source for virus isolation. Mumps virus grows well in primary monolayer cell cultures derived from monkey kidney, producing syncytial giant cells and viral hemagglutinin. Rapid diagnosis can be made by direct detection of viral antigen in pharyngeal cells or urinary sediment, and by polymerase chain reaction (PCR).
Cell culture of saliva, throat, CSF, and urine; PCR
The usual serologic tests are enzyme immunoassay (EIA) or enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence to detect IgM- and IgG-specific antibody responses. Other serologic tests are also available, such as complement fixation, hemagglutination inhibition, and neutralization. Of these, the neutralization test is the most sensitive for detection of immunity to infection.
Viral antigen detected by immunofluorescence and ELISA
ELISA serology detects IgM and IgG
PREVENTION
There is no specific treatment available for mumps. Since 1967, a live attenuated vaccine that is safe and highly effective has been available. As a result of its routine use, infections in the United States before 2005 were exceedingly rare; however, in late 2005 and into 2006, a large outbreak (>6000 proved or probable cases) developed in Iowa and eight neighboring midwestern states. Most occurred in persons 18 to 25 years of age, many of whom had been previously vaccinated at least once. The mumps strain identified was genotype G, a common strain similar to the one that involved more than 70 000 cases in the United Kingdom from 2004 to 2006. Thus, it has been reemphasized that a two-dose vaccine regimen is essential to ensure adequate immunity. The vaccine is produced by serial propagation of virus in chick embryo cell cultures. It is commonly combined with measles, rubella, and varicella (chicken pox virus) vaccines (MMRV), and given as a single injection to a child at 12 to 15 months of age. A second dose of MMRV is recommended at 4 to 6 years of age; those who have missed the second dose should receive it no later than 11 to 12 years of age. A single dose causes seroconversion in approximately 80% of recipients, and it increases only to about 90% after two doses. The vaccine must be given at least 2 to 4 weeks before exposure to be at all effective in postexposure prophylaxis. In approximately 10% of the people who have received the two doses of the vaccine and, probably, partially sero-converted could still be infected with the mumps virus because of living in close contact, such as at schools and colleges. In 2009 to 2010, a mumps outbreak occurred in the northeastern United States, spreading in a camp, followed by spread in schools and household. This infection most likely came through a boy who traveled to the United Kingdom and later joined the camp.
Live attenuated vaccine ideally given at 12 to 15 months of age, repeated at 4 to 6 years
MEASLES
 VIROLOGY
VIROLOGY
The measles virus is classified in the paramyxovirus family, genus Morbillivirus. It contains a linear, negative-sense, single-stranded RNA genome surrounded by a helical nucleocapsid protein and a lipid bilayer envelope containing two glycoprotein projections (peplomers), two envelope glycoproteins, namely hemagglutinin (H), that mediates virus adsorption to the cell surfaces, and fusion (F) protein that mediates cell fusion, hemolysis, and viral entry into the cell. On the inside of the envelope surface, there is a matrix (M) protein that plays a key role in viral assembly. The virions also contain the viral RNA polymerase (RNA-dependent RNA polymerase) required for viral RNA transcription and replication. Unlike the mumps virus, the measles virus lacks neuraminidase (N) activity. The receptor for measles virus is CD46 (membrane cofactor protein), a regulator of complement activation. Replication of measles virus is similar to other paramyxoviruses, which is described in Chapter 6 for negative-sense RNA viruses. Only a single serotype restricted to human infection is recognized; however, subtle antigenic and genetic variations among wild-type measles strains do occur. These variations can be determined by sequencing analyses, enabling more precise epidemiologic tracking of outbreaks and their origins. Such ongoing molecular surveillance is also extremely important in determining whether significant antigenic drifts evolve over time.
Enveloped, negative-sense, single-stranded RNA virus has hemagglutinin and fusion glycoproteins
CD46 is a cell receptor
![]() MEASLES INFECTION
MEASLES INFECTION
EPIDEMIOLOGY
The highest attack rates of measles have been in children, usually sparing infants less than 6 months of age because of passively acquired antibody. However, a shift in age-specific attack rates to greater involvement of adolescents and young adults was observed in the United States in the 1980s. A marked decline in measles in the United States during the early 1990s may reflect decreased transmission as increased immunization coverage takes effect. However, in developing countries, an estimated one million children still die from this disease each year. Furthermore, measles remains endemic in most countries in the world, including parts of Europe. In 2007 to 2008, large outbreaks of measles were occurring in Switzerland and Israel, resulting in imported cases leading to localized spread within the United States. In the United States, approximately 60 people are reported to have measles each year. In 2011, 222 people were infected with measles, including 40% imported from Europe and Asia involving more than a dozen outbreaks in various communities in the United States. Thus, continued vigilance is required for all who care for patients.
Although a childhood disease, infections in young adults is important in transmission
Dramatic decrease in United States, but importation of infections still a problem
Epidemics tend to occur during the winter and spring and, increasingly, are limited to one-dose vaccine failures or groups who do not accept immunizations. The infection rate among exposed susceptible subjects in a classroom or household setting is estimated at 85%, and more than 95% of those infected become ill. The period of communicability is estimated to be 3 to 5 days before appearance of the rash to 4 days afterward.
Epidemics occur in unimmunized or partially immunized groups
PATHOGENESIS
Measles is transmitted through respiratory inhalation and, after implantation of the virus in the upper respiratory tract, viral replication proceeds in the respiratory mucosal epithelium. The effect within individual respiratory cells is profound. Although measles does not directly restrict host cell metabolism, susceptible cells are damaged or destroyed by virtue of the intense viral replicative activity and the promotion of cell fusion with formation of syncytia. This results in disruption of the cellular cytoskeleton, chromosomal disorganization, and the appearance of inclusion bodies within the nucleus and cytoplasm. Replication is followed by viremic and lymphatic dissemination throughout the host to distant sites, including lymphoid tissues, bone marrow, abdominal viscera, and skin. The virus can be demonstrated in the blood during the first week after illness onset, and viruria persists for up to 4 days after the appearance of rash. Viremia also allows the infection of conjunctiva, urinary tract, small blood vessels, and the CNS. Figure 10–3 summarizes the pathogenesis, clinical disease, and immunity in measles virus infection.
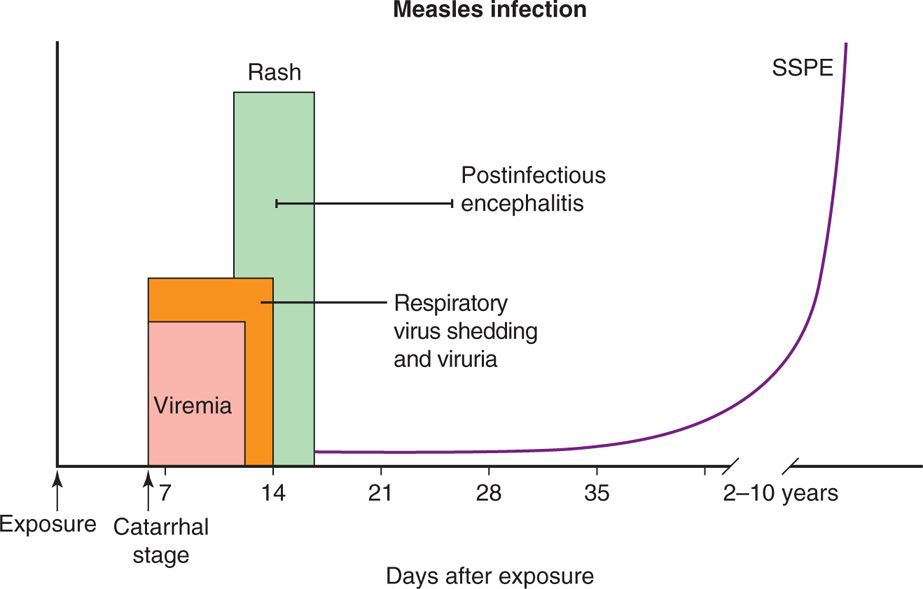
FIGURE 10–3. Pathogenesis of measles virus infection. After exposure, the virus multiplies in the respiratory tract epithelium (incubation period of 9-11 days, on average) and spreads to regional lymph nodes followed by viremia, which helps the virus to be transported throughout the body. Moreover, the virus is also shed in saliva and excreted in the urine. Koplik spots appear on the tongue before appearance of rash on head, then trunk and other extremities. Humoral immune response plays an important role in clearing the virus from the hosts, with IgM appearing early in infection followed by IgG that persists for a long time. Cell-mediated immunity plays a role in disease progression. Postinfectious encephalitis and bacterial superinfections are major complications of measles virus infection. In some patients, there is a rare persistent infection of the CNS known as subacute sclerosing panencephalitis (SSPE).
Respiratory cell multiplication disrupts cytoskeleton
Viremia disseminates to multiple sites
During the viremic phase, measles virus infects T and B lymphocytes, circulating monocytes, and polymorphonuclear leukocytes without producing cytolysis. Profound depression of cell-mediated immunity occurs during the acute phase of illness and persists for several weeks thereafter. This is believed to be a result of virus-induced downregulation of interleukin-12 (IL-12) production by monocytes and macrophages. The effect on B lymphocytes has been shown to suppress immunoglobulin synthesis; in addition, generation of natural killer cell activity appears to be impaired. Moreover, there is evidence that the capability of polymorphonuclear leukocytes to generate oxygen radicals is diminished, perhaps directly by the virus or by activated regulatory T cells. This may further explain the enhanced susceptibility to bacterial superinfections. Virion components can be detected in biopsy specimens of Koplik spots and vascular endothelial cells in the areas of skin rash.
T and B lymphocytes are infected
Leukocyte function is impaired
Susceptibility to bacterial superinfections enhanced
In addition to necrosis and inflammatory changes in the respiratory tract epithelium, several other features of measles virus infection are noteworthy. The skin lesions show vasculitis characterized by vascular dilation, edema, and perivascular mononuclear cell infiltrates. The lymphoid tissues show hyperplastic changes, and large multinucleated reticuloendothelial giant cells are often observed (Warthin-Finkeldey cells). Some of the giant cells contain intracytoplasmic and intranuclear inclusions. Similarly involved giant epithelial cells can be found in a variety of mucosal sites, the respiratory tract, skin, and urinary sediment.
Vasculitis, giant cells, and inclusions are seen
In some patients with measles, an immune-mediated postinfectious encephalitis occurs after the rash. The major findings in measles encephalitis include areas of edema, scattered petechial hemorrhages, perivascular mononuclear cell infiltrates, and necrosis of neurons. In most cases, perivenous demyelination in the CNS is also observed. The pathogenesis is thought to be related to infiltration by cytotoxic (CD8+) T cells, which react with myelin-forming or virus-infected brain cells.
Encephalitis lesions are due to cytotoxic T-cell activity
IMMUNITY
Cell-mediated immune responses to other antigens may be acutely depressed during measles infection and persist for several months. There is evidence that measles virus-specific cell-mediated immunity developing early in infection plays a role in mediating some of the features of disease, such as the rash, and is necessary to promote recovery from the illness. Antibodies to the virus appear in the first few days of illness, peak in 2 to 3 weeks, and then persist at low levels. Immunity to reinfection is lifelong and is associated with the presence of neutralizing antibody. In patients with defects in cell-mediated immunity, including those with severe protein-calorie malnutrition, infection is prolonged, tissue involvement is more severe, and complications such as progressive viral pneumonia are common.
Lifelong immunity associated with neutralizing antibody
 CLINICAL ASPECTS
CLINICAL ASPECTS
MANIFESTATIONS
Common synonyms for measles include rubeola, 5-day measles, and hard measles. The incubation period ranges from 7 to 18 days. A typical illness usually begins 9 to 11 days after exposure, with cough, coryza, conjunctivitis, and fever. One to three days after onset, pinpoint gray-white spots surrounded by erythema (grains-of-salt appearance) appear on mucous membranes. This sign, called Koplik spots, is usually most noticeable over the buccal mucosa opposite the molar teeth and persists for 1 to 2 days (Figure 10–4). Within a day of the appearance of Koplik spots, the typical measles rash begins—first on the head, then on the trunk and extremities. The rash is maculopapular and semiconfluent; it persists for 3 to 5 days before fading (Figure 10–5). Fever and severe systemic symptoms gradually diminish as the rash progresses to the extremities.
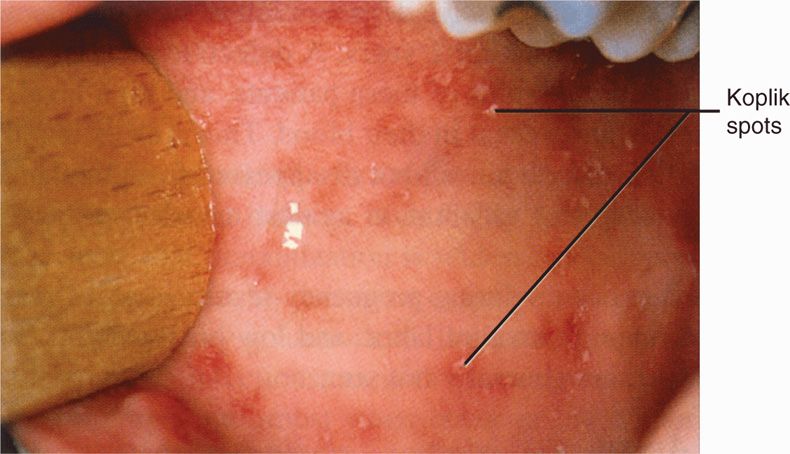
FIGURE 10–4. Oral Koplik spots on day 3 of measles. (Reproduced with permission from Nester EW: Microbiology: A Human Perspective, 6th edition. 2009.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


