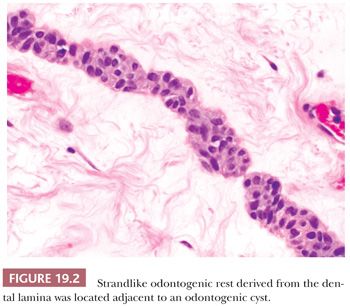
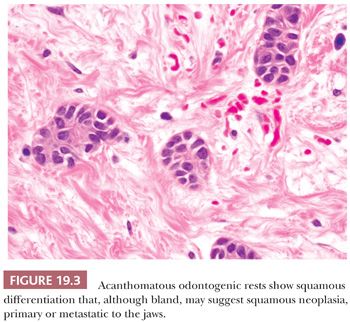
These residual odontogenic rests may be encountered in histologic evaluation of extracted teeth or in sections of tooth-bearing jaws. Odontogenic rests are typically round or strandlike in form (Fig. 19.2). Acanthotic or hyperplastic variations may be confused with a neoplastic squamous proliferation (Fig. 19.3). Rests are distinguished by their bland cytology and lack of ameloblastic differentiation of peripheral columnar cells with nuclear polarization and central stellate reticulum–like zones.
ORAL MUCOSAL LESIONS
GINGIVITIS
Gingivitis is a painless inflammatory reaction limited to the soft tissues around the teeth. It is seen as redness of the gingiva and is caused by dental plaque, a biofilm composed mainly of streptococcal bacteria (Streptococcus sanguinis and Streptococcus mutans) and anaerobes, salivary polymers, and bacterial extracellular matrix products at the marginal gingiva and in the gingival sulcus (3). Plaque-associated gingivitis generally progresses through three histologic stages: initial neutrophilic infiltrate, early T-cell–dominated lymphoid infiltrate, and late-stage infiltration by abundant B cells and plasma cells (2). Gingivitis may also be modified by nutritional deficiency (vitamins B and C), endocrine imbalances of adolescence or pregnancy, generalized infection, or drugs (e.g., phenytoin).
ACUTE NECROTIZING GINGIVITIS
An acute, necrotizing form of plaque-associated gingivitis is known by several names including acute necrotizing gingivitis (ANG), acute necrotizing ulcerative gingivitis (ANUG), ulcerative stomatitis, Vincent disease, and Vincent stomatitis. This infection is caused by fusospirochetal microbes (fusospirochetal gingivitis) usually in association with predisposing factors, such as poor oral hygiene, smoking, and stress (4,5). It occurs almost exclusively in younger age groups of late teens to midtwenties. The clinical appearance is that of highly inflamed, sore, bleeding gingiva with interdental gingival necrosis covered by a gray pseudomembrane that is easily removed, leaving exposed, raw bleeding tissue. This results in a characteristic fetid odor (trench mouth) and an accumulation of detritus. Histologically, there is necrosis of the interdental gingival papillae overlying acute purulent inflammation. ANG lesions heal with scarring and blunting of the interdental papillae. Chronic recurrent forms of ANG do occur.
PERIODONTITIS
Periodontitis is bacterial-related inflammation of the tissues of the periodontium that support the teeth, thereby resulting in loss of supporting bone, loose teeth, and eventual tooth loss (6). Periodontal disease is induced by the activity of a mixed bacterial biofilm growing under anaerobic conditions. Viruses may also play a synergistic role with bacteria in the initiation and progression of periodontitis (7). The most commonly recognized bacterial pathogens are Gram-negative anaerobes—Actinobacillus actinomycetemcomitans, Bacteroides forsythus, Campylobacter species, Capnocytophaga species, Eikenella corrodens, Fusobacterium nucleatum, Porphyromonas gingivalis, Prevotella intermedia, and oral spirochetes like Treponema denticola. Although gingivitis is inflammation limited to the gingiva and results in no bone loss, it may progress to the periodontal ligament and the surrounding alveolar bone forming the tooth socket. When the attachment of the gingival sulcus epithelium to the tooth is lost, the subjacent periodontal ligament becomes involved in the inflammatory process. Eventually, periodontal fibers that attach the tooth to bone are lost, and the formation of a so-called periodontal pocket begins along the tooth root. This creates an occult microenvironment for subgingival bacteria and their products to drive the periodontal destructive process. Bacterial plaque within pockets serves as a nidus for mineralization and calcifies to form dental calculus that is adherent to the tooth roots. Histologically, the inflammatory process is mostly chronic with superimposed acute inflammation. Occasionally, a periodontal abscess may form in a pocket. Risk factors for periodontal disease include poor oral hygiene, genetic predisposition, smoking, and diabetes mellitus (8–10).
Treatment of periodontal disease includes enhanced personal daily dental hygiene to mechanically remove plaque, regular professional scaling and planing of the root surfaces to remove subgingival plaque and calculus, antiseptic mouthwashes to alter bacterial flora, and periodontal surgery to reduce pocket depth and make them more hygienic.
PYOGENIC GRANULOMA
Pyogenic granuloma, a common red tumorlike mass of the gingiva, is a well-vascularized lesion (granulation tissue) (11). Pyogenic granuloma is a misnomer because it is neither pus producing nor granulomatous. Histologically, two types of proliferations have been called pyogenic granuloma: either a uniform granulation tissue mass or a lobular capillary proliferation with intervening fibrous connective tissue. Ulceration may or may not be present (12).
Pyogenic granulomas are reactive lesions whose growth may be modified by hormonal changes associated with pregnancy and puberty. Pyogenic granulomas may be included in some generalized gingival hyperplasias, which are associated with bacterial plaque and subgingival calculus. Generalized lesions may also be modified (enhanced) by hormonal imbalances, drugs (oral contraceptives, phenytoin, cyclosporine, nifedipine, other calcium channel blockers, and epidermal growth factor receptor inhibitor therapies), leukemia, and genetic factors. Lesions usually occur in children and young adults or pregnant women at the second or third trimester as a “pregnancy tumor” of the gingiva. They commonly involve the oral mucosa (especially maxillary labial gingiva) or skin of the head, neck, extremities, and upper trunk. In the oral cavity, it involves, in decreasing order, the gingiva, lips, tongue, buccal mucosa, and alveolus.
Pyogenic granulomas characteristically evolve relatively rapidly over a few weeks and commonly ulcerate and bleed. They are usually associated with gingivitis in the area. Occasionally untreated lesions may become fibrotic. The lesions are treated by simple excision with removal of any initiating factors (e.g., calculus, foreign body). Occasional recurrences are seen, but there is no malignant potential.
PERIPHERAL FIBROMA (FOCAL FIBROUS HYPERPLASIA)
This is the result of focal chronic gingival inflammation and represents a localized hyperplastic fibrous mass in the free or attached gingiva. Synonyms used include focal fibrous hyperplasia, peripheral ossifying fibroma, fibroid epulis (old), and fibroepithelial polyp (13). Peripheral fibromas can contain insignificant islands of ossification and are designated peripheral ossifying fibroma. Occasionally, peripheral fibromas contain stellate and multinucleated fibroblasts and have been designated giant cell fibromas. Yet another variant is the peripheral odontogenic fibroma containing strands of odontogenic epithelium and small calcifications. Unlike the other peripheral fibromas, it has a slight propensity for recurrence and is described in more detail under the section on benign mesenchymal odontogenic tumors. The reactive nature of peripheral fibromas and their variants is in contradistinction to the central ossifying fibromas of the jaws, which are fibro-osseous neoplasms.
PERIPHERAL GIANT CELL GRANULOMA
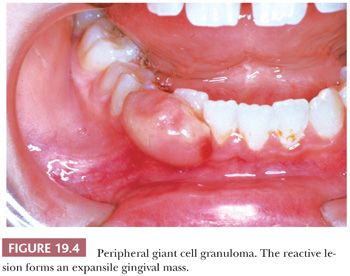
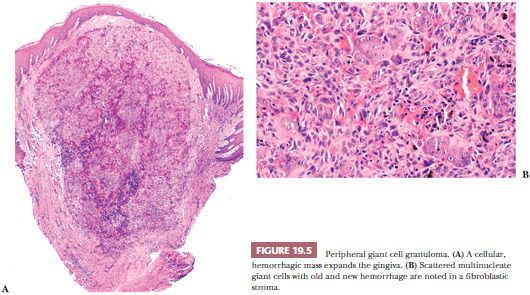
Microscopically, peripheral giant cell granuloma (PGCG) is the gingival soft tissue counterpart of central giant cell lesion (reparative granuloma) located within the jaws (14). The peripheral lesion is reactive in nature, whereas the central lesion is of disputed behavior. Clinically, PGCG is a localized erythematous mass of the gingiva similar in clinical appearance to pyogenic granuloma (Fig. 19.4). PGCG may erode subjacent alveolar bone and periodontal membrane. Histologically, PGCG is a lobular mass of fibroblasts with numerous, often clustered osteoclast-like giant cells associated with hemorrhage and hemosiderin (Fig. 19.5A,B). Occasional bone formation is found. In edentulous or partly edentulous patients, the lesions may show a characteristic erosion of alveolar bone. The treatment of PGCG is surgical excision. This includes a thorough curettage of the base of the lesion extending into the adjacent periodontal membrane or periosteum because PGCG may recur if not completely excised.
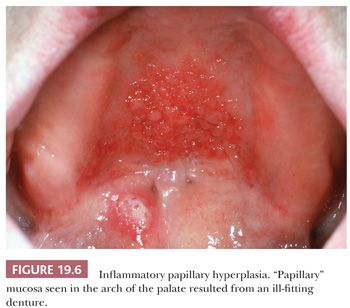
INFLAMMATORY PAPILLARY (DENTURE) HYPERPLASIA
This is a benign papillary hyperplastic lesion of the oral mucosa commonly affecting the palate, where it is also known as palatal papillomatosis (Fig. 19.6). The lesions are associated with ill-fitting dentures or partial dentures in adults and occur 10 times more often in those who wear prostheses at night and/or have poor oral hygiene (15,16).
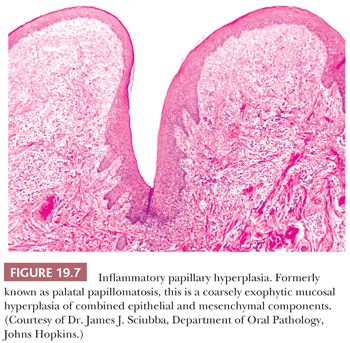
This is not a true papilloma but rather a combined epithelial and mesenchymal reactive hyperplasia. Mucosal epithelial hyperplasia, which may be in a pseudoepitheliomatous pattern, overlies hyperplastic submucosal fibrous tissue (Fig. 19.7). Occasionally, denture trauma can result in trapped islands of hyperplastic squamous mucosa embedded in the fibrous connective tissue of the submucosa. Other secondary chronic inflammatory changes may be seen in the accessory salivary glands in the palate, with sialofibrosis, acinar atrophy, and ductal sialometaplasia. There is no epithelial dysplasia and no premalignant risk associated with the lesion. Inflammatory papillary hyperplasia is treated by complete excision and avoided by construction of a well-fitting denture or partial denture.
ORAL PAPILLOMA
The most common benign intraoral squamous epithelial neoplasm is the solitary papilloma. The usual sites are posterior aspect of the hard and soft palates and uvula (34%), dorsum and lateral tongue borders (24%), gingiva (12%), lower lips (12%), and buccal mucosa (6%) (17).
These lesions are white to pink papillary surface epithelial proliferations. Three-fourths of such lesions are less than 1 cm in size. No gender preference is observed, and the average age of occurrence is approximately 38 years (17). The presence of human papillomavirus (HPV) antigens has been verified by the immunoperoxidase method in approximately 50% of oral papillomas. However, the koilocytotic epithelial changes associated with HPV infection are not always evident. HPV-6 and -11 DNA sequences have been identified in most, but not all, intraoral papillomas by in situ hybridization (18). Interestingly, a minority of cases of inflammatory papillary hyperplasia, described earlier, have also tested positive for HPV-6 by in situ hybridization (18). Despite sensitive polymerase chain reaction (PCR) for multiple HPV types, HPV DNA is not detectable in many esophageal squamous papillomas, which suggests that some oral papillomas may yet represent forms of reactive epithelial hyperplasia secondary to mucosal injury and repair but not associated with HPV as a promoter (19).
Histologically, the solitary squamous papilloma is a localized exophytic growth consisting of multiple papillary epithelial projections supported by delicate fibrovascular cores. Hyperkeratosis characterizes 82% of papillomas; 72% are surfaced by parakeratin, and the remainder is surfaced by orthokeratin or combinations of the two (17). Atypical epithelial changes may be encountered; these include hyperplasia of the basilar and parabasilar layers, hyperplasia of the prickle and granular cell layers, individual cell keratinization, and abnormal mitoses. A diagnosis of frank squamous dysplasia or papillary squamous carcinoma should be entertained in the context of a solitary squamous epithelial proliferation with papillary architecture and a significant degree of cytologic atypia and maturation abnormalities. In human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) patients, a small percentage of oral warts (papillomas) may show histologic features of dysplasia. Progression of these lesions to squamous cell carcinoma has not been documented.
Differential diagnosis of solitary intraoral papilloma-like proliferations includes the other HPV-associated oral “warts”: condyloma acuminatum, which is characterized by a more cauliflower-like acanthosis, and verruca vulgaris, which is noted for its cup-shaped margins and hyperkeratinized spires with a prominent keratohyalin granular layer. As a group, HPV-associated oral warts are prevalent in 0.5% of the general population but occur more frequently in HIV-seropositive individuals (5%), especially in those on highly active antiretroviral therapy (23%) (20). Discrimination of a more complex papilloma and ones with atypia from papillary squamous cell carcinoma and verrucous carcinoma is discussed in sections later in this chapter.
With the exception of HIV/AIDS patients, multiple papillary proliferations rarely develop in the oral cavity. Multiple lesions may be seen in HPV-associated juvenile papillomatosis of the upper aerophagic pathway and HPV-associated focal epithelial hyperplasia (Heck disease). The latter was described in 1965 by Archard et al. (21) as discrete, recurrent papillary lesions on the oral mucosa in Indian children and is now recognized in other parts of the world. Histologically, the lesions are composed of localized areas of mucosal epithelial hyperplasia characterized by marked acanthosis and parakeratosis. HPV types 1, 13, and 32 have been found in these proliferations (18).
Multiple oral papillomas that affect the lips and, less commonly, the intraoral mucosal sites are seen in focal dermal hypoplasia. Other manifestations of this X-linked dominant disorder include hypoplasia of hair, nails, or teeth; linear areas of hypoplasia of the skin; ulcers caused by congenital absence of skin; fatty herniation; bilateral syndactyly with a characteristic “lobster claw” deformity; colobomas of the iris and choroid; and strabismus (22).
Papillary lymphoid hyperplasia of the tonsils results in the clinical appearance of tonsils studded by papillary surface excrescences. This morphologic picture can be confused with epithelial papillomas on clinical examination, but microscopically, this is an unusual form of lymphoid hyperplasia surfaced by unremarkable epithelium (23). Nodular lymphoid hyperplasia may also be seen in the lateral tongue and floor of the mouth.
Oral papillomas are treated by simple excision. A low rate (4%) of recurrence and/or multiple lesions is observed (17), although the rate is higher in HIV/AIDS patients.
INTRAORAL AND OROPHARYNGEAL SQUAMOUS CELL CARCINOMA
Worldwide, oral cancer is the sixth most prevalent cancer, ranking eighth in developed countries and third in the developing world. The frequency of oral cavity malignancies has held constant over the years in the United States, accounting for an estimated 3% of all malignant tumors in men and a lesser percentage in women (24).
The incidence of oropharyngeal (tonsillar and base of tongue) cancers in the United States has increased annually since 1973 (25,26). This is discussed more fully in the section “Human Papillomavirus–Related Squamous Cell Carcinoma.” Squamous cell carcinoma (SCC) of varying grades of differentiation and some unusual microscopic variants account for more than 90% of the intraoral malignancies. The overall 5-year survival rate for oral cancer when all stages are considered has shown little improvement over the past several decades, hovering at approximately 50%. It is important to note that the oral cancer 5-year survival rate in American blacks is significantly lower than that in whites. This difference has been attributed, in part, to lifestyle habits with higher rates of tobacco and alcohol use, clinical stage at diagnosis, and limited access to healthcare (24,27,28).
ETIOLOGY
Most cases of oral cavity and oropharyngeal SCC are in men in the fifth to eighth decade of life and associated with lifestyle habits of smoking and drinking. An estimated 50% of cases of oral cancer in American men are attributable to excessive consumption of tobacco and alcohol (29). The most significant etiologic agent associated with the development of oral SCC is tobacco. The risk of oral cancer increases with larger amounts and longer durations of tobacco use (30). In Western countries, tobacco use usually takes the form of cigarette, cigar, or pipe smoking. Other forms of tobacco use, such as snuff dipping and tobacco chewing, are arguably associated with a slight increased incidence of oral SCC. However, when smokeless tobacco is combined with betel nut leaf and slaked lime as is commonly done in India and Southeast Asia, oral cancer rates are markedly increased. Carcinogens in tobacco are believed to act as initiators, as well as promoters, in the transformation process because the relative risk declines after cessation of smoking (30). Carcinogen exposure in chronic marijuana smoking has been suggested in young patients without other risk factors, and more recently, a history of regular marijuana use has been associated with oropharyngeal cancer (31,32).
Another important etiologic factor is alcohol consumption. Alcohol appears to act synergistically with tobacco as either a cocarcinogen (increasing the risk) or a promoter (decreasing the lag time) of neoplastic transformation. According to Rothman (33), heavy smoking alone carries a twofold to threefold increased risk of oral cancer, whereas the risk is two to six times greater in the heavy drinker compared with the nondrinker; the rate increases with the amount of tobacco smoked. The risk of development of SCC in the heavy drinker and smoker is 15 times greater than the risk in those who neither smoke nor drink. A study in young adults less than age 46 years indicates an oral and pharyngeal cancer risk of 20-fold for heavy smokers, 5-fold for heavy drinkers, and almost 50-fold for those with heavy combined smoking and drinking habits (34). Oral carcinomas arising in nonusers of tobacco and alcohol (13% in one series of 109 cancers) tend to affect older women, to spare the floor of the mouth, to be found at earlier stages, and to lack an association with second primary malignancies (35).
Mechanically removing dental plaque by brushing teeth does more than avoid restorative dentistry bills. Independent risk associations for oral and oropharyngeal cancers have been noted for poor long-term oral hygiene and dental status as reflected in tooth loss, infrequent tooth brushing, and infrequent dental visits (31,36). Recent studies suggest that periodontal pockets may act as a reservoir for HPV and that chronic periodontitis, a chronic bacterial infection, may be associated with tongue cancer independent of smoking history (37,38).
Independent increased risk associations are also noted for those with a history of head and neck SCC in a first-degree relative or sibling, suggesting a specific genetic basis for some cases. A kindred with a germline mutation of the tumor suppressor p16 gene, which is normally responsible for a protein inhibitor of cell proliferation, may represent the genetic basis of a familial predisposition to the development of head and neck SCC (39).
SCCs of the oral cavity occur more frequently in bone marrow transplant recipients whose immunosuppression in concert with viral exposure may be a risk factor (40,41).
Other more controversial risk factors include the chronic use of alcohol-based mouthwashes; oral mechanical irritation, such as chronic trauma from ill-fitting dentures and jagged teeth; and the potentially precancerous conditions of syphilitic glossitis, severe iron deficiency (sideropenic dysphagia or Plummer-Vinson syndrome), oral submucous fibrosis, lichen planus, and discoid lupus erythematosus (42).
HUMAN PAPILLOMAVIRUS–RELATED SQUAMOUS CELL CARCINOMA
Historically, the dominant and synergistic role of chemical toxins in tobacco and alcohol in the causation of most head and neck squamous cancers has been undisputed. But there is a newly recognized and important pathogenesis that explains a current epidemic of head and neck cancer. Despite a reduction in smoking in the United States, there has been a growing increase in the incidence of oropharyngeal carcinomas since the 1970s (43). HPV-mediated carcinogenesis is now recognized as the major cause of oropharyngeal carcinoma in developed countries reported in 40% to 90% of cases (44–46). Transcriptionally active HPV type 16 accounts for nearly all of the HPV-driven oropharyngeal carcinomas, with a minority caused by HPV types 18, 31, 33, and 35. Oral HPV DNA carriage in young men is common, with a point prevalence of 15% to 30%, and HPV-16 is the most frequent type. Oral carriage may play a significant role in HPV transmission (47).
HPV-related oropharyngeal cancers are characterized by younger age at onset, male predominance, and a strong association with oral sexual behaviors (48). They exhibit improved outcomes compared to HPV-negative cancers. The influence of coincident cigarette smoking on risk of local recurrence, distant metastasis, and outcome in HPV-positive oropharyngeal carcinoma is not entirely clear, but there is some suggestion that HPV-16 status confers no outcome benefit in smokers (49–51).
However, in oral cavity cancers, the findings of HPV prevalence are quite variable, and the impact on outcomes is less clear (52–55). Cancers of the oral tongue seem to rarely contain HPV even when p16 is expressed (56–59).
SCC of the oral cavity arising in younger patients (younger than 45 years of age) who lack evidence of HPV infection or tobacco or alcohol use appears to behave aggressively with poor prognosis. These cancers may represent yet another clinical subset of carcinoma with an as yet undefined etiology in the head and neck (60).
HPV-Induced Carcinogenesis
HPV E6 and E7 oncoproteins are essential in HPV-induced carcinogenesis and act through numerous genetic mechanisms that control the cell cycle and apoptosis targeting degradation of the p53 tumor suppressor gene protein and inactivation of the Rb apoptosis/tumor suppressor gene family of proteins. HPV-induced cellular transformation promotes uncontrolled cell replication and genomic instability predisposing to malignant transformation from additional cytogenetic alterations, including mutational events derived from chemical carcinogens and environmental and dietary mutagenic factors (61,62). One of the downstream consequences of HPV-induced carcinogenesis and E7 degradation of pRb is aberrant overexpression of p16, which can serve as a surrogate marker for transcriptionally active HPV-related disease when detected by immunohistochemistry (63). The usual immunoprofile of HPV-related SCC is therefore p53 negative and p16 positive. In contrast, the cancer phenotype driven by tobacco and/or alcohol is usually p53 positive and p16 negative. Tumors derived from the chemical carcinogenesis pathway differ in that p53 is genetically mutated but detectable and the gene CDKN2A is inactivated so that p16 is underexpressed.
Biologic Behavior of HPV-Induced Carcinomas
HPV status is a strong predictor of survival for patients with locoregionally advanced SCC of the oropharynx/tonsil and therefore determination of HPV status is expected in the workup of these cancers.
There is a suggestion that prognostic parameters of conventional staging systems may be modified by HPV infection. The presence of an HPV driver (transcriptionally active virus) may negate the significance of the usual prognostic staging factors in oropharyngeal carcinoma such as pT, higher pN, and number of positive nodes (64) as HPV-induced carcinomas exhibit a marked difference in overall 5-year survival even when presenting in advanced stages (65).
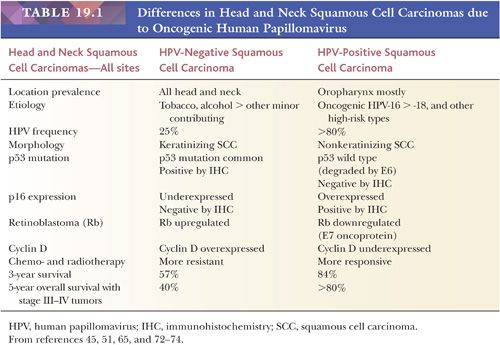
Compared to non–HPV-associated carcinomas, the locoregional recurrence rates of HPV-positive oropharyngeal carcinomas are roughly half. There is a significantly improved progression-free and disease-free survival for HPV-related oropharyngeal SCC, with a 54% better overall survival compared to patients with HPV-negative disease (65–67). Although the distant metastasis rates are similar for HPV-positive (11%) and HPV-negative (15%) cancers, distant metastases may occur at a longer interval in HPV-positive carcinoma, disseminating to more than two organs in a third of cases yet exhibiting a better survival (68). But there are exceptions that exhibit adverse outcomes. These cases have been associated with matted nodes, widespread distant metastases, and bone-only metastases (69,70). Among patients with oropharyngeal SCC, over the past three decades, the risk of a second primary malignancy—another historic adverse prognostic indicator—has decreased dramatically to the lowest risk level of any head and neck subsite (71). Table 19.1 summarizes the differences in head and neck SCCs due to oncogenic HPV (45,51,65,72–74).
CLINICAL APPEARANCE AND HISTOLOGIC CORRELATES OF PRECURSORS TO ORAL SQUAMOUS CELL CARCINOMA
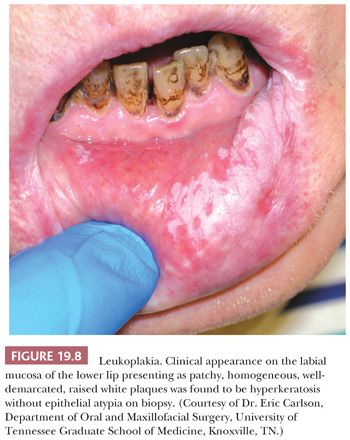
Oral squamous mucosa responds to chronic injury or carcinogenic stimuli by either hyperplasia or atrophy. Lesions related to chronic irritation are focal hyperkeratosis and represent the most common oral white lesions. Other white lesions in which the causes are unknown are called idiopathic leukoplakias. These lesions cannot be scraped off, reversed by the removal of irritants, or ascribed to another disease entity. Most oral idiopathic leukoplakias are found on the buccal mucosa, mandibular and maxillary alveolar ridges, and sulci, palate, and lip (75). Various clinical types of idiopathic leukoplakia are recognized; among these are the homogeneous, speckled, and verruciform types (Fig. 19.8). This white thickening of the normally thin pink mucosa is caused by the expansion of the keratin layer (hyperkeratosis) and/or the prickle cell layer (acanthosis) (Fig. 19.9).
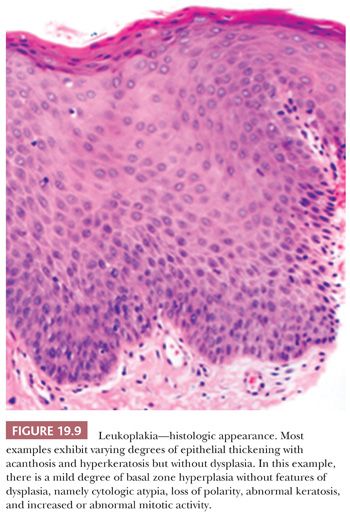
Idiopathic leukoplakia is a clinical term and should not be used microscopically because it encompasses many microscopic lesions. Approximately 80% of idiopathic leukoplakias are diagnosed as hyperkeratosis. Dysplasias make up approximately 12% of idiopathic leukoplakias, carcinoma in situ (CIS) makes up approximately 3%, and invasive carcinomas make up approximately 5%. Transformation rates of all idiopathic leukoplakias are between 5% and 15% (75–79). Significantly higher rates of malignant transformation are observed in speckled (mixed red and white) leukoplakias and verrucous-papillary hyperkeratotic lesions known as proliferative verrucous leukoplakia (79–81). More than 75% of intraoral SCCs arise in a horseshoe-shaped area of the mouth that is defined by the floor of the mouth, the lateral-ventral tongue, and the soft palate–retromolar trigone anterior tonsillar pillar region. These high-risk regions are constantly bathed in the salivary pool, with the potential for concentrated intraoral exposure to carcinogens. White lesions arising in these areas should be viewed with great suspicion. Consequently, all these oral idiopathic leukoplakias should be biopsied because microscopic features cannot be predicted from clinical appearance.
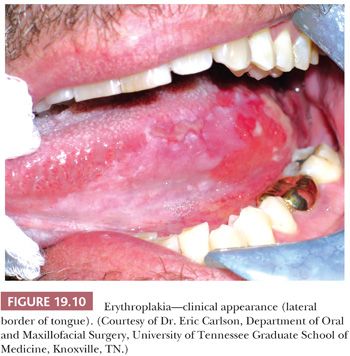
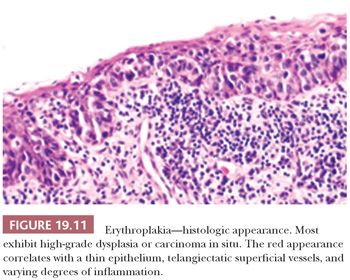
Erythroplakia, or “red patch,” is a particularly ominous oral mucosal lesion (Fig. 19.10), representing carcinoma in 51% of cases, severe dysplasia or CIS in 40% of cases, and mild to moderate dysplasia in 9% of cases (82). These bright red, velvety patches correspond to thinned, atrophic epithelium with prominent subepithelial vascular telangiectasia and inflammation (Fig. 19.11). The lesion tends to be located predominantly in the cancer-prone areas of the floor of the mouth (28%), the retromolar trigone (19%), the mandibular gingiva and sulcus (12.5%), the ventral and/or lateral tongue (12.5%), and the palate (12.5%) (82).
ORAL SQUAMOUS DYSPLASIA AND CARCINOMA IN SITU
Dysplasia refers to abnormal epithelial growth that is characterized by features of cytologic, maturational, and architectural changes. These disturbances may comprise some or all of the features defined by the World Health Organization (WHO) Collaborating Center for Oral Precancerous Lesions in 1978, and refined more recently by the WHO in 2005 (42,83), and shown in Table 19.2. The former criterion of the presence of more than one layer of cells having a basaloid appearance (basal/parabasal cell hyperplasia) has been eliminated. Histologic recognition of hyperplasia versus an early precursor lesion (dysplasia) is not always a reproducible observation, as acknowledged in the 2005 WHO publication (83): “There is a challenge in the recognition of the earliest manifestations of dysplasia and no single combination of the above features allows for consistent distinction between hyperplasia and the earliest stages of dysplasia. Dysplasia is a spectrum and no criteria exist to precisely divide this spectrum into mild, moderate and severe categories.” In fact, given the 16 histologic variables of dysplasia described by the WHO, any of which can be observed in reactive squamous epithelium, there would be 560 combinations of any three histologic criteria to observe. Clearly, the requirement to reproducibly diagnose and grade squamous dysplasia is a challenge to the practicing pathologist.

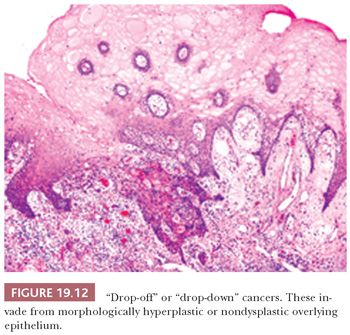
Nevertheless, the intent of histologic grading of dysplasia is to convey to the clinician a sense of the risk of development of subsequent carcinoma. In the oral cavity, biopsy samples showing only CIS have the same age and sex distribution as the invasive oral carcinomas, with a similar grouping at the high-risk sites of the floor of the mouth, the tongue, and the lips (84). Most biopsy samples containing CIS are taken from locations adjacent to an invasive cancer; therefore, the presumption is that moderate and severe degrees of dysplasia, if they are sequential, carry increasing risk. However, the relationship of increasing degrees of oral squamous dysplasia to the subsequent progression to invasive carcinoma is not well defined. In fact, some invasive cancers do not develop from an in situ continuum but rather arise as “drop-off” or “drop-down” cancers from apparently normal or minimally atypical surface epithelium (Fig. 19.12).
Histologic grading of epithelial abnormalities arising in the upper aerodigestive tract mucosa is notoriously subjective for several reasons. These include the absence of an accepted single standardized scheme or quantitative criteria for classification; the recognition that the lesser grades recognized as dysplasia may not be neoplastic at all but may rather represent reversible, reactive mucosal change; the realization that mild to moderate degrees of squamous dysplasia arising in the mucosa with lichenoid inflammatory features may be masked and difficult to discern as truly dysplastic; and the understanding that many lesions are keratinized and that they therefore never meet the CIS criteria for full-thickness abnormal proliferation. To acknowledge the histologic uncertainty in identifying true neoplasia from a reversible reactive process in cases of low-grade abnormalities or mild “dysplasia” (85), some pathologists prefer to use the term squamous atypia rather than dysplasia. However, the following caveat from WHO 1997 (86), which was lost in the 2005 WHO publication, acknowledges that histologic appearances are imperfect predictors and should be remembered when attempting to convey to the clinician the importance of a squamous abnormality in certain oral mucosal sites: “slight degrees of epithelial dysplasia do not indicate any great danger for the patient (although special reference should be made to certain high risk sites, such as floor of mouth and ventral surface of tongue where importance should be attached to even slight dysplasia).”
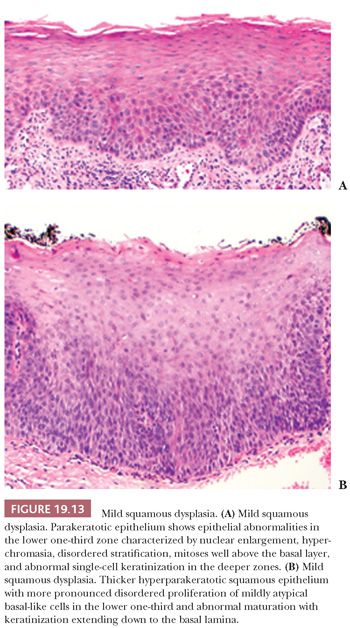
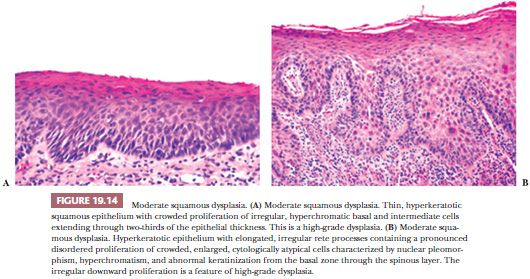
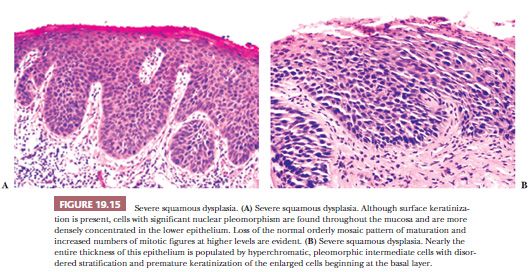
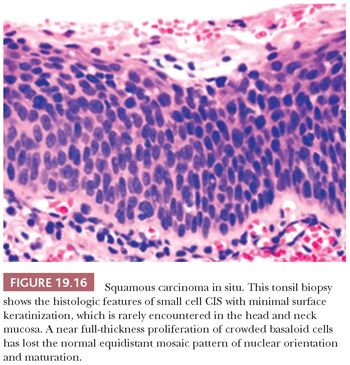
Despite these shortcomings discussed earlier and the fact that histologic grading cannot be reliably shown to offer significant prognostic value, the custom is to classify oral epithelial abnormalities into three grades of dysplasia based on a combination of the level of proliferation of cytologically atypical keratinocytes within the mucosa, the degree of cytologic atypia, the maturation, and the architectural abnormalities (87). However, this leads to innumerable histologic appearances of squamous dysplasia, making grading somewhat subjective. Some examples of squamous dysplasia in varying epithelial thicknesses are illustrated in Figures 19.13A,B (mild), 19.14A,B (moderate), and 19.15A,B (severe). A full-thickness proliferation of undifferentiated basaloid cells (classic CIS), as is commonly seen in the cervical intraepithelial neoplasia, is rarely seen in the head and neck mucosa (Fig. 19.16).
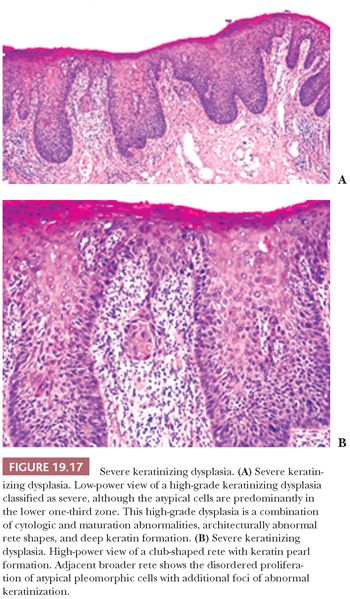
Severe dysplasias in the upper tract mucosa are commonly thickened, hyperkeratotic epithelium in which marked nuclear abnormalities, abnormal mitoses, disordered maturation with loss of orientation, and dyskeratosis confined to the lower portion of the epithelium can be seen; these are seldom full thickness in their extent and often show abnormal or drop-shaped rete (Fig. 19.17A). Shafer and Waldron (84) have suggested that the presence of single-cell keratinization and/or keratin pearl formation in oral dysplasia is often a hallmark of transition to invasive carcinoma (Fig. 19.17B). This biopsy finding of deep keratinization warrants careful examination for invasion. Some evidence from studies of laryngeal glottic mucosa indicates that severe keratinizing dysplasias may exhibit as high a rate of progression to invasive carcinoma as does classic CIS at that site. For this reason, the suggestion has been that both of these high-grade forms be categorized under the rubric of squamous intraepithelial neoplasia grade III to standardize the terminology and histologic criteria connoting the high risk of malignant transformation (88). In the oral cavity mucosa, whether these two high-grade histologic abnormalities of severe keratinizing dysplasia versus classic CIS carry different rates or periods of progression is not known.
MICROINVASIVE CARCINOMA
The primary purpose of identifying microinvasive carcinoma is to convey to the clinician a morphologic feature that should be predictive of biologic behavior and direct therapeutic intervention. However, no consensus exists on the definition of the depth or histologic form of invasion that constitutes microinvasive, early, or superficially invasive squamous carcinoma in the head and neck region. The assessment of tumor thickness or depth of invasion using Breslow-type measurements, excluding the layers of surface keratin and parakeratin, has consistently shown statistical significance and independent power in predicting the rate of cervical lymph node metastasis, recurrence, and survival (89–95). In a study of SCC from mixed oral cavity and oropharyngeal sites, tumors with depths of invasion of less than 4 mm had a metastatic rate of 8.3%, those with invasion of 4 to 8 mm had a 35% metastatic rate, and those with invasion greater than 8 mm had an 83% metastatic rate (93). However, the least depth of invasion predictive of cervical node metastasis was a 2-mm invasive cancer. Therefore, with the limited available data, a 1- to 2-mm distance from the basement membrane to define microinvasive carcinoma appears reasonable, provided that no extension into muscle or cartilage or evidence of lymphovascular space invasion is present.
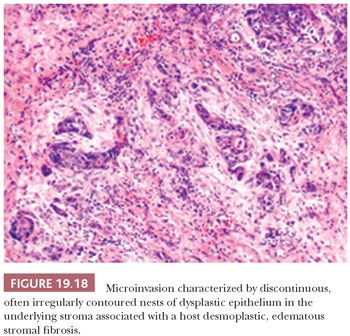
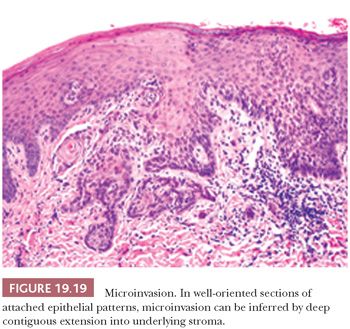
The assessment of microinvasion requires well-oriented sections that are perpendicular to the mucosa to define either of two histologic patterns of extension. The first frankly invasive pattern is that of discontinuous, often irregularly contoured nests of dysplastic epithelium in the underlying stroma that is usually associated with a host desmoplastic edematous stromal fibrosis (Fig. 19.18). The second pattern of invasion is analogous to the discrimination of actinic keratosis from SCC of skin, in which a criterion of depth of contiguous extension is used. In the head and neck mucosa, this is a much more subjective assessment that is dependent on well-oriented sections. This form of attached or “incipient” invasion may be very well differentiated, as is seen in VC, or frankly dysplastic with cytologic atypia and dyskeratosis that is evident (Fig. 19.19). Although describing this latter pattern of invasion into the stroma is appropriate for directing therapy, the biologic behavior compared with the frankly invasive pattern is undefined.
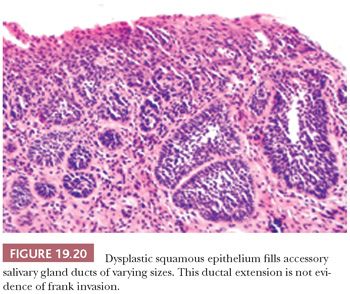
Several notable pitfalls are seen in the interpretation of invasion. The first is use of tangential sections of markedly thickened, high-grade dysplastic mucosa as a sign of invasion. Dysplastic squamous mucosa in the head and neck region may often be markedly thickened, but it still may not be invasive. Tangential sectioning may exaggerate this thickness, lending the appearance of a mass. In addition, tangential sectioning with exaggeration and elongation of the rete often populated by an apparently increased proportion of darker basal cells may mimic invasion. Furthermore, the well-contoured, detached epithelial foci without desmoplasia in the underlying stroma are often not invasive. Deeper levels through the block may “stand” the section up and show that foci that were previously “detached” and appeared invasive are nonetheless attached to the overlying epithelium. Another pitfall is overcalling invasion by misinterpreting the extension of CIS into the underlying minor salivary gland ducts and mucous glands. This spread of CIS is recognized by the rounded, filled ductal contours and preserved but expanded lobular salivary gland architecture (Fig. 19.20). These features can be confirmed with immunohistochemical stains for residual native basal and myoepithelial cells. It is advisable to section oral biopsies with atypical epithelium at several levels because many head and neck lesions are histopathologically heterogeneous.
MULTIPLE PRIMARIES
Patients with head and neck cancer are prone to the development of multiple synchronous and metachronous malignancies involving both upper and lower aerodigestive tracts. Of those patients with initial signs of oral cavity SCC, 27% have ultimately had second primary cancers (96). The occurrence of a second primary cancer is an unfavorable prognostic factor, resulting in an overall 5-year survival rate of 17% compared with a 35% survival rate in patients with only a single head and neck cancer (97). Until now, the prevailing explanation for the increased frequency of second head and neck mucosal malignancies in this population has been that of field cancerization. This proposal by Slaughter et al. (98) in 1953 postulated a multiclonal origin such that multiple, separate cancers develop in these patients from independent neoplastic events in a mucosa that has been widely activated by common exposure to carcinogens such as tobacco and alcohol. More recently, based on molecular evidence, some multiple, geographically and temporally separate squamous cancers have been proposed to arise from an initial single cancer clone that spreads laterally within the mucosa and appear as separate “primaries” but, in essence, are the extensions of one tumor (99–102). These studies somewhat negate the reliance on previously accepted histologic criteria for the diagnosis of a second primary SCC. These criteria had required biopsy proof that two synchronous tumors were anatomically separated by an intervening nonneoplastic mucosa or that a metachronous tumor had an in situ mucosal origin. Typically, however, the reappearance of tumor at or near the site of a previously treated carcinoma is not usually taken as evidence of surface origin. When the tumor is located in the deeper portions of the submucosa, its reappearance is presumed to represent persistence of the original tumor (103).
Oropharyngeal carcinomas that historically carried the highest risk for development of a synchronous second primary malignancy now carry the lowest risk in the head and neck. This appears to be related to the epidemiologic shift in oropharyngeal cancer causation from tobacco-associated field cancerization to that of a localized oncogenic HPV infection (104).
TUMOR-NODE-METASTASIS STAGING
Tumor staging parameters of primary tumor size and the extent of invasion; the size, number, and laterality of positive cervical lymph nodes; and the presence of distant metastases remain the most important factors influencing therapeutic decisions and predicting outcome in patients with oral SCC. Staging is accomplished with the tumor-node-metastasis (TNM) staging classification (105). The requirements of the pathologic classification system, which is designated pTNM and which verifies the clinical classification (cTNM), specify that resection of the primary tumor or biopsy must be adequate to evaluate the highest pathologic tumor (pT) category and that removal of regional nodes must be an effective means of validating the absence of regional lymph node metastasis and of evaluating the highest pathologic node (pN) category. For pathologic metastasis (pM), microscopic examination of a distant metastasis is required. Notable changes from the last edition include describing T4 lesions as T4a (resectable) and T4b (unresectable), so that advanced-stage disease is classified as stage IVA, advanced resectable disease; stage IVB, advanced unresectable disease; and stage IVC, advanced distant metastatic disease. Moreover, a descriptor has been added to designate nodal metastasis in the upper neck as (U) and lower neck as (L), but this has no influence on nodal staging.
The unified TNM staging classification of lip, oral cavity, and oropharynx sites defines primary tumor (T) extent using not only size of tumor in greatest dimension (T1 to T3) but also local extension of disease in T4 categories:
TX, primary tumor cannot be assessed.
T0, no evidence of primary tumor.
Tis, carcinoma in situ.
T1, tumor of 2 cm or less in greatest dimension.
T2, tumor more than 2 cm but not more than 4 cm in greatest dimension.
T3, tumor more than 4 cm in greatest dimension.
T4a (lip), tumor invading through the cortical bone*, inferior alveolar nerve, floor of mouth, or skin of face.
T4a (oral cavity), tumor invading through cortical bone into the deep (extrinsic) muscle of the tongue (genioglossus, hyoglossus, palatoglossus, and styloglossus), maxillary sinus, or skin of face.
T4a (oropharynx), tumor invades the larynx, deep/extrinsic muscles of tongue, medial pterygoid, hard palate, or mandible
T4b (lip and oral cavity), tumor invades the masticator space, pterygoid plate, or skull base or encases the internal carotid artery.
T4b (oropharynx), tumor invades lateral pterygoid, pterygoid plates, lateral nasopharynx, or skull base or encases carotid artery.
*Note that superficial erosion alone of the bone and/or tooth socket by primary gingival tumor is not sufficient to classify a tumor as T4.
REGIONAL LYMPH NODE METASTASIS
Several types of neck dissections may be encountered, classified as (a) radical neck dissection; (b) modified radical neck dissection, with internal jugular vein and/or sternocleidomastoid muscle spared; (c) selective neck dissection as specified by the surgeon as (i) supraomohyoid neck dissection, (ii) posterolateral neck dissection, (iii) lateral neck dissection, or (iv) central compartment neck dissection or other; and (d) extended radical neck dissection as specified by the surgeon.
The presence, number, and size (pT3) of regional lymph node metastases are the strongest predictors of survival in oral cavity SCC. The importance of these parameters as prognostic indicators lays in the recognition that cancer deaths in many patients stem from uncontrolled regional disease in the cervical lymph nodes that is often associated with local recurrence at the primary site.
The size and level of involved lymph nodes correlate with prognosis (106). Therefore, the surgical pathology report should document lymph node involvement by anatomic subdivisions of the submental, submandibular, or anterior triangle; the subdigastric–upper jugular, midjugular, low jugular, posterior cervical, or posterior triangle; and the supraclavicular regions.
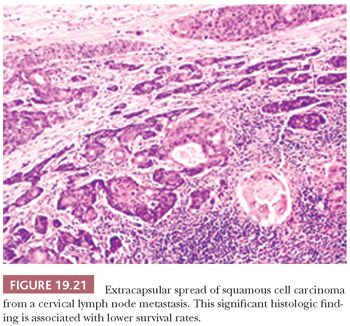
The surgical pathologic documentation of extracapsular lymph node spread of SCC in neck dissections is another significant prognostic indicator of survival (Fig. 19.21). The survival rate of patients with oral cavity SCC with metastases and intact lymph node capsules is 33% at 5 years compared with an 11% survival rate in patients with extracapsular infiltration into adjacent soft tissues (107). The communication of this finding in the surgical pathology report is important because it often modifies the treatment plan to include regional radiotherapy, radioactive seed implantation, or chemotherapy.
It is accepted that both clinical and routine pathologic staging underestimate the presence of lymph node metastases. Given that elective neck dissection for early oral cancers is an unsettled clinical issue, some have resorted to sentinel lymph node biopsy for direction. Roughly 20% of clinically negative necks (N0) will have metastases detected histologically, and routine pathologic techniques provide a negative predictive value of 96% in the assessment of neck nodes (108,109). In two single-institution studies, sentinel lymph node biopsy was used to upstage 32% to 35% of clinical N0 necks to positive necks, leading to therapeutic neck dissection for this subset of patients (110,111). This would include histopathologic findings of isolated tumor cells, micrometastases less than 2 mm, and macrometastases greater than 2 mm. The sensitivity of sentinel node examination in predicting lymph node metastasis in oral and oropharyngeal carcinomas in another study was 89%, leaving an 11% false-negative rate, which is similar to the experience in breast cancer (112,113). Currently, the use of sentinel lymph node biopsy for head and neck cancer is not standard therapy, and the prognostic significance of immunohistochemically demonstrated occult nodal micrometastases is not known. The role of sentinel node biopsy for head and neck cancers in lieu of elective neck dissection awaits multi-institutional trial validation.
The TNM classification of regional lymph node (N) involvement for oral cavity and oropharyngeal tumors is based on single versus multiple node involvement, greatest size dimension of involved node, and ipsilateral versus bilateral node involvement, specified as follows:
NX, regional lymph nodes cannot be assessed.
N0, no regional lymph node metastasis.
N1, metastasis in a single ipsilateral lymph node that is 3 cm or less at its greatest dimension.
N2, metastasis in a single ipsilateral lymph node that is more than 3 cm but not more than 6 cm in greatest dimension; or in multiple ipsilateral lymph nodes, none of which is more than 6 cm in greatest dimension; or in bilateral or contralateral lymph nodes, none of which is more than 6 cm in greatest dimension.
N2a, metastasis in a single ipsilateral lymph node of more than 3 cm but not more than 6 cm.
N2b, metastasis in multiple ipsilateral lymph nodes with none being more than 6 cm.
N2c, metastasis in bilateral or contralateral lymph nodes with none being more than 6 cm.
N3, metastasis in a lymph node that is more than 6 cm in greatest dimension.
Note: midline nodes are considered ipsilateral nodes.
DISTANT METASTASIS
In contrast to the 1923 autopsy study by Crile (114), which showed a 1% rate of distant metastasis, a significant rate of systemic metastases from oral cavity SCC is now recognized (115). This rate of distant metastasis ranges from 31% in patients who die of oral carcinoma after initially having negative results on neck dissections to 59% in patients with pathologically staged positive regional lymph node metastases (116). The most probable explanation is that improvements in local and regional tumor control have led to longer survival and thereby opportunities, especially in those with recurrent disease, for the metastatic cascade to be successfully completed and clinically recognized.
In the TNM classification, distant metastasis (M) is designated as follows:
Mx, the presence of distant metastasis cannot be assessed.
M0, no distant metastasis.
M1, distant metastasis.
The combinations of increasing T, N, and M stages result in the following stage groupings for lip, oral cavity, oropharynx, and hypopharynx sites:
Stage 0: Tis, N0, M0.
Stage I: T1, N0, M0.
Stage II: T2, N0, M0.
Stage III: T1 or T2, N1, M0; T3, N0 or N1, M0.
Stage IVA: T1 or T2 or T3, N2, M0; T4a, N0 or N1 or N2, M0.
Stage IVB: any T, N3, M0; T4b, any N, M0.
Stage IVC: any T, any N, M1.
HISTOPATHOLOGIC PARAMETERS OF PROGNOSTIC SIGNIFICANCE
Numerous studies of histopathologic features of tumor and host response parameters in oral SCC have shown variable prognostic significance. Although some studies have found tumor grade to be predictive of regional lymph node metastasis, the assessments of tumor grade encompassing traditional histologic and cytologic features of differentiation are not highly reproducible. Therefore, the tumor grade is of limited clinical value in comparison with the clinical staging parameters. Also because most SCCs are moderately differentiated, no apparent correlation of grade with tumor size has been found.
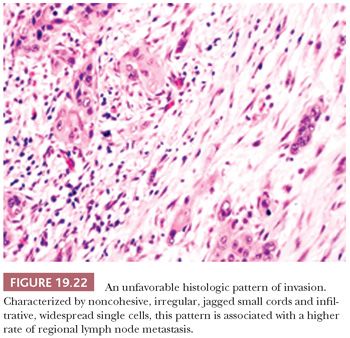
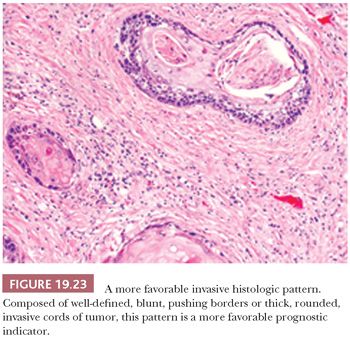
Two of the more important histopathologic observations in oral SCC are the pattern or mode of tumor invasion within the stroma and the depth or thickness of tumor invasion. The patterns of noncohesive, irregular, jagged small cords and infiltrative, widespread single cells (Fig. 19.22) are associated with a higher rate of regional lymph node metastasis than are patterns composed of well-defined, blunt pushing borders or thick, rounded invasive cords of tumor (Fig. 19.23) (89,93,117–120). When the pattern of invasion is subjected to multivariate analysis, it is a significant histologic feature that surpasses that of the conventional tumor grade as a prognostic indicator (89). Depth of invasion is an independent predictor of lymph node metastasis for cancers of numerous oral sites.
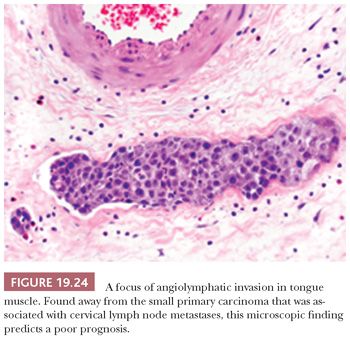
Additional microscopic findings associated with increased rates of cervical lymph node metastasis and poor prognosis that are recommended for inclusion in the surgical pathology report by the Association of Directors of Anatomic and Surgical Pathology (121) are the presence of angiolymphatic invasion (Fig. 19.24) (89,93,122) and perineural invasion (123). Other assessments of lymph node reaction patterns and host inflammatory and desmoplastic response have added no consistent or independent discriminating information (93). In the upcoming sections, the significance of these histopathologic parameters as they pertain to the specific oral cavity and oropharyngeal sites of SCC is elaborated.
EVALUATION OF RESECTION MARGINS
The pathologic evaluation and documentation of the completeness of primary excision margins provide significant information for the intraoperative and postsurgical treatment options and prognosis. The definition of a positive margin is usually restricted to the finding of invasive carcinoma at the microscopic margin (124,125). The observations of CIS and a close margin of less than 5 mm from the resection line are noteworthy because patients with high-grade dysplasia and close margins also appear to be at risk for locally recurrent carcinoma (126).
When defining a positive margin as either in situ or invasive SCC, Byers et al. (124) have noted an 80% local recurrence rate for oral cavity and oropharynx tumors with positive margins, compared with rates of 12% for oral cavity SCC and 18% for oropharyngeal cancers with initially free margins (124). In some studies, the frequency of positive margins in head and neck cancer appears to correlate with increased tumor size or the stage of disease and, therefore, with the potential to resect the tumor surgically without sacrificing vital structures or the tissue required for closure (125). Postoperative radiotherapy for positive margins appears to be ineffectual, and the 2-year survival rate is dismal in this group of patients (124,125).
The intraoperative consultation assessment of margin adequacy is a time-consuming endeavor but has been shown to be reliable and accurate and represents a prime opportunity for assuring surgical eradication of the disease. In practice, techniques of sectioning margins include full-thickness, serial, and bread-loaf types, with inked surgical margins, as well as parallel, shave margins from the edges. The latter are often used in the examination of separately submitted marginal tissue and additional tissue taken from previously positive or close margins.
SITE-SPECIFIC CONSIDERATIONS
No distinct histopathologic differences in SCC have been identified for the various intraoral sites. Oropharyngeal carcinomas are addressed separately in the following texts. In general, larger oral cavity and oropharyngeal tumors exhibit a higher rate of metastasis such that the risk from tumors less than 3 cm is low, the risk from tumors that are 3 to 4 cm is intermediate, and the risk from tumors more than 4 cm is the highest (127). An analysis of 898 SCCs of the oral cavity and oropharynx has shown that same-size tumors of the lip, floor of the mouth, buccal mucosa, hard palate, and gingiva have approximately the same risk for metastasis to regional lymph nodes (127). Some site-specific differences in biologic behavior are recognized that may relate to innate biologic differences, the associated clinical symptoms leading to timely diagnosis at earlier stages, and variations in local anatomy that are associated with lymphatic drainage. These differences and other pertinent histopathologic observations for SCC involving specific intraoral sites are enumerated in the following sections.
SQUAMOUS CELL CARCINOMA OF THE LIP
The most common oral cancer, which accounts for 42% of all cases, is SCC of the lip (128). Roughly 90% of these SCCs arise in the lower lip, usually along the mucocutaneous junction or vermilion border. Unlike other oral SCCs, chronic sunlight exposure is a prime etiologic agent, in addition to tobacco exposure in the form of pipe and cigarette smoking and poor oral hygiene. The pattern of metastasis from lower lip SCC is to the ipsilateral submandibular and submental lymph nodes, with potential for bilateral metastases in midline lesions. The lymphatic drainage from SCCs of the upper lip is to the preauricular and infraparotid lymph nodes. The rate of metastasis from SCCs of the lip is directly related to size, with a 5% rate of metastasis in SCCs less than 2 cm, 50% in tumors of 2 to 4 cm, and 73% in SCCs larger than 4 cm. Other pathologic observations that are predictive of subsequent regional metastasis include a high tumor grade, tumor thickness of more than 6 mm, an aggressive pattern of invasion, and the presence of perineural invasion (90). These histologic parameters are best assessed in the deeper portions of the neoplasm. The survival rates for patients with lip SCC decline with advancing tumor size, the presence of lymph node metastases, and high tumor grade.
SQUAMOUS CELL CARCINOMA OF THE TONGUE
SCC of the tongue accounts for 22% of intraoral SCCs, which is practically equal to the total incidence of SCC at all other sites of intraoral carcinoma if the lips are excluded (128). The neoplasm most often develops as an area of leukoplakia, chronic ulcer, or erythroplakia on the lateral aspect of the middle one-third of the tongue (Fig. 19.25). SCC involving the anterior two-thirds, or the mobile tongue, has a higher tendency to metastasize than do other intraoral carcinomas, with the exception of carcinomas of the tongue base (posterior one-third) and oropharynx, which have historically exhibited the most aggressive behavior (127). This is changing in the era of HPV-induced SCC. Overall, 70% of patients with carcinoma of the tongue have unilateral or bilateral metastases at their first examination. Tongue SCC characteristically metastasizes to the ipsilateral subdigastric, submandibular, and midjugular lymph nodes. Bilateral nodal involvement is more common in tumors of the midline and base of the tongue, as opposed to lateral tongue tumors. Whereas most intraoral primary SCCs metastasize initially to the nearest draining nodes (level I or II) with progressive overflow to neighboring nodes, tongue cancers may show a more aggressive pattern with skip lesions and peppering of lymph nodes (129). Tongue base cancers may show isolated level IV metastasis and, overall, have a high rate of level IV nodal involvement that translates to a worse 5-year survival (130). Because of their posterior location and, at times, endophytic growth pattern, the patients usually are not aware of early-stage tongue base carcinomas; 90% are first evaluated in the advanced stages III and IV, compared with 37% of patients with mobile tongue cancers (131).
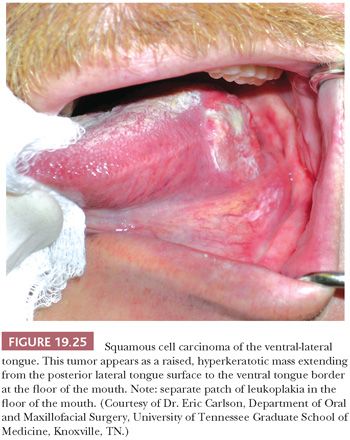
Histologically, posterior tongue SCCs tend to be less differentiated than those arising in the mobile tongue, but the microscopic pattern or mode of invasion is the feature that predicts for nodal metastasis. Also, pathologic determination of depth of invasion is a significant predictor of regional lymph node metastasis for tongue cancers. Assessment of tumor thickness is a very good indicator of the presence of occult cervical node metastasis and of poor outcomes in early-stage I/II oral tongue cancer (132). Tongue cancers that exceed 5 mm in tumor depth have a metastatic rate of approximately 51% to 65% compared with approximately 6% to 8% for those less than or equal to 5 mm (133,134).
SQUAMOUS CELL CARCINOMA OF THE FLOOR OF MOUTH
The third most common site of intraoral SCC is the floor of the mouth, which comprises 17% of intraoral carcinomas (128). This is the most common site of intraoral carcinoma in African Americans (135). The area of the floor of the mouth that is most often affected is the anterior region of the caruncles of the submaxillary gland and the lingual frenum, where it can appear as either an elevated leukoplakia or a velvety erythroplakic lesion. On the initial examination, SCC of the floor of the mouth has invaded one or more contiguous structures in 75% of cases (136). The submandibular triangle and subdigastric lymph nodes are most commonly involved, but submental nodes are rarely affected, even in anterior tumors. In tumors of the floor of the mouth, palpable nodes designated as positive on clinical examination have shown a false-positive rate of up to 56%; in tumors with negative results on clinical evaluation, the false-negative rate is 24% after histologic examination (137). This high rate of erroneous clinical assessment is attributed to confusion with the obstructive enlargement of the sublingual and submandibular glands as well as to reactive lymph node hyperplasia caused by inflammation.
In general, the frequency of lymph node metastasis is related to the tumor size, with most notable differences being seen between T1 and T3 and T4 tumors. Histopathologic assessment of the pattern of invasion and the depth of tumor invasion in T2 lesions of the floor of the mouth appears to have discriminatory power in predicting lymph node metastasis, with no metastases being noted from superficially invasive tumors and a 44% rate of metastases with confluent, nodular, or deeply invasive cancers (137). Histologic measurements of the tumor thickness, independent of tumor size, may be a stronger predictor for the subsequent development of nodal metastases from the floor of the mouth, with a 2% metastatic rate from SCCs measuring less than 1.5 mm compared with a 33% rate for tumor thicknesses of 1.6 to 3.5 mm and a 60% rate for lesions thicker than 3.6 mm (92). The importance of tumor thickness has been corroborated by another study applying multivariate analysis to cancers of the floor of the mouth and tongue that showed that tumor thickness less than 2 mm was the most significant favorable predictor of recurrence and survival (94).
SQUAMOUS CELL CARCINOMA OF THE GINGIVA
Carcinoma of the gingiva accounts for 6% of intraoral carcinomas and usually occurs in the mandibular bicuspid and molar areas (128). In nonedentulous patients, the lesions develop at the free gingival margin, whereas in edentulous patients, the lesions are located on the alveolar ridge (Fig. 19.26). Carcinoma of the gingiva is often clinically misdiagnosed as one of the many inflammatory lesions of the periodontium (e.g., pyogenic granuloma, PGCG, localized gingivitis/periodontitis), papilloma, or even fibroid epulis (inflammatory hyperplasia).
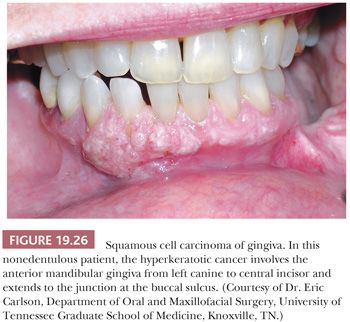
SCC of the gingiva is usually a well-differentiated carcinoma that tends to invade bone. These patients often have loose teeth because of tumor invasion of adjacent periodontal ligament and alveolar bone. Metastasis is often to the submandibular lymph nodes, where it may demonstrate a less differentiated histopathologic appearance. Although rarely seen, the gingiva (and hard palate) is the intraoral site of predilection for melanoma.
SQUAMOUS CELL CARCINOMA OF THE BUCCAL MUCOSA
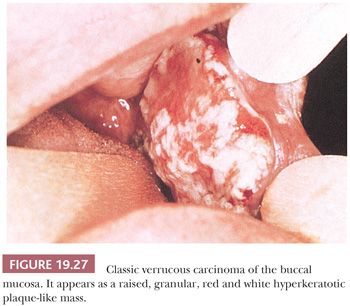
SCC of the buccal mucosa accounts for 2% of intraoral carcinomas (128). Buccal mucosal carcinoma, like SCC arising at other oral cavity sites, predominates among men. Some American series from southern states, however, are heavily weighted toward women because of the more commonplace habit of tobacco chewing or oral snuff dipping (138). Many of these people have verrucous carcinoma in the buccal mucosa or gingiva (Fig. 19.27). SCC of the buccal mucosa may extend to involve the contiguous soft tissue and bony structures that is directly related to tumor size. Regional metastasis, which is usually limited to the submandibular lymph nodes, is typically a late-stage phenomenon.
In addition to tumor stage, observations of anterior-posterior tumor location and histopathologic factors have prognostic value. Five-year cure rates decline with more posterior buccal mucosal sites: 40% for tumors located in the anterior buccal, 17% for the middle third, and 10% for the posterior third (139). The rates of recurrence and survival are significantly related to a depth of invasion less than 3 mm and tumor thickness of less than 6 mm (140). The latter observation is an independent variable in predicting survival regardless of stage.
SQUAMOUS CELL CARCINOMA OF THE PALATE
SCC arising from the oral surfaces of the palate is more commonly localized to the soft palate (141). It accounts for 5.5% of intraoral SCC but is the most common malignant neoplasm of the palate (128,141). A male predominance of 2.5 to 1 is observed, with no gender predilection between the hard and soft palates. More than half of palatal SCCs extend beyond the anatomic confines of the palate at the initial examination (142). The resection specimens of hard palate should be decalcified to evaluate the extent of invasion into underlying bone. The lymphatic drainage pathway of the hard and soft palates is through the retromolar triangle to the lymph nodes of the internal jugular chain, the submandibular, and the retropharyngeal regions. A third of patients have cervical node metastases initially, with a similar incidence for both hard and soft palate lesions. Bilateral cervical metastases and distant metastases are uncommon. The tumor size and histologic grade correlate with survival, but the stage of the disease at presentation is the most important prognostic indicator (141,142).
SQUAMOUS CELL CARCINOMA OF THE OROPHARYNX (PALATINE TONSIL AND BASE OF TONGUE)
In the United States, the palatine tonsil is the most common primary site of SCC in the oropharynx, followed by the base of tongue, the soft palate and/or uvula, and the pharyngeal wall (143). Historically, tonsillar SCC has had a demographic profile and associated etiologic factors similar to those of intraoral carcinomas. In these smoking- and alcohol-driven cancers, a poor prognosis is associated with an advanced stage, which is related to large tumor size, presence of regional lymph node metastasis, and involvement of the base of the tongue (144). The tumor’s pattern of invasion and mitotic index as assessed by histopathologic observations are correlated with survival, although these microscopic features are of lesser prognostic value (89). More recent studies have shown that HPV-16–positive tonsillar carcinomas tend to arise in younger individuals without smoking or drinking risk factors and exhibit a better overall and disease-specific survival compared with HPV-negative tonsillar carcinomas (145).
Tonsillar carcinomas have a high overall rate of lymph node metastasis. They usually metastasize to the ipsilateral subdigastric lymph nodes, but they also involve the middle and lower jugular nodes as well as the posterior cervical triangle nodes (144). Occasionally, node metastases may exhibit a cystic and, at times, deceptively bland histologic appearance that simulates a branchial cyst or a branchiogenic carcinoma (146). Most cystic cervical metastases are HPV positive and are derived from the tonsil or base of tongue (147). Branchiogenic carcinoma, however, is exceedingly rare, and blind tonsillar, base of tongue, or nasopharyngeal biopsies have led to the correct diagnosis of metastatic carcinoma in many purported cases.
In general, SCC arising in the oropharynx (palatine tonsil and base of tongue) has a tendency to be nonkeratinizing, and formerly, this was considered a less differentiated carcinoma with attendant poor outcomes compared to the conventional keratinizing SCC seen in the more proximal oral sites. This has changed as HPV-associated oropharyngeal carcinomas become more frequent.
MORPHOLOGIC TYPES OF HPV-RELATED OROPHARYNGEAL CARCINOMA
There is a wide morphologic spectrum of HPV-associated disease in the head and neck that is not well reflected morphologically by the WHO classification of well, moderately, and poorly differentiated SCC. This scheme works best for keratinizing SCC. A better fit to describe the predominantly nonkeratinizing morphology of the oropharyngeal carcinomas (tonsil and base of tongue) is a modified classification scheme identifying keratinizing, nonkeratinizing, nonkeratinizing with maturation, and undifferentiated SCC, analogous to that used for nasopharyngeal carcinomas (148).
KERATINIZING SQUAMOUS CELL CARCINOMA
This is the conventional infiltrating, variably keratinizing SCC, usually with stromal desmoplastic reaction. It accounts for about 25% of oropharyngeal carcinomas. This form of carcinoma is less likely to be HPV positive (about 20%), responds less well to chemo and radiotherapy, and has poorer survival than the nonkeratinizing forms (148).
NONKERATINIZING SQUAMOUS CELL CARCINOMA
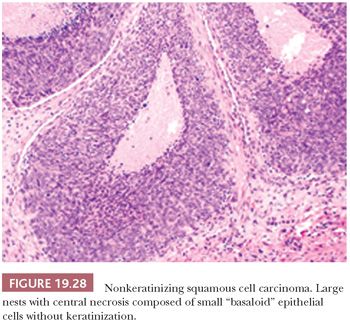
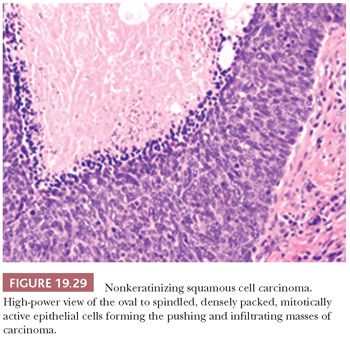
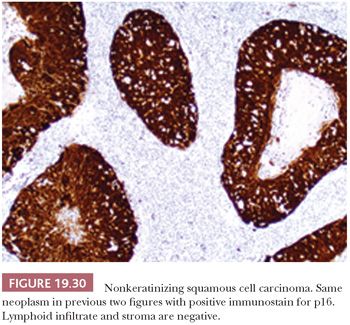
This is the most common form, about 50%, of oropharyngeal SCC. The appearance is that of pushing garlands, ribbons, and large nests, often with central necrosis (Fig. 19.28), composed of a dense proliferation of “basaloid” epithelial cells that are ovoid to spindle shaped (Fig. 19.29). Minimal keratinization is present. This type is significantly more likely, over 90%, to be HPV and p16 positive (Fig. 19.30) and have a better survival. In earlier literature, this type has been referred to as basaloid, basal-like, and poorly differentiated SCC (148).
NONKERATINIZING SQUAMOUS CELL CARCINOMA WITH MATURATION
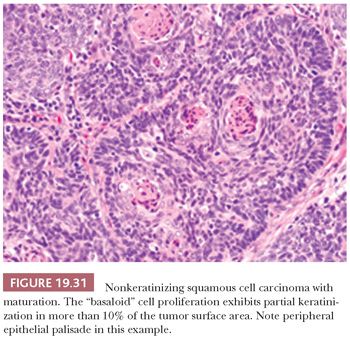
Another favorable type of oropharyngeal carcinoma, accounting for roughly 25%, is a nonkeratinizing carcinoma with maturation (partially keratinizing). It is hybrid both in morphology and in HPV association (roughly 74% to 84% positive) and in survival compared to the conventional keratinizing SCC and the purely nonkeratinizing SCC. It has been defined as having keratinizing features in greater than 10% of the tumor surface area (Fig. 19.31) (148,149). Identification of the HPV-positive nonkeratinizing and hybrid carcinomas is important, as they are more responsive to chemoradiation and radiation therapy than the conventional HPV-negative keratinizing carcinomas.
UNDIFFERENTIATED CARCINOMA
Undifferentiated carcinoma (nasopharyngeal type) is uncommon in the oropharynx. Roughly 90% are HPV and p16 positive but EBV negative, in contrast to the identical-appearing nasopharyngeal counterpart. Over 80% of patients are smokers and present with nodal metastases. HPV status is associated with a more favorable disease-specific survival analogous to other HPV-positive oropharyngeal carcinomas (150,151).
Other variant forms of SCC in the oropharynx that may show HPV positivity include papillary, adenosquamous, basaloid squamous, small cell (neuroendocrine), and adenoid cystic-like carcinomas. A rare spindle cell carcinoma may also be HPV positive. The influence of HPV status on biologic behavior of these SCC variants is discussed in the following text.
UNUSUAL VARIANTS OF SQUAMOUS CELL CARCINOMA
VERRUCOUS CARCINOMA
Verrucous carcinoma (VC) of the oral cavity was first described by Friedell and Rosenthal (152) in 1941 and was further elaborated as a locally invasive, nonmetastasizing variant of SCC by Ackerman (153) in 1948. It accounts for approximately 5% of oral cancers. The name of this clinicopathologic entity derives from its exophytic, warty clinical appearance (Fig. 19.27).
Clinical Features
In the oral cavity, the most common sites of occurrence of VC in decreasing order are the buccal mucosa, gingiva, tongue, palate, and tonsillar pillar. The average demographic profile is the same as most patients with oral cancer, with a predominance being seen among men late in the seventh decade of life (154). The oral tumors range from 1 to 10 cm in size (155). The tumor may extend by blunt invasion into the surrounding soft tissue and periosteum, and it may invade bone. Common symptoms include difficult mastication (because of a mass), ulcer, pain, and tenderness.
At one time, the association between long-term smokeless tobacco use and VC was thought to be quite strong (154), but this has not been substantiated with controlled clinical studies. Nonetheless, smokeless tobacco use is generally regarded as a risk factor for oral cancer, although the level of risk has likely been exaggerated. However, it has been well substantiated that intense smokers are at significant risk for developing verrucous and other types of oral SCCs (154,155). Alcohol abuse has not been implicated as a major etiologic agent in oral VC. The role of HPV in VC is not clear and needs more study as this time. In an older study, nearly 40% of 159 VCs tested were HPV positive, with 47% involving HPV-6/-11, 35.3% involving HPV-16/-18, and 17.7% involving HPV-2 (40). More recent studies find no correlation or a very low frequency of HPV without p16 overexpression in VC, suggesting no oncogenic role (156–158).
Morphologic Features
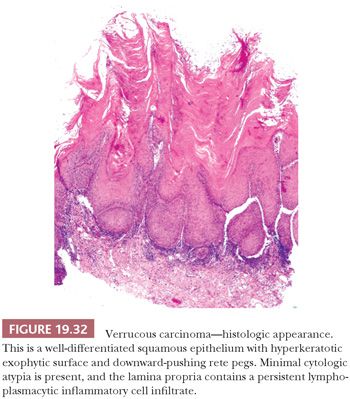
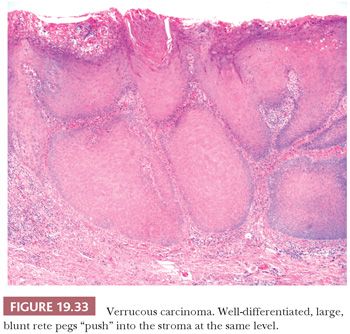
On clinical examination, VC appears as a relatively well-circumscribed, elevated, nodular mass with a surface that may be pebbled, papillary, verrucous, or smooth. Depending on the degree of surface keratinization, the VC varies in color from white to red to admixtures of both. The histologic picture of the tumor is well-differentiated squamous epithelium that exhibits orderly maturation in both upward and downward hyperplastic growth. The exophytic surface is covered by abundant orthokeratin and/or parakeratin that also fill the crevices of deep surface invaginations (Fig. 19.32). Because the lesions are well differentiated, superficial biopsies often do not yield a diagnosis. Clinical correlation can be of great value because lesions tend to be more impressive visually than microscopically. Typically, the epithelial downgrowth is made up of broad, blunt rete pegs that appear to be “pushing” into submucosa (Fig. 19.33). The advancing edge of the squamous epithelium is very well differentiated and may contain many mitotic figures that are rarely atypical. Unless the lesion is acutely inflamed, cytologic atypia is minimal. The lamina propria commonly contains a mild to moderate chronic inflammatory cell infiltrate.
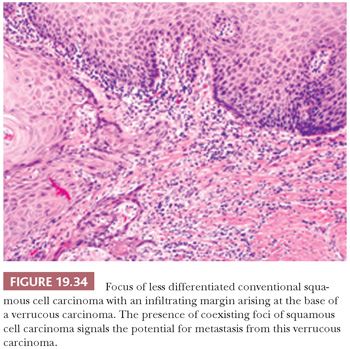
In 104 oral VCs, Medina et al. (159) identified 21 (20%) hybrid oral tumors that were characterized by VC patterns with foci of less differentiated infiltrating SCCs. This finding within an otherwise typical VC should lead to a diagnosis of “VC with a coexisting focus of SCC” to alert the clinician to the risk of metastasis (Fig. 19.34). Observing strict histologic criteria, VC is locally invasive but is unlikely to metastasize unless there is evidence of transformation to a conventional invasive SCC. Trauma and infection of bulky VCs may result in the reactive enlargement of regional lymph nodes, thus mimicking metastatic disease. Some recurrences of VC have been noted to take the form of less differentiated, nonverrucal carcinomas (160).
Differential Diagnosis
The clinical and histologic differential diagnosis of VC includes benign and malignant squamous proliferations. The benign lesions that should be considered are condyloma acuminatum, robust broad-based squamous papilloma, intraoral keratoacanthoma, pseudoepitheliomatous hyperplasia, and hyperkeratoses (leukoplakias) with warty, exophytic surfaces (proliferative verrucous leukoplakia and verrucous hyperplasia). Malignant lesions simulating VC include hyperkeratotic, bulky squamous dysplasias and well-differentiated SCCs exhibiting a papillary or verrucoid surface growth.
Broad-based squamous papillomas may be hyperkeratotic and have somewhat thick, club-shaped rete ridges, but they do not demonstrate acanthotic, downward pushing into underlying submucosa. Rarely seen oral keratoacanthomas exhibit the same architecture as their actinic cutaneous counterparts, most notable a symmetric cup-shaped morphology with a central keratin plug. The base may exhibit a blunt edge as is seen in VC, but it often has irregular tongues of epithelium that mimic invasion patterns of well-differentiated SCC. Intraoral pseudoepitheliomatous hyperplasia is typically seen in association with granular cell tumors, papillary hyperplasia of the palate, mycotic infections, and hyperplastic gingivitis. Although this form of hyperplasia may occasionally be hyperkeratotic, its irregular and pseudoinfiltrative character differs from the broad, pushing margins of VC.
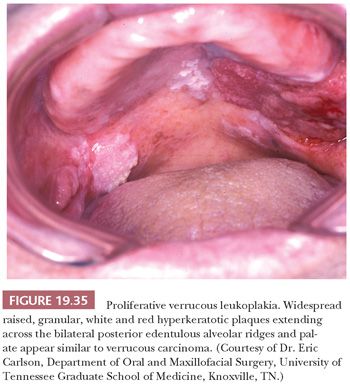
Although hyperkeratotic white lesions (leukoplakias) may have a shaggy, verrucoid surface simulating VC superficially, they do not show any expansion into submucosa. The intraoral hyperkeratoses known as proliferative verrucous leukoplakia and verrucous hyperplasia represent slow-growing, persistent, irreversible, and often multifocal proliferations that simulate VC on clinical (Fig. 19.35) and histologic grounds (Fig. 19.36) (161). These lesions are entirely exophytic and superficial to the adjacent normal epithelium, lacking the downward proliferation of the rete pegs beyond the level of the adjacent squamous mucosa. This is best evaluated in complete excisional biopsy specimens taken from the margins of the lesion. Proliferative verrucous leukoplakia is, however, a premalignant lesion that can transform into VC or SCC. It exhibits a significant association with coexisting VC (29%); conventional SCC, either coexisting or separate (10% to 37%); and epithelial dysplasia (26% to 66%) (162,163). With long-term follow-up, Silverman and Gorsky (81) have demonstrated the high-risk precancerous nature of proliferative verrucous leukoplakia, with SCC developing in 70% of patients in a mean time of 7.7 years. Interestingly, oral examples of proliferative verrucous leukoplakia have either tested negative for HPV by PCR (164,165) or have an HPV frequency, roughly 25% with HPV-18 or -16, similar to cases of conventional nonverrucous oral leukoplakia (166).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


