Chapter 66 1. List the common human respiratory viruses and modes of transmission. 2. Differentiate between viral antigenic shift and antigenic drift. Explain how each occurs, its effect on the production of vaccine, and why is it an important consideration in the study of the influenza virus. 3. Define the term “pandemic” and identify historical pandemics within the past century, including the latest influenza pandemic. 4. List the serotypes of rhinovirus and explain how testing for rhinovirus is accomplished and how it differs from testing for the other respiratory viruses. 5. List some of the most common human arboviruses. 6. Define arbovirus and describe the mode of transmission. 7. List the viruses responsible for viral encephalitis. 8. Name the most common sexually transmitted viral diseases. 9. Define tissue tropism associated with human papillomavirus (HPV) and explain the relationship between HPV and cervical cancer. 10. Define skin exanthema and identify the most common types affecting children. 11. Compare human gastrointestinal viruses, stating the types that affect adults more frequently and those that affect children. 12. Define hanta pulmonary syndrome; identify the disease-causing virus and the mode of transmission. 13. Name the family of viruses responsible for the skin eruptions orf and molluscum contagiosum. 14. List the family of viruses responsible for outbreaks of severe disease among military recruits and describe the recommended preventive measures. 15. Define the viral proteins hemagglutinin and neuraminidase; explain how these proteins function to ensure the transmissibility and reproducibility of the influenza virus. 16. Correlate the agents of specific infections shown in the following box with diseases and pathologic manifestations, including routes of transmission and appropriate diagnostic tests. Viruses of medical importance to humans comprise seven families of deoxyribonucleic acid (DNA) viruses and fourteen families of ribonucleic acid (RNA) viruses. This chapter examines the specific families of viruses, including the diseases and the symptoms associated with the viral infection. Tables 66-1 and 66-2 present a quick reference to the viral families and syndromes caused by these viruses. Table 66-1 divides the virus families according to the makeup of the viral genome, either RNA or DNA. Table 66-2 lists some of the common human viral infections. TABLE 66-1 DNA and RNA Viruses That Cause Serious Disease in Humans CMV, Cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; HTLV, human T-lymphotropic viruses; RSV, respiratory syncytial virus; SARS, severe acute respiratory syndrome; VZV, varicella-zoster virus. TABLE 66-2 Viral Syndromes and Common Viral Pathogens CMV, Cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; HPV, human papillomavirus; HSV, herpes simplex virus; HTLV, human T-lymphotropic viruses; RSV, respiratory syncytial virus; SARS, severe acute respiratory syndrome; VZV, varicella-zoster virus. Adenoviruses (Table 66-3) are medium-sized (70 to 90 nm), icosahedral, nonenveloped, double-stranded, linear DNA viruses. This virus was first isolated from cultures of human adenoids and tonsils in the early 1950s, hence the name adenovirus. The adenoviruses belong to the family Adenoviridae and are widely distributed in nature. However, only members of the genus Mastadenovirus cause human infection. Currently, 52 serotypes of human adenoviruses have been described. Most human disease is associated with one third of the viral types. These types are then divided into seven species, A through G, with species B subdivided into two subspecies; virus serotypes are then numbered within the species classification. The viruses can cause a broad range of disease in humans. Respiratory and gastrointestinal diseases are the most common clinical manifestation associated with adenovirus infection. TABLE 66-3 Arenaviruses, of the family Arenaviridae, include 29 spherical, enveloped RNA viruses that have T-shaped glycoprotein spikes 7 to 10 nm long surrounding the surface membrane of the virion (Table 66-4). The viruses can readily infect a variety of mammalian species, especially rodents and bats, often resulting in a deleterious effect on the reservoir rodent host. Human transmission usually occurs through inhalation of aerosols of infected rodent excrement (urine, saliva, feces, nasal secretions) or by direct contact with infected rodents. Disease in humans clinically displays a broad range of symptoms, from asymptomatic (no symptoms) to fever, prostration, headache and vomiting, to the more severe cases of meningitis and hemorrhagic fever. TABLE 66-4 Bunyaviruses, first detected in Bunyamwera, Uganda, belong to the family Bunyaviridae (Table 66-5). The virus is an RNA virus consisting of three, single-stranded RNA segments enclosed in a helical nucleocapsid that is surrounded by a lipid envelope. A unique feature of this family of viruses is their tripartite genome. The genomic structure provides a mechanism for genetic reassortment in nature, much like the orthomyxovirus family of viruses. Bunyaviruses comprise a large, diverse group of viruses (approximately 300 total members with 12 human pathogens), most of which are transmitted by mosquitoes (arboviruses). TABLE 66-5 Caliciviruses are small (30 to 38 nm), rounded, nonenveloped, single-stranded, positive RNA viruses that cause acute gastroenteritis in humans. Caliciviruses (Table 66-6) have been previously recognized as major animal pathogens and have a broad host range and disease manifestation. The virus causes respiratory disease in cats, a vesicular disease in swine, and a hemorrhagic disease in rabbits. Not until the 1990s did the taxonomic status of noroviruses (formerly known as Norwalk-like viruses, named after Norwalk, Ohio) and hepatitis E virus result in classification in the family Caliciviridae. Hepatitis E virus has since been removed from the calicivirus family and included in a new family, the Hepeviridae. (Hepatitis E virus is discussed later in this chapter.) TABLE 66-6 EIA, Enzyme immunoassay; EM, electron microscopy; RT-PCR, reverse transcriptase polymerase chain reaction. The family Coronaviridae includes the genera Torovirus and Coronavirus (CoV) and contains many species of both human and animal origin (Table 66-7). Once considered a harmless virus capable of causing the human “cold,” the CoVs cause a wide variety of disease in animals and birds. Interest in this virus and its relationship with animals and humans was renewed after the global outbreak of the novel coronavirus severe acute respiratory syndrome (SARS) in 2002 that resulted in severe respiratory distress in the human population. (The SARS outbreak is discussed in detail later in this chapter.) Coronaviruses are pleomorphic, roughly spherical, medium-sized, enveloped RNA viruses. The prefix corona- results from the viral structure and the crownlike surface projections on the external surface of the virus that can be seen with electron microscopy. Human respiratory coronaviruses cause colds and occasionally pneumonia in adults. Together the rhinoviruses and coronaviruses cause more than 55% of the “common colds” in the human populations. Viral transmission is person to person via contaminated respiratory secretions or aerosols. The virus is present in the highest concentration in the nasal passages, where it infects the nasal epithelial cells. Coronaviruses are thought to cause diarrhea in infants based on the presence (as seen with electron microscopy) of coronavirus-like particles in the stool of symptomatic patients. Although antigen detection is available, the technique lacks sensitivity compared with nucleic acid–based testing. No practical diagnostic methods other than electron microscopy and RT-PCR are available. Many CoVs do not grow in routine cell culture. Modified cell cultures have been useful when confirmatory testing with antigen- or nucleic acid–based methods are used. TABLE 66-7 EM, Electron microscopy; RT-PCR, reverse transcriptase polymerase chain reaction; SARS, severe acute respiratory syndrome. The Filoviridae family of viruses (Table 66-8) is considered the most pathogenic of the hemorrhagic fever viruses. The term filo means threadlike, referring to the virus’s long, filamentous structural morphology seen with electron microscopy. The viruses are pleomorphic, enveloped, nonsegmented, single-stranded, negative sense RNA viruses. The filamentous morphology appears in many forms or configurations under the electron microscope, such as the number “6,” “U,” or circular. Marburg hemorrhagic fever virus displays the characteristic “shepherd’s hook” morphology. The term “viral hemorrhagic fever” is used to describe a severe multisystem syndrome in which multiple organ systems are affected throughout the body. The patient’s vascular system becomes damaged, and the body’s ability to regulate itself is impaired. Infection with the Marburg or Ebola virus, endemic in Africa, results in severe hemorrhages, vomiting, abdominal pain, myalgia, pharyngitis, conjunctivitis, and proteinuria. Human case fatality rates for Ebola virus infection exceed 80%; the toll for Marburg virus infection is somewhat lower, with a case fatality rate of 23% to 25%. These diseases have no cure or established drug treatment. TABLE 66-8 The flaviviruses (family Flaviviridae; Table 66-9) include viruses that cause arbovirus diseases, such as yellow fever, dengue, West Nile viral encephalitis, and Japanese and St. Louis encephalitis. Hepatitis C virus (HCV) is a flavivirus but not an arbovirus. Flaviviruses are small, single-stranded, positive sense RNA, enveloped, icosahedral viruses. The name is derived from the Latin word flavus, which means yellow. The first disease identified in this group was yellow fever, which causes yellow jaundice in humans. Diseases in this viral group are transmitted to humans through the bite of an infected arthropod, usually the mosquito. TABLE 66-9 The hepatitis C virus causes hepatitis. Worldwide, an estimated 170 million people are HCV carriers, and about 4 million of those live in the United States. Acute infection with HCV progresses to a chronic infection in 50% to 90% of infected individuals (Figure 66-1). The acute infection with HCV often goes undiagnosed, because it is often asymptomatic. When clinical illness is present, it is generally mild. Chronic infection with HCV is an important cause of liver disease and is associated with the development of end-stage liver disease and hepatocellular carcinoma. The virus is transmitted predominantly by exposure to infected blood, such as during intravenous drug use and administration of contaminated blood products. The screening of blood products for HCV has eliminated the risk of transmission through contaminated blood products. Less efficient modes of transmission include sexual contact with infected partners, acupuncture, tattooing, and sharing of razors. Hepatitis E virus (HEV) is the type species of the new genus Hepevirus, in the family Hepeviridae (Table 66-10). Previously classified in the family of caliciviruses, HEV is a small, nonenveloped virus with a single-stranded RNA genome. The only other member of this virus family is an avian HEV known to cause enlarged liver and spleen disease in chickens. Several genetic and antigenic variants or strains of HEV exist and are referred to as genotypes. The different viral strains are common to different geographic locations. Genotype 3 is the strain found in the United States. HEV has also been isolated from swine worldwide and from wild deer in Japan. This indicates that the potential for transmission from animal to humans, resulting in a zoonotic human infection. TABLE 66-10 Hepatitis B virus (HBV) (Table 66-11) is the prototype virus of the Hepadnaviridae family (hepa- from hepatitis and dna from the genome type). The virus has long been recognized as a significant cause of liver damage associated with morbidity and mortality. Other mammalian and avian hepadnaviruses are known to exist. Hepadnavirus is a pleomorphic, enveloped virus containing circular, partially double-stranded DNA that replicates through an RNA intermediate. Replication occurs by means of reverse transcription and then DNA replication. TABLE 66-11 The incubation period for an acute HBV infection usually is 1 to 3 months but may be considerably longer. The initial symptoms of acute infection often are nonspecific, much like mild, flulike symptoms (Figure 66-2). Many cases are asymptomatic, especially in children. The infection presents as an acute or chronic hepatitis with a pathologic effect on the liver, resulting in self-limited or fatal outcomes. Fatal disease is most likely to occur in people co-infected with the hepatitis D virus (delta agent), a deficient RNA virus capable of replication in cells infected with HBV. Chronic HBV infection remains a significant worldwide cause of liver cirrhosis and hepatocellular carcinoma despite the availability of an effective vaccine. Eight human herpes group viruses have been described (Table 66-12). Herpes viruses are widely disseminated among animal species. However, the zoonotic forms of herpes do not infect humans, except for herpes B virus from nonhuman primates (not counted among the eight human herpes viruses). Herpes B virus causes a severe, usually fatal encephalitis in humans. Human herpes viruses include HSV types 1 and 2 (HSV-1 and HSV-2), varicella-zoster virus (VZV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV). More recently detected herpes viruses include human herpes virus (HHV) types 6 (HHV-6), 7 (HHV-7), and 8 (HHV-8). HHV-6 and HHV-7 are lymphotropic viruses acquired early in life. HHV-8, Kaposi’s sarcoma–associated herpes virus (KSHV), causes a tumor of the connective tissue. HHV-6 and HHV-7 are associated with the childhood disease roseola (exanthem subitum). The disease is characterized by a short period of fever and a skin rash. TABLE 66-12 EIA, Enzyme immunoassay; FA, fluorescent antibody; HDF, human diploid fibroblast; HIV, human immunodeficiency virus; IH, iron hematoxylin; PCR, polymerase chain reaction; WBCs, white blood cells.
Viruses in Human Disease
Viruses in Human Disease
Family
Viral Members
DNA Viruses
Adenoviridae
Human adenoviruses
Hepadnaviridae
Hepatitis B virus
Herpesviridae
HSV types I and II, VZV, CMV, EBV, human herpes viruses 6, 7, and 8
Papillomaviridae
Human papilloma viruses
Parvoviridae
Parvovirus B-19
Polyomaviridae
BK and JC polyomaviruses
Poxviridae
Variola, vaccinia, orf, molluscum contagiosum, monkeypox viruses
RNA Viruses
Arenaviridae
Lymphocytic choriomeningitis virus, Lassa fever virus
Astroviridae
Gastroenteritis-causing astroviruses
Bunyaviridae
Arboviruses, including California encephalitis and Lacrosse viruses; nonarboviruses, including sin nombre and related hantaviruses
Caliciviridae
Noroviruses and hepatitis E virus
Coronaviridae
Coronaviruses, including SARS coronavirus
Filoviridae
Ebola and Marburg hemorrhagic fever viruses
Flaviviridae
Arboviruses, including yellow fever, dengue, West Nile, Japanese encephalitis, and St. Louis encephalitis viruses; nonarboviruses, including hepatitis C virus
Orthomyxoviridae
Influenza A, B, and C viruses
Paramyxoviridae
Parainfluenza viruses, mumps virus, measles virus, RSV, metapneumovirus, Nipah virus
Picornaviridae
Polio viruses, coxsackie A viruses, coxsackie B viruses, echoviruses, enteroviruses 68-71, enterovirus 72 (hepatitis A virus), rhinoviruses
Reoviridae
Rotavirus spp., Colorado tick fever virus
Retroviridae
HIV types 1 and 2, HTLV types 1 and 2
Rhabdoviridae
Rabies virus
Togaviridae
Eastern, Western, and Venezuela equine encephalitis viruses, rubella virus
Viral Syndrome
Viral Pathogens
Infants and Children
Upper respiratory tract infection
Rhinovirus, coronavirus, parainfluenza, adenovirus, RSV, influenza
Pharyngitis
Adenovirus, coxsackie A, HSV, EBV, rhinovirus, parainfluenza, influenza
Croup
Parainfluenza, RSV, metapneumovirus
Bronchitis
Parainfluenza, RSV, metapneumovirus
Bronchiolitis
RSV, parainfluenza, metapneumovirus
Pneumonia
RSV, adenovirus, influenza, parainfluenza
Gastroenteritis
Rotavirus, adenovirus 40-41, calicivirus, astrovirus
Congenital and neonatal disease
HSV-2, echovirus, and other enteroviruses, CMV, parvovirus B-19, VZV, HIV, hepatitis viruses
Adults
Upper respiratory tract infection
Rhinovirus, coronavirus, adenovirus, influenza, parainfluenza, EBV
Pneumonia
Influenza, adenovirus, sin nombre virus (hantavirus), SARS coronavirus
Pleurodynia
Coxsackie B
Gastroenteritis
Noroviruses
All Patients
Parotitis
Mumps, parainfluenza
Myocarditis/pericarditis
Coxsackie B and echoviruses
Keratitis/conjunctivitis
HSV, VZV, adenovirus, enterovirus 70
Pleurodynia
Coxsackie B
Herpangina
Coxsackie A
Febrile illness with rash
Echoviruses and coxsackie viruses
Infectious mononucleosis
EBV, CMV
Meningitis
Echoviruses and coxsackie viruses; mumps, lymphocytic choriomeningitis viruses; HSV-2
Encephalitis
HSV-1, togaviruses, bunyaviruses, flaviviruses, rabies virus, enteroviruses, measles virus, HIV, JC virus
Hepatitis
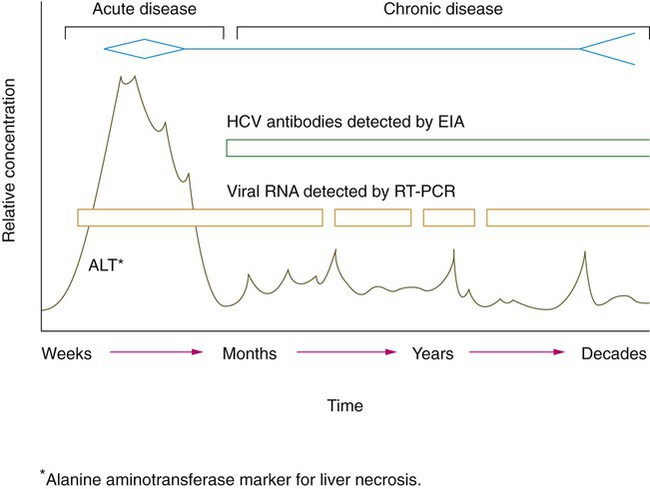
Hepatitis A, B, C, D (delta agent), E, and non-A, B, C, D, E viruses
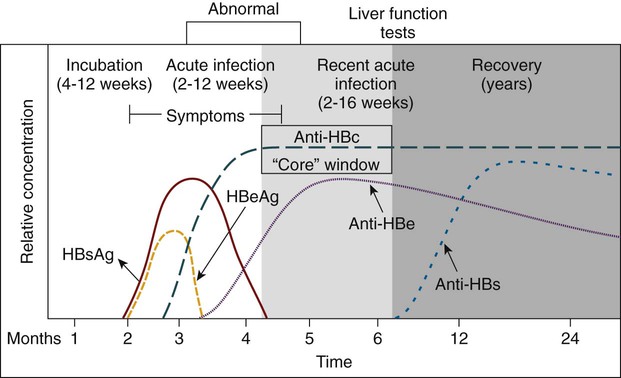
Hemorrhagic cystitis
Adenovirus, BK virus
Cutaneous infection with or without rash
HSV types 1 and 2; VZV; enteroviruses; measles, rubella viruses; parvovirus B-19; human herpes virus 6 and 7; HPV; poxviruses, including smallpox, monkeypox, molluscum contagiosum, and orf
Hemorrhagic fever
Ebola, Marburg, Lassa, yellow fever, dengue, and other viruses
Generalized, no specific target organ
HIV-1, HIV-2, HTLV-1
Adenoviruses
Family
Adenoviridae
Common name
Adenovirus
Virus
Adenovirus
Characteristics
Double-stranded DNA genome; icosahedral capsid, no envelope; approximately 50 human serotypes
Transmission
Respiratory, fecal-oral, and direct contact (eye)
Site of latency
Replication in oropharynx
Disease
Pharyngitis, pharyngoconjunctival fever, keratoconjunctivitis, pneumonia, hemorrhagic cystitis, disseminated disease, and gastroenteritis in children
Diagnosis
Cell culture (HEp-2 and other continuous human epithelial lines), enzyme immunoassay (EIA) for gastroenteritis serotypes 40-41
Treatment
Supportive
Prevention
Vaccine (adenovirus serotypes 4 and 7) for military recruits
Arenaviruses
Family
Arenaviridae
Common name
Arenavirus
Virus
Lymphocytic choriomeningitis (LCM) and Lassa fever (Lassa, Nigeria) viruses
Characteristics
Enveloped, irregular-shaped capsid containing a two-segmented (each segment is circular), single-stranded RNA genome
Transmission
From rodent to human through contamination of human environment with rodent urine; virus enters through skin abrasions or inhalation
Disease
LCM causes asymptomatic to influenza-like to aseptic meningitis–type disease; Lassa fever virus causes influenza-like disease to severe hemorrhagic fever
Diagnosis
Serology, polymerase chain reaction
Treatment
Supportive for LCM; ribavirin and immune plasma for Lassa fever
Prevention
Avoid contact with virus, institute rodent control; isolation and barrier nursing prevent nosocomial spread
Bunyaviruses
Family
Bunyaviridae
Common name
Bunyavirus
Virus
Arboviruses,* including the California encephalitis group containing Lacrosse virus, and non–arthropod-borne viruses, including hantaviruses (containing sin nombre virus)
Characteristics
Segmented, single-stranded, RNA genome; spherical or pleomorphic capsid with envelope
Transmission
Mosquito, tick, and sandfly vectors, except for hantaviruses, which are zoonoses transmitted by contact with rodent host and/or their excretions
Disease
Encephalitis for arboviruses; pneumonia or hemorrhagic fever for hantaviruses
Diagnosis
Serology and antibody detection in cerebrospinal fluid, reverse transcriptase polymerase chain reaction (RT-PCR) for hantaviruses (serology [IgM and IgG]) also available for hantavirus (sin nombre virus)
Treatment
Supportive
Prevention
Avoid contact with arthropod vector. Vector control programs; hantaviruses, avoid rodent urine and feces
Caliciviruses
Family
Caliciviridae
Common name
Calicivirus
Virus
Noroviruses
Characteristics
Nonenveloped, icosahedral capsid surrounding single-stranded RNA genome
Transmission
Fecal-oral
Disease
Nausea, vomiting, and diarrhea
Diagnosis
EM, RT-PCR, EIA for noroviruses
Treatment
Supportive
Prevention
Avoid contact with virus
Coronaviruses
Family
Coronaviridae
Common name
Coronaviruses
Virus
Coronavirus
Characteristics
Single-stranded, RNA genome; helical capsid with envelope
Transmission
Unknown, probably direct contact or aerosol
Disease
Common cold; possibly gastroenteritis, especially in children; SARS
Diagnosis
EM, RT-PCR
Treatment
Supportive
Prevention
Avoid contact with virus
Filoviruses
Family
Filoviridae
Common name
Filovirus
Virus
Ebola (or Ebola-Reston) and Marburg viruses
Characteristics
Enveloped, long, filamentous and irregular capsid forms with single-stranded RNA
Transmission
Transmissible to humans from monkeys and, presumably, other wild animals; human-to-human transmission via body fluids and respiratory droplets
Disease
Severe hemorrhage and liver necrosis; mortality as high as 90%
Diagnosis
Electron microscopy, cell culture in monkey kidney cells; Biosafety Level 4 required
Treatment
Supportive
Prevention
Avoid contact with virus; export prohibitions on wild monkeys
Flaviviruses
Family
Flaviviridae
Common name
Flavivirus
Characteristics
Single-stranded RNA genome surrounded by spherical and icosahedral capsid with envelope
Virus
Arboviruses,* including yellow fever, dengue, West Nile, Japanese encephalitis, and St. Louis encephalitis viruses
Transmission
Arthropod vector, usually mosquito
Disease
St. Louis and West Nile encephalitis, dengue and yellow fever
Diagnosis
Serology and antibody detection in cerebrospinal fluid; reverse transcriptase polymerase chain reaction (RT-PCR) for dengue and yellow fever
Treatment
Supportive
Prevention
Avoid contact with vector; vector control programs
Virus
Hepatitis C virus
Transmission
Parenteral or sexual
Disease
Acute and chronic hepatitis; strong correlation between chronic HCV infection and hepatocellular carcinoma
Diagnosis
Serology, RT-PCR and viral genotyping
Treatment
Supportive, interferon
Prevention
Avoid contact with virus; blood supply screened for antibody to hepatitis C virus
Hepevirus
Family
Hepeviridae
Common name
Hepatitis E
Virus
Hepevirus
Characteristics
Nonenveloped, icosahedral capsid surrounding single-stranded RNA genome
Transmission
Fecal-oral
Disease
Hepatitis similar to that caused by hepatitis A virus except for extraordinarily high case fatality rate (10% to 20%) among pregnant women
Diagnosis
Serology
Treatment
Supportive
Prevention
Avoid contact with virus
Hepadnaviruses
Family
Hepadnaviridae
Common name
Hepadnavirus
Virus
Hepatitis B virus (HBV)
Characteristics
Partly double-stranded DNA genome; icosahedral capsid with envelope; virion (also called Dane particle); surface antigen originally termed “Australia antigen”
Transmission
Humans are reservoir and vector; spread by direct contact, including exchange of body secretions, recipient of contaminated blood products, percutaneous injection of virus, and perinatal exposure
Site of latency
Liver
Disease
Acute infection with resolution (90%); fulminant hepatitis, most co-infected with delta virus (1%); chronic hepatitis, persistence of hepatitis B surface antigen (HBsAg) (9%) followed by resolution (disappearance of HBsAg), asymptomatic carrier state, chronic persistent (systemic disease without progressive liver disease), or chronic active disease (progressive liver damage)
Diagnosis
Serology, viral antigen detection, and polymerase chain reaction (PCR)
Oncogenic
Liver cancer
Treatment
Antivirals and liver transplant for fulminant disease
Prevention
HBV vaccine; hepatitis B immune globulin
Herpes Viruses
Family
Herpesviridae
Common name
Herpesvirus
Characteristics
Double-stranded DNA genome; icosahedral capsid with envelope; at least eight human herpes viruses known: HSV-1, HSV-2, VZV, Epstein-Barr virus (EBV), CMV, HHV-6, HHV-7, and HHV-8
Virus
Herpes simplex virus types I and II (HSV-1 and HSV-2)
Transmission
Direct contact with infected secretions
Site of latency
Sensory nerve ganglia
Disease
Predominant virus in parentheses. Gingivostomatitis (HSV-1), pharyngitis (HSV-1), herpes labialis (HSV-1), genital infection (HSV-2), conjunctivitis (HSV-1), keratitis (HSV-1), herpetic whitlow (HSV-1 and HSV-2), encephalitis (HSV-1 in adults), disseminated disease (HSV-1 or HSV-2 in neonates)
Detection
Cell culture (HDF, others), EIA, FA stain, IH stain, PCR
Treatment
Acyclovir, valacyclovir, famciclovir
Prevention
Avoid contact
Virus
Varicella-zoster virus (VZV)
Transmission
Close personal contact, especially respiratory
Site of latency
Dorsal root ganglia
Disease
Chicken pox (varicella), shingles (zoster)
Detection
FA stain, cell culture (HDF), shell vial culture, PCR
Treatment
Acyclovir and famciclovir
Prevention
Vaccine
Virus
Epstein-Barr virus (EBV)
Transmission
Close contact with infected saliva
Site of latency
B lymphocytes
Disease
Infectious mononucleosis, progressive lymphoreticular disease, oral hairy leukoplakia in patients with HIV
Detection
Serology, PCR
Oncogenic
Burkitt’s lymphoma, nasopharyngeal carcinoma
Treatment
Supportive
Prevention
Avoid contact
Virus
Cytomegalovirus (CMV)
Transmission
Close contact with infected secretions, blood transfusions (WBCs), organ transplants, transplacental
Site of latency
WBCs, endothelial cells, cells in a variety of organs
Disease
Asymptomatic infection, congenital disease of newborn, symptomatic disease of immunocompromised host, heterophile-negative infectious mononucleosis
Diagnosis
Cell culture (HDF), shell vial culture, CMV antigenemia, FA stain, PCR
Treatment
Supportive; decrease immune suppression; ganciclovir and foscarnet
Prevention
Use CMV antibody-negative blood and tissue for transfusion and transplantation, respectively
Virus
Human herpesviruses 6 and 7 (HHV-6 and HHV-7)
Transmission
Most likely close contact via respiratory route; almost all children infected by age 2 to 3 years
Site of latency
T lymphocytes (CD4 cells)
Disease
Roseola (exanthem subitum), fever, malaise, rash, leukopenia, and interstitial pneumonitis in organ transplant recipients
Detection
Detection of virus in peripheral blood specimens by PCR, cell culture using lymphocyte lines
Treatment
Susceptible to ganciclovir and foscarnet
Prevention
None practical
Virus
Human herpesvirus 8 (HHV-8)
Transmission
Not known; much less widely disseminated than other herpes viruses
Site of latency
Viral genome found in Kaposi’s tumor cells, endothelial cells, and tumor-infiltrating leukocytes
Disease
Kaposi’s sarcoma
Detection
PCR or in situ by hybridization
Treatment
None known
Prevention
Avoid contact with virus
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine