1. List the general characteristics within the genus Haemophilus, including general habitat, atmosphere, and temperature requirements. 2. Describe the infections caused by Haemophilus influenzae and Haemophilus ducreyi. 3. Describe the difference in the typeable and nontypeable categories of Haemophilus, their virulence factors, and the disease they cause. 4. Describe the Gram stain and colonial morphology of the various Haemophilus species. 5. Describe the isolation requirements necessary for optimal recovery of Haemophilus, including any special specimen processing or transport requirements. 6. Explain the satellite phenomenon and the chemical basis for the phenomenon. 7. List the X and V factor requirements for H. influenzae, H. parainfluenzae, and H. ducreyi. 8. Explain the principle of the porphyrin test. 9. Explain why routine susceptibility testing of clinical isolates for H. influenzae is only necessary on strains of clinical significance (i.e., sterile sites). 10. Correlate patient signs, symptoms, and laboratory data to identify the most probable etiologic agent associated with an infection. As presented in Table 32-1, except for Haemophilus ducreyi, Haemophilus spp. normally inhabit the upper respiratory tract of humans. Asymptomatic colonization with H. influenzae type b is rare. Although H. ducreyi is only found in humans, the organism is not part of our normal flora, and its presence in clinical specimens indicates infection. TABLE 32-1 Production of a capsule and factors that mediate bacterial attachment to human epithelial cells are the primary virulence factors associated with Haemophilus spp. In general, infections caused by Haemophilus influenzae are often systemic and life threatening, whereas infections caused by nontypeable (do not have a capsule) strains are usually localized (Table 32-2). The majority of serious infections caused by H. influenzae type b are typically biotypes I and II. The development and use of the conjugate vaccine in children since 1993 has reduced the infection rate by 95% in children younger than 5 years old in the United States. TABLE 32-2 Pathogenesis and Spectrum of Diseases Chancroid is the sexually transmitted disease caused by H. ducreyi (see Table 32-2). The initial symptom is the development of a painful genital ulcer and inguinal lymphadenopathy. Although small outbreaks of this disease have occurred in the United States, this disease is more common among socioeconomically disadvantaged populations inhabiting tropical environments. Epidemics of disease are associated with poor hygiene, prostitution, drug abuse, and poor socioeconomic conditions. Haemophilus spp. can be isolated from most clinical specimens. The collection and transport of these specimens are outlined in Table 5-1, with emphasis on the following points. First, Haemophilus spp. are susceptible to drying and temperature extremes. Therefore, specimens suspected of containing these organisms should be inoculated to the appropriate media immediately. Specimens susceptible to contamination with normal flora such as a lower respiratory specimen should be collected by bronchioalveolar lavage. In cases of pneumonia or cerebrospinal fluid (CSF) infection or suspected infection of any other normally sterile body fluid, blood cultures should also be collected. Gram stain is generally used for the direct detection of Haemophilus in clinical material (Figure 32-1). However, in some instances the acridine orange stain (AO; see Chapter 6 for more information on this technique) is used to detect smaller numbers of organisms that may be undetectable by gram staining. To increase the sensitivity of direct Gram stain examination of body fluid specimens, especially CSF, specimens may be centrifuged (2000 rpm for 10 minutes) and the smear is prepared from the pellet deposited in the bottom of the tube. Most laboratories are now equipped with a cytocentrifuge (10,000 × g for 10 minutes) used for concentration of specimens. This is highly recommended over traditional centrifugation for non-turbid specimens. This concentration step can increase the sensitivity of direct microscopic examination from five to tenfold. Moreover, cytocentrifugation of the specimen, in which clinical material is concentrated by centrifugation directly onto microscope slides, reportedly increases sensitivity of the Gram stain by as much as 100-fold (see Chapter 71 for information on infections of the central nervous system). Table 32-3 presents Haemophilus influenzae and H. parainfluenzae biotypes. TABLE 32-3 Differentiation of Haemophilus influenzae and H. parainfluenzae Biotypes
Haemophilus
Epidemiology
Organism
Habitat (Reservoir)
Mode of Transmission
Haemophilus influenzae
Normal flora: upper respiratory tract
Person-to-person: respiratory droplets
Endogenous strains
Haemophilus ducreyi
Not part of normal human flora; only found in humans during infection
Person-to-person: sexual contact
Other Haemophilus spp.
H. parainfluenzae
H. parahaemolyticus
Normal flora: upper respiratory tract
Endogenous strains
Pathogenesis and Spectrum of Disease
Organism
Virulence Factors
Spectrum of Disease and Infections
Haemophilus influenzae
Capsule:
Antiphagocytic, type b most common
Additional cell envelope factors
Mediate attachment to host cells
Unencapsulated strains:
pili and other cell surface factors mediate attachment
Encapsulated strains:
Meningitis
Epiglottitis
Cellulitis with bacteremia
Septic arthritis
Pneumonia
Nonencapsulated strains
Localized infections
Otitis media
Sinusitis
Conjunctivitis
Immunocompromised patients:
Chronic bronchitis
Pneumonia
Bacteremia
Haemophilus influenzae
Uncertain; probably similar to those of other H. influenzae
Purulent conjunctivitis single strain identified as the Brazilian purpuric fever, high mortality in children between ages 1 and 4; infection includes purulent meningitis, bacteremia, high fever, vomiting, purpura (i.e., rash), and vascular collapse
Haemophilus ducreyi
Uncertain, but capsular factors, pili, and certain toxins are probably involved in attachment and penetration of host epithelial cells
Chancroid; genital lesions progress from tender papules (i.e., small bumps) to painful ulcers with several satellite lesions; regional lymphadenitis is common
Other Haemophilus spp. and Aggregatibacter spp.
Uncertain; probably of low virulence.
Opportunistic pathogens
Associated with wide variety of infections similar to H. influenzae; A. aphrophilus is an uncommon cause of endocarditis and is the H member of the HACEK group of bacteria associated with slowly progressive (subacute) bacterial endocarditis
Laboratory Diagnosis
Specimen Collection and Transport
Direct Detection Methods
Direct Observation
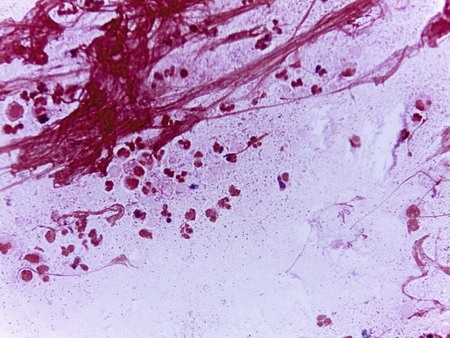
Organism and Biotype
Indole
Ornithine Decarboxylase
Urease
H. influenzae
I
pos
pos
pos
II
pos
pos
neg
III
neg
pos
neg
IV
neg
pos
pos
V
pos
neg
pos
VI
neg
pos
neg
VII
pos
neg
neg
VIII
neg
neg
neg
H. parainfluenzae
I
neg
neg
pos
II
neg
pos
pos
III
neg
pos
neg
IV
pos
pos
pos
V
neg
neg
neg
VI
pos
neg
pos
VII
pos
pos
neg
VIII
pos
neg
neg ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Haemophilus
